Alzheimer’s Disease, and Breast and Prostate Cancer Research: Translational Failures and the Importance to Monitor Outputs and Impact of Funded Research
Simple Summary
Abstract
1. Introduction
2. Three Biomedical Research Areas Characterized by a High Rate of Translational Failure: Alzheimer’s Disease, Breast Cancer, and Prostate Cancer
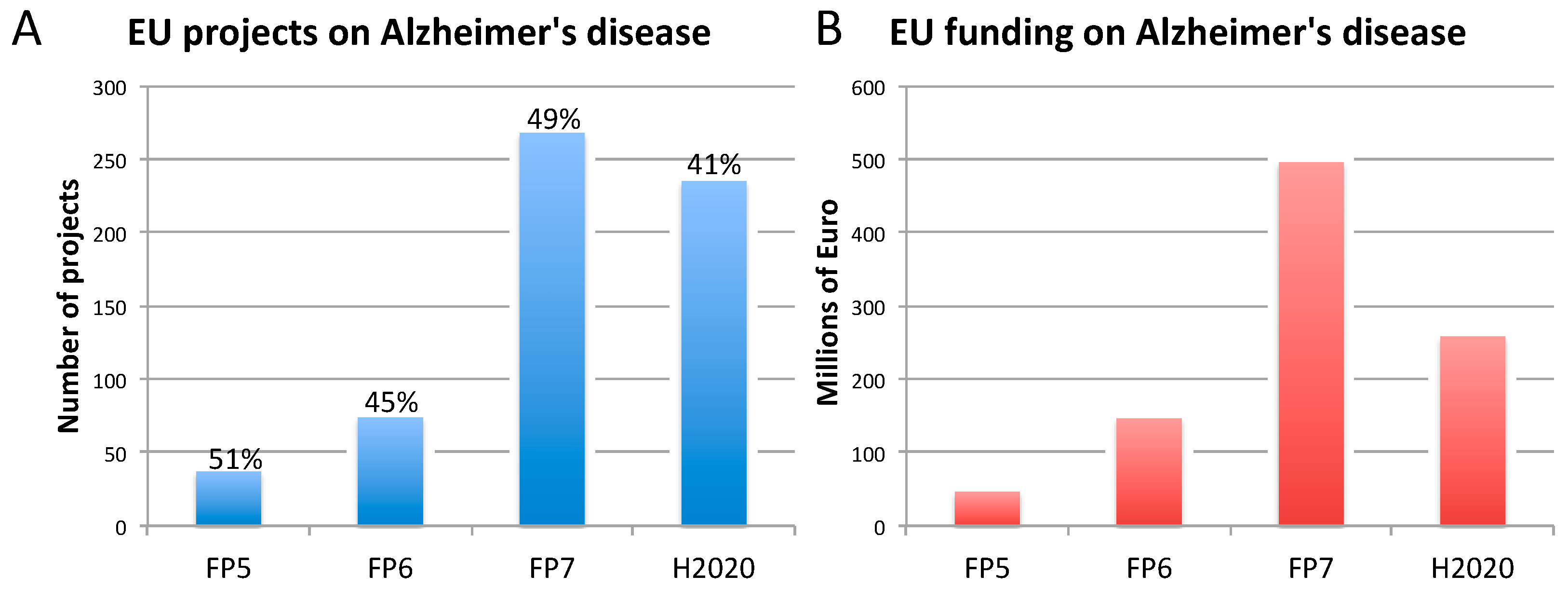
2.1. Alzheimer’s Disease
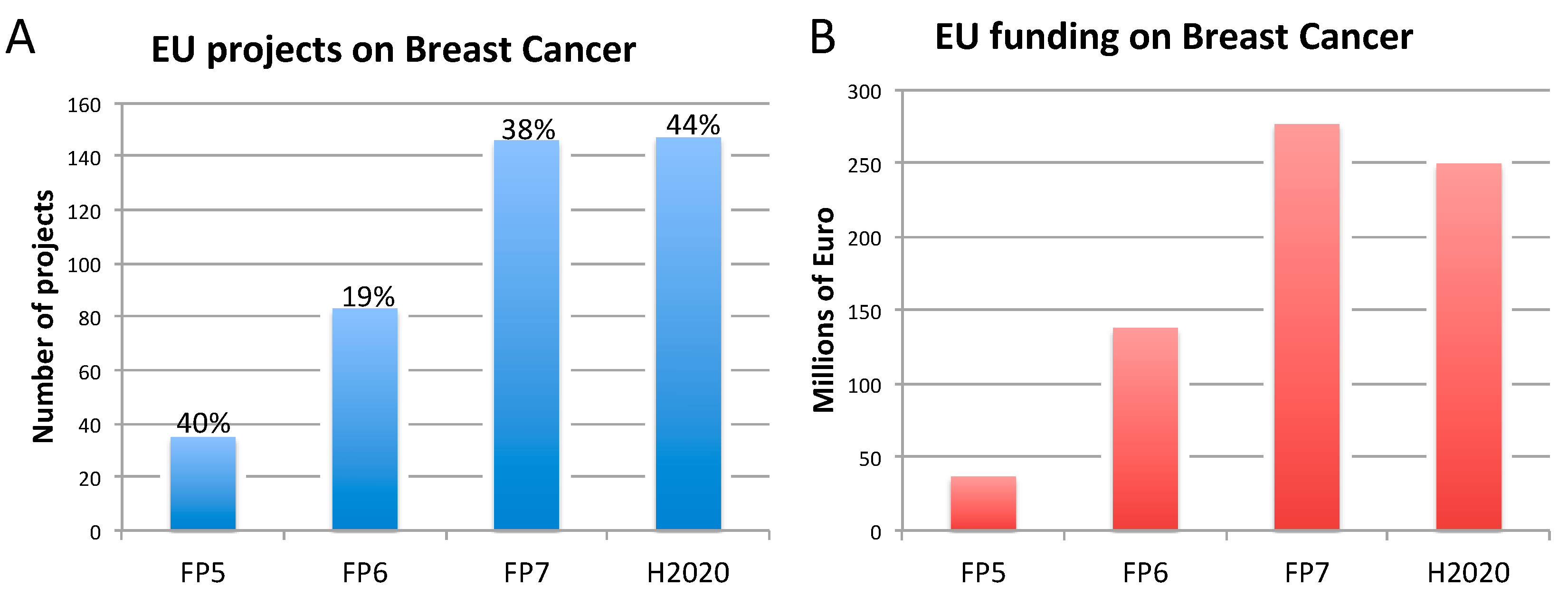
2.2. Breast Cancer
2.3. Prostate Cancer
3. The Need for Indicators to Monitor Innovation and Impact of Funded Biomedical Research
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EC. Noncommunicable Diseases—NCDs. Available online: https://ec.europa.eu/knowledge4policy/foresight/topic/shifting-health-challenges/non-communicable-diseases-ncds_en (accessed on 23 April 2020).
- OECD. Health at a Glance: Europe 2018; OECD: Paris, France, 2018. [Google Scholar]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer Dement 2019, 15, 321–387. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death. Available online: https://http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 23 April 2020).
- Deaths Registered in England and Wales (Series DR): 2017. Available online: https://http://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredinenglandandwalesseriesdr/2017 (accessed on 29 June 2020).
- Is Europe Ready for Alzheimer’s? Available online: http://www.aal-europe.eu/is-europe-ready-for-alzheimers/ (accessed on 29 June 2020).
- Worldwide Cancer Data. Available online: https://http://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data (accessed on 23 April 2020).
- EC. Cancer Statistics—Specific Cancers. Available online: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/39738.pdf (accessed on 29 June 2020).
- Simmons, D. The use of animal models in studying genetic disease: Transgenesis and induced mutation. Nat. Educ. 2008, 1, 70. [Google Scholar]
- Labant, M. Animal Models Evolve to Satisfy Emerging Needs. Available online: https://http://www.genengnews.com/insights/animal-models-evolve-to-satisfy-emerging-needs/ (accessed on 29 June 2020).
