Productive Results, Oxidative Stress and Contaminant Markers in European Sea Bass: Conventional vs. Organic Feeding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Conditions
2.2. Animal Sampling and Samples Preparation
2.3. Histochemistry
2.4. Tissue Microarray
2.5. Immunohistochemistry
2.6. Melanomacrophage Centers (MMCs) Count
2.7. Glutathione (GSH)
2.8. Qualitative Reverse Transcription/PCR and Quantitative Real-Time PCR
2.9. Proximate Composition and FA Analysis
2.10. Statistical Analyses
3. Results
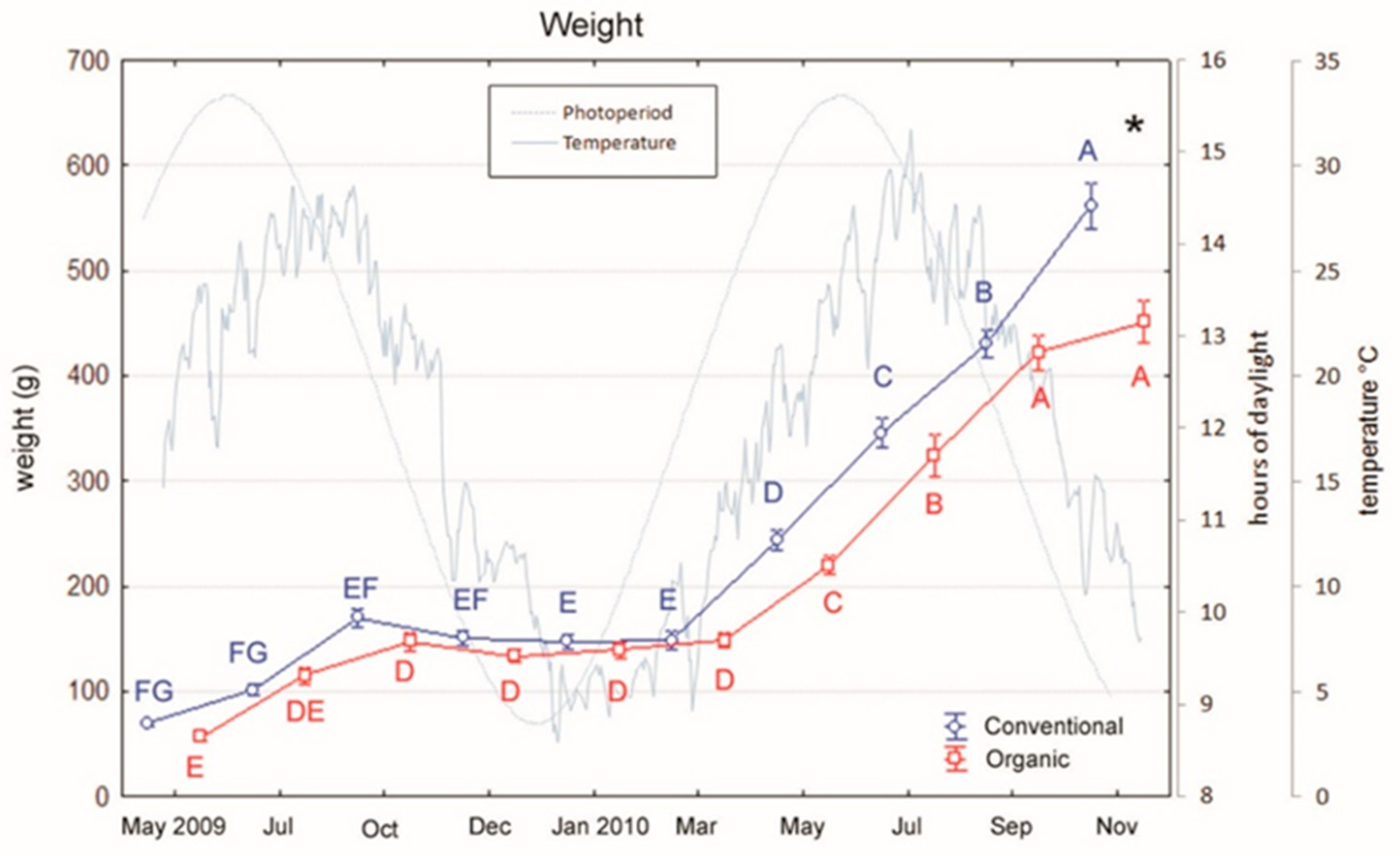
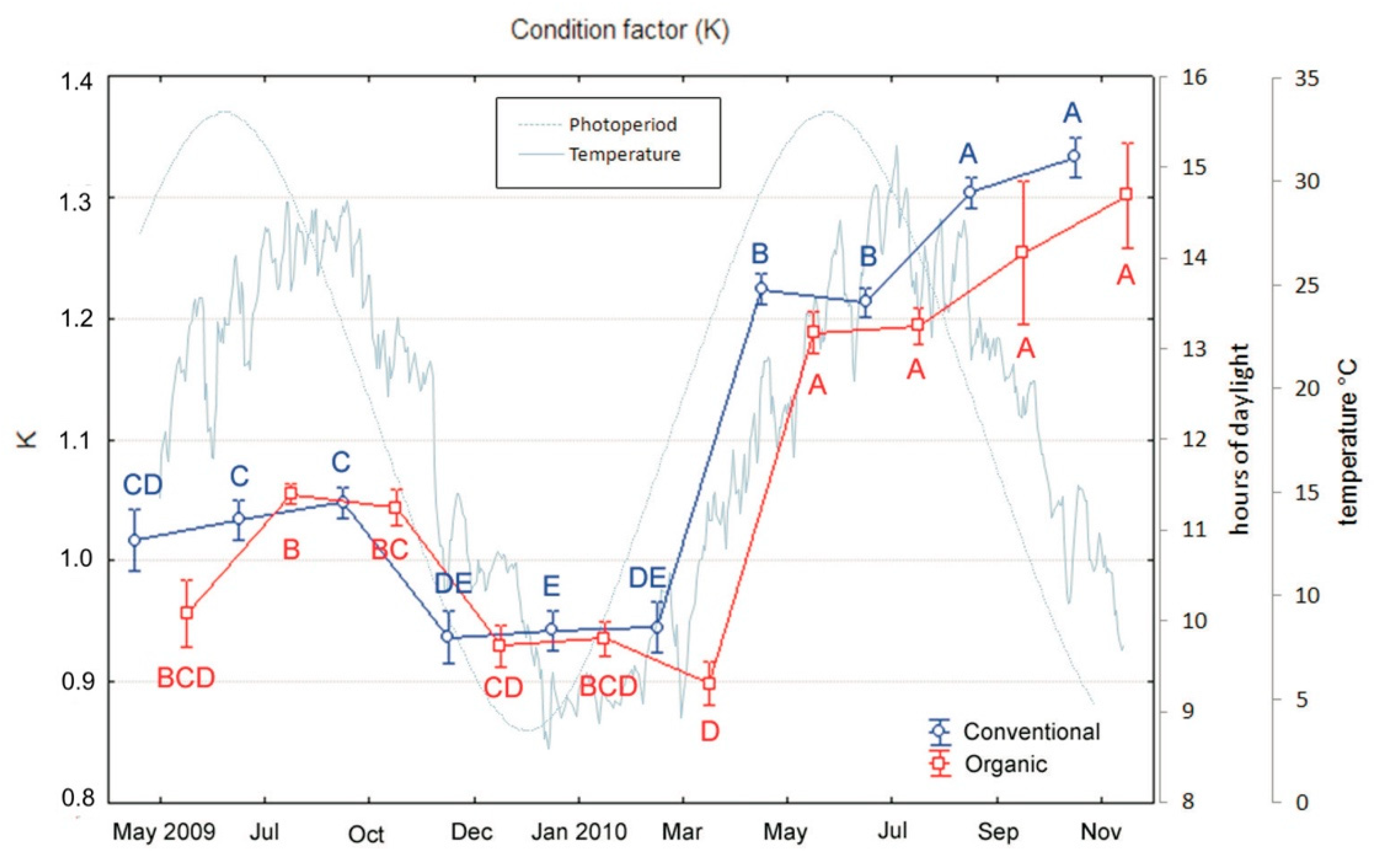
