Long-Term Effect of Environmental Enrichment on Reproductive Performance of Swiss Webster Mice and Their Female Offspring
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
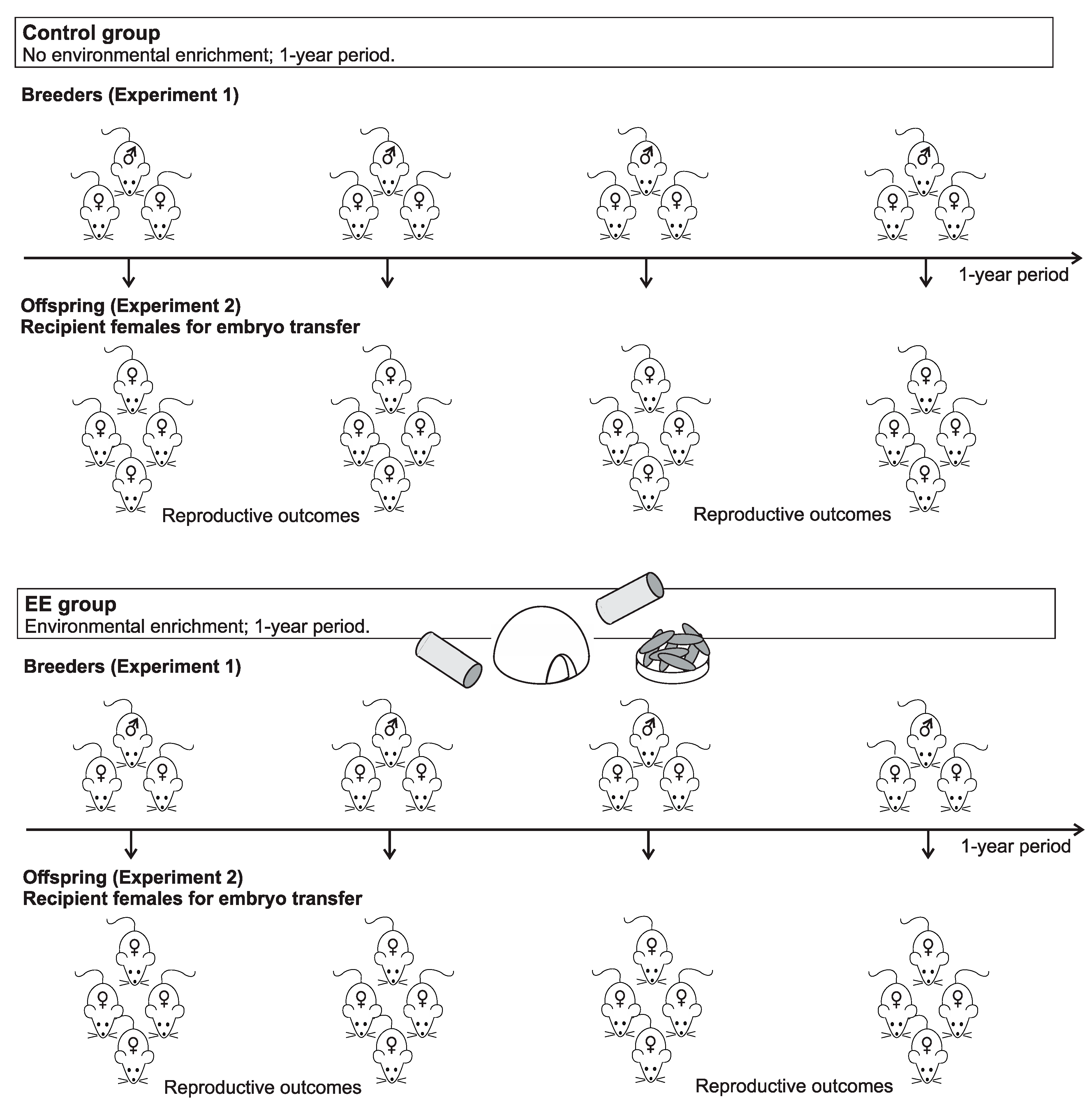
2.2. Experimental Design
2.3. Enrichment Protocol
2.4. Experiment 1: Breeding Mice
2.5. Experiment 2: Female Offspring Used as Embryo Recipients
2.6. Embryo Transfer in Recipient Females
2.7. Statistical Analysis
3. Results
3.1. Experiment 1: Breeding Colony
3.2. Experiment 2: Female Offspring Used as Embryo Recipients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NRC. Guide for the Care and Use of Laboratory Animals; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- OIE. Terrestrial Animal Health Code; World Organization for Animal Health: Paris, France, 2019. [Google Scholar]
- Baumans, V. Environmental enrichment for laboratory rodents and rabbits: Requirements of rodents, rabbits, and research. ILAR J. 2005, 46, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Environmental Enrichment for Captive Animals; Universities Federation for Animal Welfare: Wheathampstead, UK, 2003. [Google Scholar]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017, 220, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Available online: http://books.google.com/books?id=j75qAAAAMAAJ (accessed on 15 January 2020).
