Intra-Group Lethal Gang Aggression in Domestic Pigs (Sus scrofa domesticus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Farm Information
2.3. Victim Information
2.4. Data Analysis
2.4.1. Analyses of Farm Survey Data (n = 42)
2.4.2. Analyses of Victim Records (n = 316)
2.4.3. Analyses of Victim Data Obtained from Images (n = 91)
3. Results
3.1. Incidence of Gang Aggression on Affected Farms
3.2. Potential Causes
3.3. The Victim
4. Discussion
4.1. Survey Sample
4.2. More Aggression on Straw Bedding
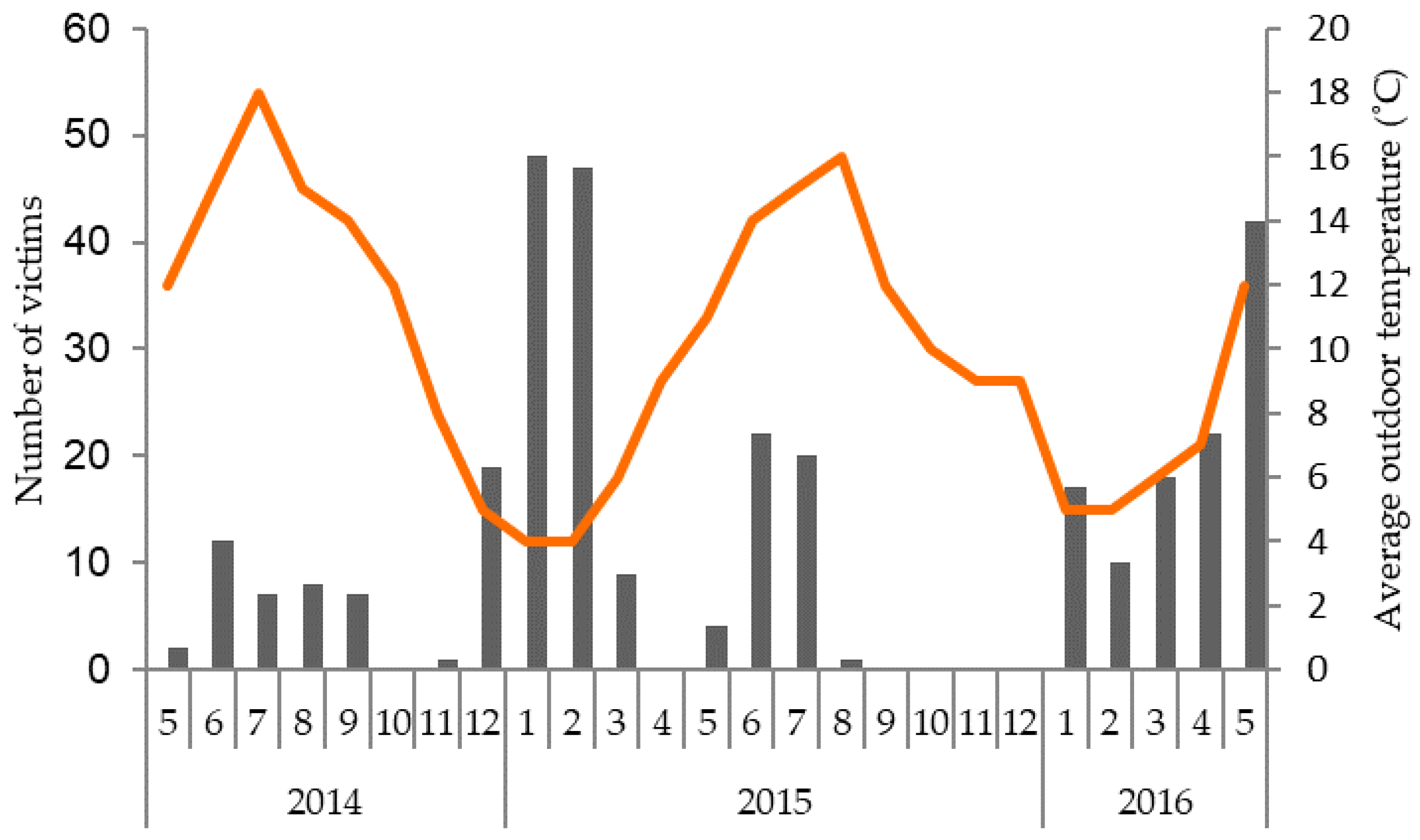
4.3. Seasonal Influences
4.4. Lethal Aggression in Female-Only Groups
4.5. Instability in Dominance Relationships
4.6. Lethal Aggression and Genetics
4.7. Nutrition
4.8. Welfare Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Question | Type |
|---|---|
| Aggression in stable (not recently mixed) groups of pigs There has been an increase in reports about aggression in stable groups of pigs, whereby the group can turn towards one individual. The Animal Behaviour & Welfare team at SRUC (a UK-based research institute) is looking at the possible causes of this type of aggression. We would be very grateful if you could help us with the following information. Your answers will be anonymous and used for research purposes only. Many thanks for your input. | Introduction |
| 1. Please describe the situation at your farm regarding aggression between pigs in stable groups | Open |
| 2. How often does severe aggression in stable groups (not recently mixed) occur? Occasionally, a few times a year; About 1–2× a month; Several times a month; In phases | Single choice |
| 3. What is the average age of the pigs in which this type of aggression occurs? | Open |
| 4. What are the results to the victim? Severe skin lesions on whole of body; Severe skin lesions on the front mainly; Severe skin lesions on the rear mainly; Lameness; Death | Multiple choice |
| 5. What type of breed/genetic line was used at the time when this aggression occurred? (optional to specify genetics company) | Open |
| 6. What is the average group size in which the aggression occurs? | Open |
| 7. What is the housing type for the affected pigs? Indoor, deep litter straw; Indoor with minimum bedding or without bedding; Outdoor; Outdoor access | Single choice |
| 8. What is the group composition? Only boars; Only gilts; Boars and gilts; Rearing gilts | Multiple choice |