3.1. Growth
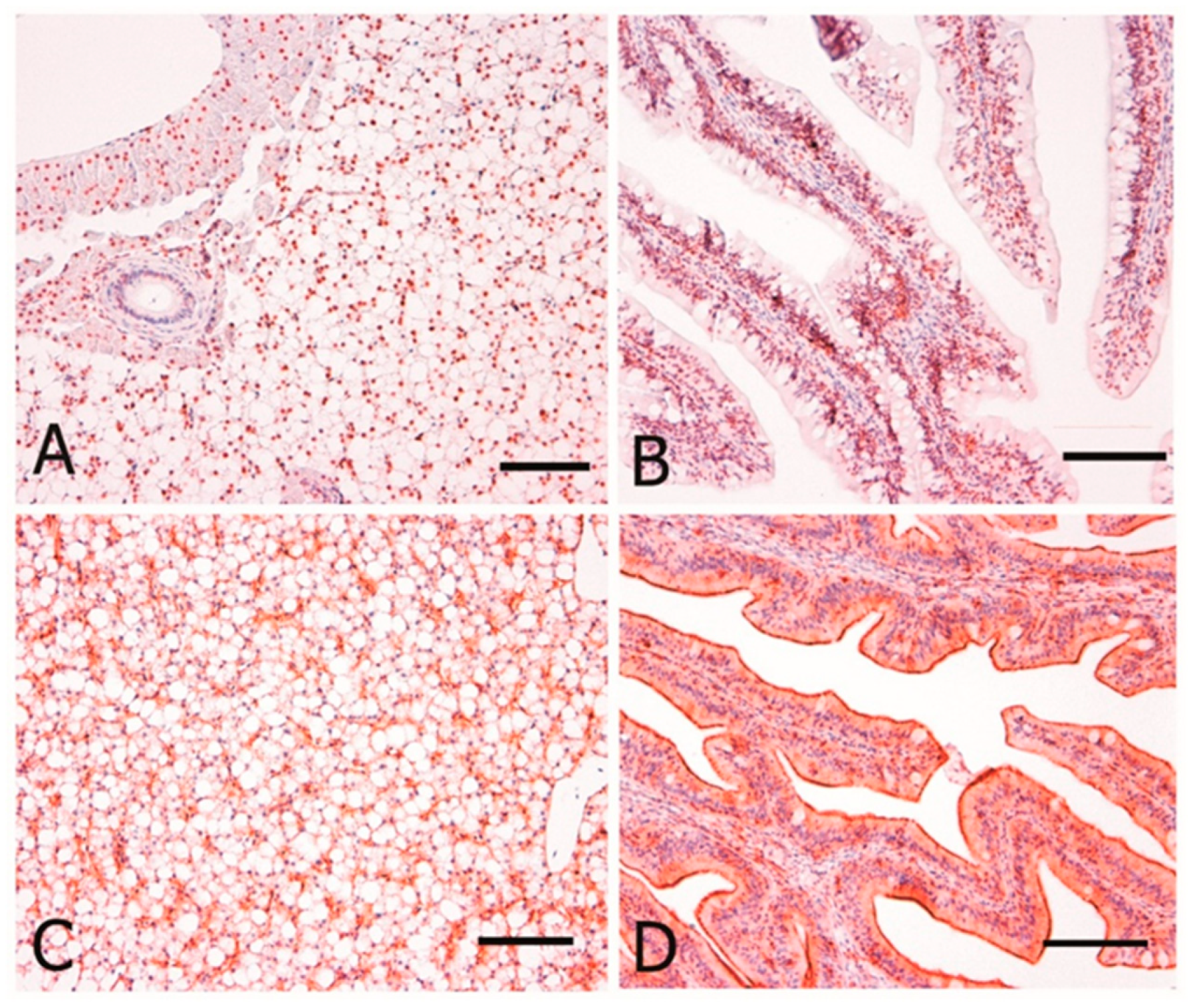
3.2. Immunohistochemistry
3.3. MMCs Count
3.4. Total Glutathione (GSH)
3.5. IGF-I and IGF-II expression
3.6. Proximate Composition and FA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Willer, H.; Schlatter, B.; Trávníček, J.; Kemper, L.; Lernoud, J. The world of organic agriculture 2020: Summary. In World of Organic Agriculture. Statistics and Emerging Trends 2020; Willer, H., Schlatter, B., Trávníček, J., Kemper, L., Lernoud, J., Eds.; Research Institute of Organic Agriculture (FiBL), Frick, and IFOAM–Organics International: Bonn, Germany, 2020; pp. 20–30. [Google Scholar]
- Di Marco, P.; Petochi, T.; Mariño, G.; Priori, A.; Finoia, M.; Tomassetti, P.; Porrello, S.; Giorgi, G.; Lupi, P.; Bonelli, A.; et al. Insights into organic farming of European sea bass Dicentrarchus labrax and gilthead sea bream Sparus aurata through the assessment of environmental impact, growth performance, fish welfare and product quality. Aquaculture 2017, 471, 92–105. [Google Scholar] [CrossRef]
- EUR-Lex, European Union Low. Organic Farming Regulation n.889/2008. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R0889/ (accessed on 25 March 2020).