- Novaes, L.S.; Dos Santos, N.B.; Batalhote, R.F.P.; Malta, M.B.; Camarini, R.; Scavone, C.; Munhoz, C.D. Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology 2017, 113, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A. Environmental enrichment reduces chronic psychosocial stress-induced anxiety and ethanol-related behaviors in mice. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 77, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Novaes, L.S.; Dos Santos, N.B.; Perfetto, J.G.; Goosens, K.A.; Munhoz, C.D. Environmental enrichment prevents acute restraint stress-induced anxiety-related behavior but not changes in basolateral amygdala spine density. Psychoneuroendocrinology 2018, 98, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Brandt, Y.; Rodriguez-Martinez, H.; Madej, A. Conference lecture: Influence of stress on estrus, gametes and early embryo development in the sow. Theriogenology 2008, 70, 1197–1201. [Google Scholar] [CrossRef]
- Martin, B.; Golden, E.; Carlson, O.D.; Egan, J.M.; Mattson, M.P.; Maudsley, S. Caloric restriction: Impact upon pituitary function and reproduction. Ageing Res. Rev. 2008, 7, 209–224. [Google Scholar] [CrossRef]
- Whitaker, J.W.; Moy, S.S.; Pritchett-Corning, K.R.; Fletcher, C.A. Effects of Enrichment and Litter Parity on Reproductive Performance and Behavior in BALB/c and 129/Sv Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 387–399. [Google Scholar]
- Whitaker, J.; Moy, S.S.; Godfrey, V.; Nielsen, J.; Bellinger, D.; Bradfield, J. Effects of cage size and enrichment on reproductive performance and behavior in C57BL/6Tac mice. Lab. Anim. 2009, 38, 24–34. [Google Scholar] [CrossRef]
- Leidinger, C.S.; Thone-Reineke, C.; Baumgart, N.; Baumgart, J. Environmental enrichment prevents pup mortality in laboratory mice. Lab. Anim. 2019, 53, 53–62. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Pritchett-Corning, K.R.; Gordon, C.J.; Pajor, E.A.; Lucas, J.R.; Davis, J.K.; Garner, J.P. Energy reallocation to breeding performance through improved nest building in laboratory mice. PLoS ONE 2013, 8, e74153. [Google Scholar] [CrossRef]
- Fisch, J.; Oliveira, I.V.d.; Fank, J.; Paim, L.M.G.; Zandoná, M.R.; Lopes, E.F.; Mello, F.B.d.; Oliveira, A.T.D.d. Effects of environmental enrichment on reproductive performance and quantity and morphology of cumulus-oocyte complexes obtained from Rattus norvegicus. Theriogenology 2017, 94, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Araújo, A.J.; Farias, D.F.; Rocha-Bezerra, L.C.B.; Cavalheiro, M.G. Development and reproductive performance of Swiss mice in an enriched environment. Braz. J. Biol. 2009, 69, 153–160. [Google Scholar] [CrossRef]
- Shair, H.N.; Nunez, Y.; Osman, M.M. Enrichment materials do not negatively affect reproductive success and offspring survival and weight in mice. Lab. Anim. 2011, 41, 14. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.N.; Schlapp, G.; Menchaca, A.; Crispo, M. Minimum volume Spatula MVD vitrification method improves embryo survival compared to traditional slow freezing, both for in vivo and in vitro produced mice embryos. Cryobiology 2018, 84, 77–81. [Google Scholar] [CrossRef]
- Schlapp, G.; Goyeneche, L.; Fernandez, G.; Menchaca, A.; Crispo, M. Administration of the nonsteroidal anti-inflammatory drug tolfenamic acid at embryo transfer improves maintenance of pregnancy and embryo survival in recipient mice. J. Assist. Reprod. Genet. 2015, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version. Available online: infostat.dirienzo.com.ar/estudiantil/ (accessed on 18 August 2020).