| 9. Please describe the feeding strategy (feeder type; solid/liquid feed; feed company; mineral supplementation etc.) | Open |
| 10. Please provide any additional information that you deem relevant. | Open |
Appendix B
| Respondent | Quote |
|---|---|
| A (2) | “Without any recognizable signs or reasons all of the sudden all pigs of a group start to bit one specific member of the group to death…Within the last 7 years I had not a single case of tail biting or any other signs of abnormal behaviour.” |
| “It takes not even several hours before a victim is dead. In most cases it takes about ½ hour but not more than one hour.” | |
| B (3) | “Target pig exhibits high pitched squeal when attacked. If a pig is not removed immediately, result is death within 24 h. After segregation/death, pen behaviour returns to normal.” |
| “We have the same pigs in our conventional system (fully indoor, concrete slats) and they do not have this issue.” | |
| “I observed one of the targeted pigs stand up during a period of respite and immediately 2 of the bully pigs ran into the target at full speed to knock it back down to the floor.” | |
| “We have found that the best way to fight the problem is to have growers immediately segregate any pigs that are vocalizing a high pitched/painful sounding squeal.” | |
| C (4) | “You could watch them and all of a sudden they would pick on one pig and they would set about it and within a few minutes it would be dead if we didn’t get to it first.” |
| D (25) | “…[We have] no tail biting, only what I call savaging, large group 200 to 300 head in shed with outside feeding[,] floor all concrete…usually compact fat barrow most likely to be victim, but not always.” |
| E (28) | “The group will target one pig and kill it.” |
| F (32) | “They choose one pig and bully it to death. Later on sometimes they choose a next animal to bully.” |
References
- Lorenz, K. On Aggression; Methuen Publishing Ltd.: London, UK, 1966. [Google Scholar]
- Newton-Fisher, N.E.; Thompson, M.E. Comparative evolutionary perspectives on violence. In The Oxford Handbook of Evolutionary Perspectives on Violence, Homicide, and War; Oxford University Press: New York, NY, USA, 2012; pp. 41–60. [Google Scholar]
- Parker, G.A. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 1974, 47, 223–243. [Google Scholar] [CrossRef]