- Satoh, S.; Hernandez, A.; Tokoro, T.; Morishita, Y.; Kiron, V.; Watanabe, T. Comparison of phosphorus retention efficiency between rainbow trout (Oncorhynchus mykiss) fed a commercial diet and a low fish meal based diet. Aquaculture 2003, 224, 271–282. [Google Scholar] [CrossRef]
- Wang, X.; Kim, K.-W.; Bai, S.C.; Huh, M.-D.; Cho, B.-Y. Effects of the different levels of dietary vitamin C on growth and tissue ascorbic acid changes in parrot fish (Oplegnathus fasciatus). Aquaculture 2003, 215, 203–211. [Google Scholar] [CrossRef]
- Albrektsen, S.; Mundheim, H.; Aksnes, A. Growth, feed efficiency, digestibility and nutrient distribution in Atlantic cod (Gadus morhua) fed two different fish meal qualities at three dietary levels of vegetable protein sources. Aquaculture 2006, 261, 626–640. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions*. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Shamblott, M.J.; Chen, T.T. Age-related and tissue-specific levels of five forms of insulin-like growth factor mRNA in a teleost. Mol. Mar. Biol. Biotechnol. 1993, 2, 351–361. [Google Scholar] [PubMed]
- Funkenstein, B.; Almuly, R.; Chan, S. Localization of IGF-I and IGF-I Receptor mRNA in Sparus aurata Larvae. Gen. Comp. Endocrinol. 1997, 107, 291–303. [Google Scholar] [CrossRef]
- Patruno, M.; Sivieri, S.; Poltronieri, C.; Sacchetto, R.; Maccatrozzo, L.; Martinello, T.; Funkenstein, B.; Radaelli, G. Real-time polymerase chain reaction, in situ hybridization and immunohistochemical localization of insulin-like growth factor-I and myostatin during development of Dicentrarchus labrax (Pisces: Osteichthyes). Cell Tissue Res. 2007, 331, 643–658. [Google Scholar] [CrossRef]
- Carnevali, O.; Cardinali, M.; Maradonna, F.; Parisi, M.; Olivotto, I.; Mosconi, G.; Funkenstein, B.; Polzonetti-Magni, A.M. Hormonal regulation of hepatic IGF-I and IGF-II gene expression in the Marine Teleost Sparus aurata. Mol. Reprod. Dev. 2005, 71, 12–18. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, C.; Bern, H.A. Insulin-like growth factor signaling in fish. Intern. Rev. Cytol. 2005, 243, 215–285. [Google Scholar] [CrossRef]
- Patruno, M.; Maccatrozzo, L.; Funkenstein, B.; Radaelli, G. Cloning and expression of insulin-like growth factors I and II in the shi drum (Umbrina cirrosa). Comp. Biochem. Physiol. B 2006, 144, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, G.; Poltronieri, C.; Bertotto, D.; Funkenstein, B.; Simontacchi, C. Cellular localization of insulin-like growth factor-II protein in the sea bass (Dicentrarchus labrax) from hatching to adult. Histol. Histopathol. 2008, 23, 523–530. [Google Scholar] [PubMed]
- Sargent, J.R.; Bell, J.G.; Bell, M.V.; Henderson, R.J.; Tocher, D.R. The metabolism of phospholipids and polyunsaturated fatty acids in fish. In Coastal and Estuarine Studies; American Geophysical Union (AGU): Washington, DC, USA, 1993; Volume 43, pp. 103–124. [Google Scholar]
- Sargent, J.R.; Bell, J.G.; Bell, M.V.; Henderson, R.J.; Tocher, D.R. Requirement criteria for essential fatty acids. J. Appl. Ichthyol. 1995, 11, 183–198. [Google Scholar] [CrossRef]
- Sargent, J.; McEvoy, L.; Bell, J. Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 1997, 155, 117–127. [Google Scholar] [CrossRef]
- Conte, F. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Poli, B.M. Farmed fish welfare-suffering assessment and impact on product quality. Ital. J. Anim. Sci. 2009, 8, 139–160. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Aldini, G.; Dalle-Donne, I.; Facino, R.M.; Milzani, A.D.G.; Carini, M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007, 27, 817–868. [Google Scholar] [CrossRef]
- Stegeman, J.J.; Hahn, M.E. Biochemistry and molecular biology of monooxygenases: Current perspective on forms, functions, and regulation of cytochrome P450 in aquatic species. In Aquatic toxicology; Molecular, Biochemical and Cellular Perspectives; Malins, D.C., Ostrander, G.K., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 87–203. [Google Scholar]
- Ploch, S.A.; Lee, Y.P.; MacLean, E.; Di Giulio, R.T. Oxidative stress in liver of brown bullhead and channel catfish following exposure to tert-butyl hydroperoxide. Aquat. Toxicol. 1999, 46, 231–240. [Google Scholar] [CrossRef]