- Yeshurun, S.; Short, A.K.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Paternal environmental enrichment transgenerationally alters affective behavioral and neuroendocrine phenotypes. Psychoneuroendocrinology 2017, 77, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kubota, M.; Watanabe, H. Significant improvement in survival of tabby jimpy mutant mice by providing folded-paper nest boxes. Scan. J. Lab. Anim. Sci. 2009, 36, 243–249. [Google Scholar]
- Galani, R.; Jarrard, L.E.; Will, B.E.; Kelche, C. Effects of postoperative housing conditions on functional recovery in rats with lesions of the hippocampus, subiculum, or entorhinal cortex. Neurobiol. Learn. Mem. 1997, 67, 43–56. [Google Scholar] [CrossRef]
- Berrocal, Y.; Pearse, D.D.; Singh, A.; Andrade, C.M.; McBroom, J.S.; Puentes, R.; Eaton, M.J. Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J. Neurotrauma 2007, 24, 1761–1772. [Google Scholar] [CrossRef]
- Pham, T.M.; Hagman, B.; Codita, A.; Van Loo, P.L.P.; Strömmer, L.; Baumans, V. Housing environment influences the need for pain relief during post-operative recovery in mice. Physiol. Behav. 2010, 99, 663–668. [Google Scholar] [CrossRef] [PubMed]
| Variables | Enrichment | Control | p-Value |
|---|---|---|---|
| No. of females | 18 | 18 | - |
| No. of litters | 59 ± 0.8 | 57 ± 0.7 | NS |
| No. of litters/female | 3.3 ± 0.3 | 3.2 ± 0.3 | NS |
| Litter size (No. of pups) | 7.1 ± 0.5 | 6.9 ± 0.4 | NS |
| Time (d) to 1st litter | 22.8 ± 0.4 | 24.4 ± 1.4 | NS |
| Interlitter interval (d) | 32.7 ± 1.6 | 36.5 ± 2.6 | NS |
| No. of pups weaned | 417 | 388 | - |
| Male | 220 | 203 | - |
| Female | 197 | 185 | - |
| M: F ratio (%) | 53:47 | 52:48 | NS |
| Pup weight at weaning (g) | 14.4 ± 0.1 | 13.8 ± 0.1 | <0.01 |
| Male | 14.6 ± 0.2 | 14.2 ± 0.2 | 0.07 |
| Female | 14.1 ± 0.2 | 13.4 ± 0.2 | <0.01 |
| Items | Enrichment | Control | p-Value |
|---|---|---|---|
| Pregnancy rate | 57.1% (20/35) | 68.0% (17/25) | NS |
| Litter size (No. of pups/female) | 2.4 ± 0.4 | 3.5 ± 0.7 | NS |
| No. of pups weaned | 48 | 59 | - |
| Male | 19 | 26 | - |
| Female | 29 | 33 | - |
| M: F ratio (%) | 40:60 | 44:56 | NS |
| Birth rate in total transferred females | 5.7% (48/846) | 10.3% (59/575) | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meikle, M.N.; Arévalo, A.P.; Schlapp, G.; Fernández-Graña, G.; Menchaca, A.; Crispo, M. Long-Term Effect of Environmental Enrichment on Reproductive Performance of Swiss Webster Mice and Their Female Offspring. Animals 2020, 10, 1438. https://doi.org/10.3390/ani10081438
Meikle MN, Arévalo AP, Schlapp G, Fernández-Graña G, Menchaca A, Crispo M. Long-Term Effect of Environmental Enrichment on Reproductive Performance of Swiss Webster Mice and Their Female Offspring. Animals. 2020; 10(8):1438. https://doi.org/10.3390/ani10081438
Chicago/Turabian StyleMeikle, María Noel, Ana Paula Arévalo, Geraldine Schlapp, Gabriel Fernández-Graña, Alejo Menchaca, and Martina Crispo. 2020. "Long-Term Effect of Environmental Enrichment on Reproductive Performance of Swiss Webster Mice and Their Female Offspring" Animals 10, no. 8: 1438. https://doi.org/10.3390/ani10081438
APA StyleMeikle, M. N., Arévalo, A. P., Schlapp, G., Fernández-Graña, G., Menchaca, A., & Crispo, M. (2020). Long-Term Effect of Environmental Enrichment on Reproductive Performance of Swiss Webster Mice and Their Female Offspring. Animals, 10(8), 1438. https://doi.org/10.3390/ani10081438