- Maynard Smith, J. Game theory and the evolution of behaviour. Proc. R. Soc. B. 1979, 205, 475–488. [Google Scholar] [CrossRef]
- Sherrow, H.M. Violence across animals and within early Hominins. In The Oxford Handbook of Evolutionary Perspectives on Violence, Homicide, and War; Oxford University Press: New York, NY, USA, 2012; pp. 23–40. [Google Scholar]
- Gilby, I.C.; Brent, L.J.; Wroblewski, E.E.; Rudicell, R.S.; Hahn, B.H.; Goodall, J.; Pusey, A.E. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 2013, 67, 373–381. [Google Scholar] [CrossRef]
- Feldblum, J.T.; Manfredi, S.; Gilby, I.C.; Pusey, A.E. The timing and causes of a unique chimpanzee community fission preceding Gombe’s “Four-Year War”. Am. J. Phys. Anthropol. 2018, 166, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior; Harvard University Press: Cambridge, UK, 1986. [Google Scholar]
- Watts, D.P.; Muller, M.; Amsler, S.J.; Mbabazi, G.; Mitani, J.C. Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am. J. Primatol. 2006, 68, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, K.; Muhumuza, G. Death of a wild chimpanzee community member: Possible outcome of intense sexual competition. Am. J. Primatol. 2000, 51, 243–247. [Google Scholar] [CrossRef]
- Kaburu, S.S.; Inoue, S.; Newton-Fisher, N.E. Death of the alpha: Within-community lethal violence among chimpanzees of the Mahale Mountains National Park. Am. J. Primatol. 2013, 75, 789–797. [Google Scholar] [CrossRef]
- De Villiers, M.S.; Richardson, P.R.; Van Jaarsveld, A.S. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J. Zool. 2003, 260, 377–389. [Google Scholar] [CrossRef]
- Silk, J.B.; Alberts, S.C.; Altmann, J. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim. Behav. 2004, 67, 573–582. [Google Scholar] [CrossRef]
- Meese, G.B.; Ewbank, R. A note on instability of the dominance hierarchy and variations in level of aggression within groups of fattening pigs. Anim. Sci. 1972, 14, 359–362. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Turner, S.P. The natural behaviour of the pig. In The Welfare of Pigs; Marchant-Forde, J.N., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 13–45. [Google Scholar]
- Barrette, C. Fighting behavior of wild Sus scrofa. J. Mammal. 1986, 67, 177–179. [Google Scholar] [CrossRef]