- Mourente, G.; Díaz-Salvago, E.; Tocher, D.; Bell, J. Effects of dietary polyunsaturated fatty acid/vitamin E (PUFA/tocopherol ratio on antioxidant defence mechanisms of juvenile gilthead sea bream (Sparus aurata L., Osteichthyes, Sparidae). Fish. Physiol. Biochem. 2000, 23, 337–351. [Google Scholar] [CrossRef]
- Mourente, G.; Díaz-Salvago, E.; Bell, J.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Berntssen, M.; Julshamn, K.; Lundebye, A.K. Chemical contaminants in aquafeeds and Atlantic salmon (Salmo salar) following the use of traditional-versus alternative feed ingredients. Chemosphere 2010, 78, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Bilotto, S.; Nazzaro, M.; Russo, G.; Di Stasio, M.; Volpe, M. Effects of conventional and organic feed on the mineral composition of cultured European sea bass (Dicentrarchus labrax). Aquac. Nutr. 2016, 23, 796–804. [Google Scholar] [CrossRef]
- Nebert, D.W.; Gonzalez, F.J. P450 genes: Structure, evolution, and regulation. Ann. Rev. Biochem. 1987, 56, 945–993. [Google Scholar] [CrossRef] [PubMed]
- Sarasquete, C.; Segner, H. Cytochrome P4501A (CYP1A) in teleostean fishes. A review of immunohistochemical studies. Sci. Total. Environ. 2000, 247, 313–332. [Google Scholar] [CrossRef]
- Ribecco, C.; Hardiman, G.; Šášik, R.; Vittori, S.; Carnevali, O. Teleost fish (Solea solea): A novel model for ecotoxicological assay of contaminated sediments. Aquatic Toxicol. 2012, 109, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Meinelt, T.; Krüger, R.; Pietrock, M.; Osten, R.; Steinberg, C. Mercury pollution and macrophage centres in pike (Esox lucius) tissues. Environ. Sci. Pollut. Res. 1997, 4, 32–36. [Google Scholar] [CrossRef]
- Suresh, N. Effect of cadmium chloride on liver, spleen and kidney melano macrophage centres in Tilapia mossambica. J. Environ. Biol. 2009, 30, 505–508. [Google Scholar]
- Pascoli, F.; Negrato, E.; Di Giancamillo, A.; Bertotto, D.; Domeneghini, C.; Simontacchi, C.; Mutinelli, F.; Radaelli, G. Evaluation of oxidative stress biomarkers in Zosterisessor ophiocephalus from the Venice Lagoon, Italy. Aquatic Toxicol. 2011, 101, 512–520. [Google Scholar] [CrossRef]
- Passantino, L.; Santamaria, N.; Zupa, R.; Pousis, C.; Garofalo, R.; Cianciotta, A.; Jirillo, E.; Acone, F.; Corriero, A. Liver melanomacrophage centres as indicators of Atlantic bluefin tuna, Thunnus thynnus L. well-being. J. Fish. Dis. 2013, 37, 241–250. [Google Scholar] [CrossRef]
- Bucke, D.; Vethaak, A.; Lang, T. Quantitative assessment of melanomacrophage centres (MMCs) in dab Limanda limanda along a pollution transect in the German Bight. Mar. Ecol. Prog. Ser. 1992, 91, 193–196. [Google Scholar] [CrossRef]
- Roberts, R.J. Melanin-containing cells of the teleost fish and their relation to disease. In The Pathology of Fishes; Ribelin, W.E., Migaki, G., Eds.; University of Wisconsin Press: Madison, WI, USA, 1975; pp. 399–428. [Google Scholar]
- Agius, C. Preliminary studies on the ontogeny of the melano-macrophages of teleost haemopoietic tissues and age-related changes. Dev. Comp. Immunol. 1981, 5, 597–606. [Google Scholar] [CrossRef]
- Kranz, H. Changes in splenic melano-macrophage centres of dab Limanda limanda during and after infection with ulcer disease. Dis. Aquat. Org. 1989, 6, 167–173. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion of the panel on animal health and welfare on a request from the European commission on animal welfare aspects of husbandry systems for farmed European seabass and Gilthead seabream. EFSA J. 2008, 844, 1–21. [Google Scholar]
- Costa, C.; Menesatti, P.; Rambaldi, E.; Argenti, L.; Bianchini, M.L. Preliminary evidences of colour differences in European sea bass reared under organic protocols. Aquac Eng. 2013, 57, 82–88. [Google Scholar] [CrossRef]
- Fulton, T.W. Rate of growth of sea fishes. Sci. Invest. Fish. Div. Scotl. Reprod. 1902, 20, 1–22. [Google Scholar]
- EFSA, European Food Safety Authority. Available online: http://www.efsa.europa.eu/ (accessed on 2 April 2013).