- Camerlink, I.; Arnott, G.; Farish, M.; Turner, S.P. Complex contests and the influence of aggressiveness in pigs. Anim. Behav. 2016, 121, 71–78. [Google Scholar] [CrossRef]
- Rushen, J.; Pajor, E. Offence and defence in fights between young pigs (Sus scrofa). Aggress. Behav. 1987, 13, 329–346. [Google Scholar] [CrossRef]
- Peden, R.S.; Turner, S.P.; Boyle, L.A.; Camerlink, I. The translation of animal welfare research into practice: The case of mixing aggression between pigs. Appl. Anim. Behav. Sci. 2018, 204, 1–9. [Google Scholar] [CrossRef]
- Desire, S.; Turner, S.P.; D’Eath, R.B.; Doeschl-Wilson, A.B.; Lewis, C.R.G.; Roehe, R. Genetic associations of short- and long-term aggressiveness identified by skin lesions with growth, feed efficiency and carcass characteristics in growing pigs. J. Anim. Sci. 2015, 93, 3303–3312. [Google Scholar] [CrossRef]
- Haller, J.; Mikics, E.; Halasz, J.; Tóth, M. Mechanisms differentiating normal from abnormal aggression: Glucocorticoids and serotonin. Eur. J. Pharmacol. 2005, 526, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Mikics, E.; Tulogdi, A.; Aliczki, M.; Haller, J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm. Behav. 2011, 60, 28–36. [Google Scholar] [CrossRef]
- Turner, S.P.; Horgan, G.W.; Edwards, S.A. Effect of social group size on aggressive behaviour between unacquainted domestic pigs. Appl. Anim. Behav. Sci. 2001, 74, 203–215. [Google Scholar] [CrossRef]
- Ewbank, R.; Bryant, M.J. Aggressive behaviour amongst groups of domesticated pigs kept at various stocking rates. Anim. Behav. 1972, 20, 21–28. [Google Scholar] [CrossRef]
- Turner, S.P.; Ewen, M.; Rooke, J.A.; Edwards, S.A. The effect of space allowance on performance, aggression and immune competence of growing pigs housed on straw deep-litter at different group sizes. Livest. Prod. Sci. 2000, 66, 47–55. [Google Scholar] [CrossRef]
- Arey, D.S.; Edwards, S.A. Factors influencing aggression between sows after mixing and the consequences for welfare and production. Livest. Prod. Sci. 1998, 56, 61–70. [Google Scholar] [CrossRef]
- Arey, D.S.; Franklin, M.F. Effects of straw and unfamiliarity on fighting between newly mixed growing pigs. Appl. Anim. Behav. Sci. 1995, 45, 23–30. [Google Scholar] [CrossRef]
- Greenwood, E.C.; Plush, K.J.; van Wettere, W.H.; Hughes, P.E. Hierarchy formation in newly mixed, group housed sows and management strategies aimed at reducing its impact. Appl. Anim. Behav. Sci. 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Livesey, C.T. Occurrence and significance of mycotoxins in forage crops and silage: A review. J. Sci. Food Agric. 1998, 77, 1–17. [Google Scholar] [CrossRef]
- Nordkvist, E.; Häggblom, P. Fusarium mycotoxin contamination of cereals and bedding straw at Swedish pig farms. Anim. Feed Sci. Technol. 2014, 198, 231–237. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Robert, H.; Payros, D.; Pinton, P.; Théodorou, V.; Mercier-Bonin, M.; Oswald, I.P. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Env. Heal. B 2017, 20, 249–275. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Leterrier, C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: A review. Physiol. Behav. 2019, 112658. [Google Scholar] [CrossRef]
- Kirchoff, N.S.; Udell, M.A.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef] [PubMed]
- Parois, S.P.; Duttlinger, A.W.; Richert, B.T.; Lindemann, S.R.; Johnson, J.S.; Marchant-Forde, J.N. Effects of three distinct 2-week long diet strategies after transport on weaned pigs’ short and long-term welfare markers, behaviors, and microbiota. Front. Vet. Sci. 2020, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.K.; DeChant, M.T.; Perry, E.B. When the nose doesn’t know: Canine olfactory function associated with health, management, and potential links to microbiota. Front. Vet. Sci. 2018, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, L.C.; Arthur, R.D.; Rosenthal, T.L. Predictors of social dominance and aggression in gilts. Appl. Anim. Behav. Sci. 1999, 63, 121–129. [Google Scholar] [CrossRef]
- Patterson, J.; Foxcroft, G. Gilt management for fertility and longevity. Animals 2019, 9, 434. [Google Scholar] [CrossRef]
- Vyas, S.; Briant, C.; Chemineau, P.; Le Danvic, C.; Nagnan-Le Meillour, P. Oestrus pheromones in farm mammals, with special reference to cow. Indian J. Anim. Sci. 2020, 82, 256–267. [Google Scholar]
- Wrubel, K.M.; Moon-Fanelli, A.A.; Maranda, L.S.; Dodman, N.H. Interdog household aggression: 38 cases (2006–2007). J. Am. Vet. Med. Assoc. 2011, 238, 731–740. [Google Scholar] [CrossRef]
- Meese, G.B.; Ewbank, R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav. 1973, 21, 326–334. [Google Scholar] [CrossRef]