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: Edinburgh, UK, 2008. [Google Scholar]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish. Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Richard, J.; Pascoli, F.; Simontacchi, C.; Radaelli, G. Whole body cortisol and expression of HSP70, IGF-I and MSTN in early development of sea bass subjected to heat shock. Gen. Comp. Endocrinol. 2011, 174, 44–50. [Google Scholar] [CrossRef]
- Trocino, A.; Xiccato, G.; Majolini, D.; Tazzoli, M.; Bertotto, D.; Pascoli, F.; Palazzi, R. Assessing the quality of organic and conventionally-farmed European sea bass (Dicentrarchus labrax). Food Chem. 2012, 131, 427–433. [Google Scholar] [CrossRef]
- Pascoli, F.; Lanzano, G.; Negrato, E.; Poltronieri, C.; Trocino, A.; Radaelli, G.; Bertotto, D. Seasonal effects on hematological and innate immune parameters in sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 2011, 31, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Pastoureaud, A. Influence of starvation at low temperatures on utilization of energy reserves, appetite recovery and growth character in sea bass, Dicentrarchus labrax. Aquaculture 1991, 99, 167–178. [Google Scholar] [CrossRef]
- Escobar-Aguirre, S.; Felip, A.; Mazón, M.J.; Ballester-Lozano, G.; Pérez-Sánchez, J.; Björsson, B.T.; Zanuy, S.; Carrillo, M. Long-term feeding of a maintenance ration affects the release of Igf-1 and leptin, and delays maturation in a male teleost fish, Dicentrarchus labrax L. Aquaculture 2020, 587, 735467. [Google Scholar] [CrossRef]
- Leggatt, R.A.; Brauner, C.J.; Iwama, G.K.; Devlin, R.H. The glutathione antioxidant system is enhanced in growth hormone transgenic coho salmon (Oncorhynchus kisutch). J. Comp. Physiol. B 2007, 177, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Mitić, S.S.B.; Radovanović, T.B.; Gavrilović, B.R.; Despotović, S.G.; Gavrić, J.P.; Saicic, Z. Seasonal variations of the activity of antioxidant defense enzymes in the Red Mullet (Mullus barbatus l.) from the Adriatic Sea. Mar. Drugs 2010, 8, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Pascual, P.; Pedrajas, J.R.; Toribio, F.; López-Barea, J.; Peinado, J. Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chem. Biol. Interact. 2003, 145, 191–199. [Google Scholar] [CrossRef]
- Fiocchi, E.; Civettini, M.; Carbonara, P.; Zupa, W.; Lembo, G.; Manfrin, A. Development of molecular and histological methods to evaluate stress oxidative biomarkers in sea bass (Dicentrarchus labrax). Fish. Physiol. Biochem. 2020, 1–12. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M.; Tort, L.; Robaina, L.; Vergara, J. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish. Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Magrone, T.; Fontana, S.; Laforgia, F.; Dragone, T.; Jirillo, E.; Passantino, L. Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labraxL.) modulates intestinal and spleen immune responses. Oxid. Med. Cell. Longev. 2015, 2016, 1–11. [Google Scholar] [CrossRef]