- McGlone, J.J. A quantitative ethogram of aggressive and submissive behaviors in recently regrouped pigs. J. Anim. Sci. 1985, 61, 556–566. [Google Scholar] [CrossRef]
- Turner, S.P.; Farnworth, M.J.; White, I.M.; Brotherstone, S.; Mendl, M.; Knap, P.; Lawrence, A.B. The accumulation of skin lesions and their use as a predictor of individual aggressiveness in pigs. Appl. Anim. Behav. Sci. 2006, 96, 245–259. [Google Scholar] [CrossRef]
- Skinner, J.E. The role of the central nervous system in sudden cardiac death: Heartbeat dynamics in conscious pigs during coronary occlusion, psychologic stress and intracerebral propranolol. Integr. Psychol. Behav. Sci. 1994, 29, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Judge, M.D.; Eikelenboom, G.; Zuidam, L.; Sybesma, W. Blood acid-base status and oxygen binding during stress-induced hyperthermia in pigs. J. Anim. Sci. 1973, 37, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Parrott, R.F.; Vellucci, S.V.; Forsling, M.L.; Goode, J.A. Hyperthermic and endocrine effects of intravenous prostaglandin administration in the pig. Domest. Anim. Endocrinol. 1995, 12, 197–205. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; De Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.N.; Grommers, F.J. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Veroude, K.; Zhang-James, Y.; Fernàndez-Castillo, N.; Bakker, M.J.; Cormand, B.; Faraone, S.V. Genetics of aggressive behavior: An overview. Am. J. Med. Genet. B 2016, 171, 3–43. [Google Scholar] [CrossRef]
- Tiihonen, J.; Rautiainen, M.R.; Ollila, H.M.; Repo-Tiihonen, E.; Virkkunen, M.; Palotie, A.; Saarela, J. Genetic background of extreme violent behavior. Mol. Psychiatry 2015, 20, 786–792. [Google Scholar] [CrossRef]
- Muráni, E.; Ponsuksili, S.; D’Eath, R.B.; Turner, S.P.; Kurt, E.; Evans, G.; Wimmers, K. Association of HPA axis-related genetic variation with stress reactivity and aggressive behaviour in pigs. BMC Genet. 2010, 11, 74. [Google Scholar] [CrossRef]
- Manson, J.H.; Wrangham, R.W. Intergroup aggression in chimpanzees and humans. Curr. Anthropol. 1991, 32, 369–390. [Google Scholar] [CrossRef]
- Smith, J.E.; Van Horn, R.C.; Powning, K.S.; Cole, A.R.; Graham, K.E.; Memenis, S.K.; Holekamp, K.E. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 2010, 21, 284–303. [Google Scholar] [CrossRef]
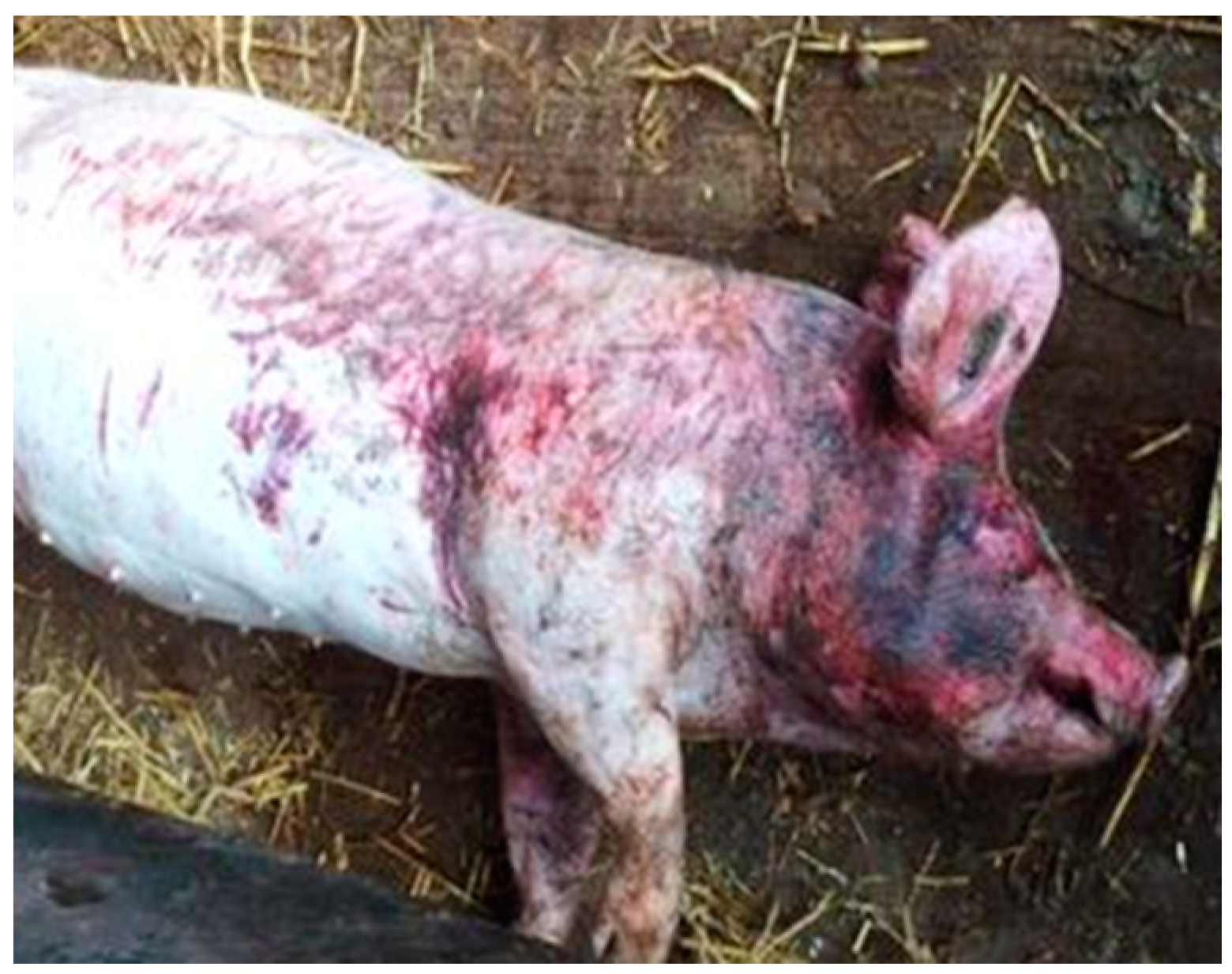
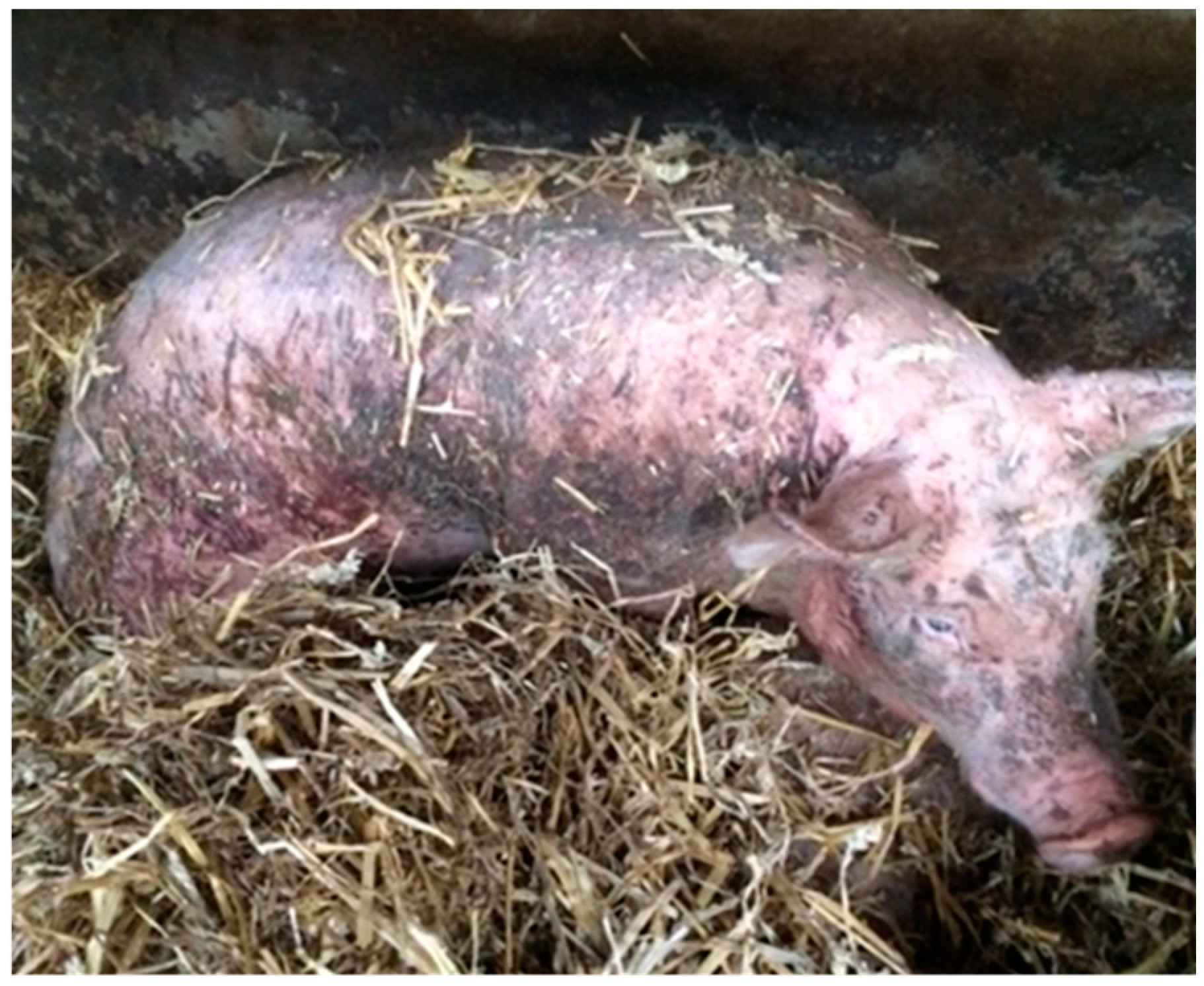