- Arciuli, M.; Fiocco, D.; Fontana, S.; Arena, M.P.; Frassanito, M.A.; Gallone, A. Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labrax L.): Kidney melanomacrophages response. Fish. Shellfish. Immunol. 2017, 68, 404–410. [Google Scholar] [CrossRef]
- Jacobs, M.; Covaci, A.; Schepens, P. Investigation of selected persistent organic pollutants in farmed Atlantic salmon (Salmo salar), salmon aquaculture feed, and fish oil components of the feed. Environ. Sci. Technol. 2002, 36, 2797–2805. [Google Scholar] [CrossRef]
- Hites, R.A.A.; Foran, J.; Carpenter, D.O.; Hamilton, M.C.; Knuth, B.A.; Schwager, S.J. Global assessment of organic contaminants in farmed salmon. Science 2004, 303, 226–229. [Google Scholar] [CrossRef]
- Berntssen, M.; Olsvik, P.; Torstensen, B.E.; Julshamn, K.; Midtun, T.; Goksøyr, A.; Johansen, J.; Sigholt, T.; Joerum, N.; Jakobsen, J.-V.; et al. Reducing persistent organic pollutants while maintaining long chain omega-3 fatty acid in farmed Atlantic salmon using decontaminated fish oils for an entire production cycle. Chemosphere 2010, 81, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Olli, J.J.; Breivik, H.; Mørkøre, T.; Ruyter, B.; Johansen, J.; Reynolds, P.; Thorstad, O.; Berge, G. Removal of persistent organic pollutants from Atlantic salmon (Salmo salar L.) diets: Influence on growth, feed utilization efficiency and product quality. Aquaculture 2010, 310, 145–155. [Google Scholar] [CrossRef]
- Crampton, V.; Nanton, D.; Ruohonen, K.; Skjervold, P.O.; El-Mowafi, A. Demonstration of salmon farming as a net producer of fish protein and oil. Aquac. Nutr. 2010, 16, 437–446. [Google Scholar] [CrossRef]
- Grigorakis, K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: A review. Aquaculture 2007, 272, 55–75. [Google Scholar] [CrossRef]
- Turchini, G.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Bermejo-Nogales, A.; Karampatos, A.I.; Ballester-Lozano, G.F.; Navarro, J.C.; Diez, A.; Bautista, J.M.; Bell, J.G.; Tocher, D.R.; Obach, A.; et al. Modelling the predictable effects of dietary lipid sources on the fillet fatty acid composition of one-year-old gilthead sea bream (Sparus aurata L.). Food Chem. 2011, 124, 538–544. [Google Scholar] [CrossRef]




| Rearing System (R) | Organic | Conventional |
|---|---|---|
| Proximate composition | ||
| Water (%) | 7.10 | 6.32 |
| Ether extract (%) | 20.3 | 17.2 |
| Crude protein (%) | 40.7 | 44.5 |
| Crude fibre (%) | 0.73 | 1.18 |
| Ash (%) | 12.3 | 7.73 |
| Fatty acid profile | ||
| C14:0 | 5.03 | 3.05 |
| C15:0 | 0.37 | 0.23 |
| C16:0 | 13.1 | 12.3 |
| C17:0 | 0.35 | 0.32 |
| C18:0 | 2.50 | 3.82 |
| C20:0 | 0.21 | 0.28 |
| Other SFAs | 0.77 | 0.53 |
| Total SFAs | 22.4 | 20.6 |
| C16:1n-7 | 3.90 | 3.03 |
| C18:1n-7 | 3.93 | 3.99 |
| C18:1n-9 | 18.5 | 21.3 |
| C20:1n-9 | 2.76 | 0.84 |
| C22:1n-9 | 0.71 | 0.13 |
| C22:1n-11 | 8.08 | 0.62 |
| C24:1n-9 | 0.46 | 0.19 |
| Other MUFAs | 0.81 | 0.35 |
| Total MUFAs | 39.0 | 30.4 |
| C18:3n-3 | 2.92 | 4.96 |
| C18:4n-3 | 5.82 | 0.79 |
| C20:5n-3 | 4.47 | 5.96 |
| C22:5n-3 | 0.91 | 0.74 |
| C22:6n-3 | 5.90 | 3.10 |
| PUFAs n-3 | 20.0 | 15.6 |
| C18: 2n-6 | 13.3 | 29.3 |
| C20:4n-6 | 0.24 | 0.31 |