| Score | Description |
|---|---|
| 1 | 0–10 lesions, mostly unaffected skin |
| 2 | Approximately 1/3 of the body area is covered with lesions |
| 3 | Approximately 1/2 of the body area is covered with lesions |
| 4 | At least ¾ of the body area is covered with lesions |
| 5 | At least ½ of the body area is covered with lesions including patches of uncountable or deep lesions |
| Farm Characteristic | with Gang Aggression | without Gang Aggression | p-Value |
|---|---|---|---|
| Housing conditions | n = 22 | n = 17 | 0.01 |
| Indoor barren | 27.3 | 76.5 | |
| Deep litter straw | 59.1 | 5.9 | |
| Outdoor/outdoor access | 13.6 | 17.7 | |
| Group size | n = 23 | n = 17 | 0.80 |
| Small groups (≤30) | 34.8 | 70.6 | |
| Medium groups (31–100) | 30.4 | 29.4 | |
| Large groups (>100) | 17.4 | 0 | |
| Both small and large groups | 17.4 | 0 | |
| Group composition | n = 23 | n = 18 | 0.17 |
| Females only | 43.5 | 16.7 | |
| Males only | 4.4 | 0 | |
| Mixed groups | 52.2 | 83.3 | |
| Genetics | n = 20 | n = 13 | 0.42 |
| Purebred | 20.0 | 7.7 | |
| Cross bred | 60.0 | 53.9 | |
| Duroc cross bred | 20.0 | 38.5 | |
| Breeding company | n = 22 | n = 16 | 0.54 |
| A | 22.7 | 12.5 | |
| B | 27.3 | 12.5 | |
| C | 9.1 | 12.5 | |
| D | 27.3 | 0 | |
| E | 4.6 | 12.5 | |
| F | 0 | 12.5 | |
| G | 9.1 | 36.5 | |
| Feed type | n = 19 | n = 14 | 0.85 |
| Dry feed | 73.7 | 85.7 | |
| Wet (liquid) feed | 21.1 | 14.3 | |
| Both dry and wet feed | 5.3 | 0 | |
| Milling own feed | n = 9 | n = 9 | 0.35 |
| Yes | 55.6 | 33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerlink, I.; Chou, J.-Y.; Turner, S.P. Intra-Group Lethal Gang Aggression in Domestic Pigs (Sus scrofa domesticus). Animals 2020, 10, 1287. https://doi.org/10.3390/ani10081287
Camerlink I, Chou J-Y, Turner SP. Intra-Group Lethal Gang Aggression in Domestic Pigs (Sus scrofa domesticus). Animals. 2020; 10(8):1287. https://doi.org/10.3390/ani10081287
Chicago/Turabian StyleCamerlink, Irene, Jen-Yun Chou, and Simon P. Turner. 2020. "Intra-Group Lethal Gang Aggression in Domestic Pigs (Sus scrofa domesticus)" Animals 10, no. 8: 1287. https://doi.org/10.3390/ani10081287
APA StyleCamerlink, I., Chou, J.-Y., & Turner, S. P. (2020). Intra-Group Lethal Gang Aggression in Domestic Pigs (Sus scrofa domesticus). Animals, 10(8), 1287. https://doi.org/10.3390/ani10081287