| PUFAs n-6 | 13.5 | 29.6 |
| Ratio of n-3 to n-6 PUFAs | 1.48 | 0.53 |
| Other PUFAs | 1.00 | 0.67 |
| Total PUFAs | 34.5 | 45.8 |
| Unknown FAs | 4.15 | 3.21 |
| Rearing System (R) | Organic | Conventional | Probability | RSD |
|---|---|---|---|---|
| Live weight (g) | 432 | 473 | 0.18 | 57 |
| Proximate composition | ||||
| Water (%) | 71.3 | 69.3 | 0.08 | 0.60 |
| Ether extract (%) | 7.93 | 9.72 | 0.06 | 0.47 |
| Crude protein (%) | 19.5 | 19.5 | 0.92 | 0.37 |
| Ash (%) | 1.14 | 1.16 | 0.41 | 0.01 |
| Fatty acid profile | ||||
| C14:0 | 4.32 | 3.30 | 0.10 | 0.34 |
| C15:0 | 0.53 | 0.40 | 0.15 | 0.06 |
| C16:0 | 16.6 | 17.1 | 0.66 | 0.94 |
| C17:0 | 0.54 | 0.49 | 0.32 | 0.04 |
| C18:0 | 7.45 | 7.00 | 0.84 | 2.10 |
| Other SFAs | 4.18 | 2.90 | 0.22 | 0.72 |
| Total SFAs | 33.6 | 31.2 | 0.62 | 4.16 |
| C16:1n-7 | 4.50 | 4.52 | 0.59 | 0.03 |
| C18:1n-9 | 19.6 | 22.8 | 0.05 | 0.77 |
| C18:1n-7 | 2.24 | 2.44 | 0.04 | 0.04 |
| C20:1n-9 | 4.24 | 1.75 | < 0.001 | 6.15 |
| C22:1n-11 | 3.41 | 0.69 | < 0.001 | 7.40 |
| Other MUFAs | 3.41 | 1.54 | 0.03 | 0.34 |
| Total MUFAs | 37.4 | 33.8 | 0.02 | 0.53 |
| C18:3n-3 | 1.71 | 1.77 | 0.75 | 0.00 |
| C18:4n-3 | 1.04 | 0.73 | 0.09 | 0.10 |
| C20:5n-3 | 2.72 | 4.41 | 0.10 | 0.56 |
| C22:5n-3 | 0.70 | 0.96 | 0.29 | 0.18 |
| C22:6n-3 | 3.36 | 3.67 | 0.79 | 1.06 |
| PUFAs n-3 | 10.1 | 12.0 | 0.45 | 2.10 |
| C18: 2n-6 | 11.0 | 14.3 | 0.04 | 0.69 |
| C20:4n-6 | 0.34 | 0.46 | 0.08 | 0.04 |
| PUFAs n-6 | 12.0 | 15.8 | 0.04 | 0.79 |
| Ratio of n-3 to n-6 PUFAs | 0.83 | 0.76 | 0.64 | 0.12 |
| Other PUFAs | 3.03 | 3.63 | 0.10 | 0.20 |
| Total PUFAs | 23.8 | 29.9 | 0.18 | 2.96 |
| Unknown FAs | 5.14 | 5.13 | 0.99 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carminato, A.; Pascoli, F.; Trocino, A.; Locatello, L.; Maccatrozzo, L.; Palazzi, R.; Radaelli, G.; Ballarin, C.; Bortoletti, M.; Bertotto, D. Productive Results, Oxidative Stress and Contaminant Markers in European Sea Bass: Conventional vs. Organic Feeding. Animals 2020, 10, 1226. https://doi.org/10.3390/ani10071226
Carminato A, Pascoli F, Trocino A, Locatello L, Maccatrozzo L, Palazzi R, Radaelli G, Ballarin C, Bortoletti M, Bertotto D. Productive Results, Oxidative Stress and Contaminant Markers in European Sea Bass: Conventional vs. Organic Feeding. Animals. 2020; 10(7):1226. https://doi.org/10.3390/ani10071226
Chicago/Turabian StyleCarminato, Antonio, Francesco Pascoli, Angela Trocino, Lisa Locatello, Lisa Maccatrozzo, Renato Palazzi, Giuseppe Radaelli, Cristina Ballarin, Martina Bortoletti, and Daniela Bertotto. 2020. "Productive Results, Oxidative Stress and Contaminant Markers in European Sea Bass: Conventional vs. Organic Feeding" Animals 10, no. 7: 1226. https://doi.org/10.3390/ani10071226
APA StyleCarminato, A., Pascoli, F., Trocino, A., Locatello, L., Maccatrozzo, L., Palazzi, R., Radaelli, G., Ballarin, C., Bortoletti, M., & Bertotto, D. (2020). Productive Results, Oxidative Stress and Contaminant Markers in European Sea Bass: Conventional vs. Organic Feeding. Animals, 10(7), 1226. https://doi.org/10.3390/ani10071226