- Pound, P.; Ritskes-Hoitinga, M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 2018, 16, 304. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Gould, S.E.; Junttila, M.R.; De Sauvage, F.J. Translational value of mouse models in oncology drug development. Nat. Med. 2015, 21, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Mullane, K.; Williams, M. Preclinical Models of Alzheimer’s Disease: Relevance and Translational Validity. Curr. Protoc. Pharmacol. 2019, 84, e57. [Google Scholar] [CrossRef]
- Cavanaugh, S.E.; Pippin, J.J.; Barnard, N.D. Animal models of Alzheimer disease: Historical pitfalls and a path forward. Altex 2014, 31, 279–302. [Google Scholar] [CrossRef]
- Manning, H.C.; Buck, J.R.; Cook, R.S. Mouse Models of Breast Cancer: Platforms for Discovering Precision Imaging Diagnostics and Future Cancer Medicine. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57 (Suppl. 1), 60S–68S. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Sflomos, G.; Brisken, C. The challenges of modeling hormone receptor-positive breast cancer in mice. Endocr. -Relat. Cancer 2018, 25, R319–R330. [Google Scholar] [CrossRef]
- Rea, D.; Del Vecchio, V.; Palma, G.; Barbieri, A.; Falco, M.; Luciano, A.; De Biase, D.; Perdona, S.; Facchini, G.; Arra, C. Mouse Models in Prostate Cancer Translational Research: From Xenograft to PDX. Biomed. Res. Int. 2016, 2016, 9750795. [Google Scholar] [CrossRef] [PubMed]
- Hensley, P.J.; Kyprianou, N. Modeling prostate cancer in mice: Limitations and opportunities. J. Androl. 2012, 33, 133–144. [Google Scholar] [CrossRef] [PubMed]
- BIO, Biomedtracker, Amplion. Development Success Rates 2006–2015. Available online: https://www.bio.org/sites/default/files/legacy/bioorg/docs/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf (accessed on 23 April 2020).
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Cummings, J.; Reiber, C.; Kumar, P. The price of progress: Funding and financing Alzheimer’s disease drug development. Alzheimer’s Dement. 2018, 4, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, B.; Braeken, D.; Li, Y.E. Brain-on-a-chip Devices for Drug Screening and Disease Modeling Applications. Curr. Pharm. Des. 2018, 24, 5419–5436. [Google Scholar] [CrossRef]
- Eglen, R.M.; Reisine, T. Human iPS Cell-Derived Patient Tissues and 3D Cell Culture Part 2: Spheroids, Organoids, and Disease Modeling. Slas Technol. 2019, 24, 18–27. [Google Scholar] [CrossRef]
- Essayan-Perez, S.; Zhou, B.; Nabet, A.M.; Wernig, M.; Huang, Y.A. Modeling Alzheimer’s disease with human iPS cells: Advancements, lessons, and applications. Neurobiol. Dis. 2019, 130, 104503. [Google Scholar] [CrossRef]
- Shirotani, K.; Matsuo, K.; Ohtsuki, S.; Masuda, T.; Asai, M.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Kondo, T.; Inoue, H.; et al. A simplified and sensitive method to identify Alzheimer’s disease biomarker candidates using patient-derived induced pluripotent stem cells (iPSCs). J. Biochem. 2017, 162, 391–394. [Google Scholar] [CrossRef]
- Zanoni, M.; Pignatta, S.; Arienti, C.; Bonafe, M.; Tesei, A. Anticancer drug discovery using multicellular tumor spheroid models. Expert Opin. Drug Discov. 2019, 14, 289–301. [Google Scholar] [CrossRef]
- Lee, I.C. Cancer-on-a-chip for Drug Screening. Curr. Pharm. Des. 2018, 24, 5407–5418. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.K.; Schmidt, H.; Anastasiadou, E.; Altucci, L.; Angelini, M.; Badimon, L.; Balligand, J.L.; Benincasa, G.; Capasso, G.; Conte, F.; et al. Molecular networks in Network Medicine: Development and applications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, e1489. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Hong, X.; Li, S.; Zhang, Y.; Zhao, Q.; Du, W.; Wang, Y.; Ni, J. Urine-Based Biomarkers for Alzheimer’s Disease Identified Through Coupling Computational and Experimental Methods. J. Alzheimer’s Dis. JAD 2018, 65, 421–431. [Google Scholar] [CrossRef] [PubMed]
- EC. FET Open. Available online: https://ec.europa.eu/programmes/horizon2020/en/h2020-section/fet-open (accessed on 23 April 2020).
- EC. Funding & Tender Opportunities. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-search;freeTextSearchKeyword=;typeCodes=1;statusCodes=31094501,31094502;programCode=H2020;programDivisionCode=31047826;focusAreaCode=null;crossCuttingPriorityCode=null;callCode=Default;sortQuery=openingDate;orderBy=asc;onlyTenders=false;topicListKey=topicSearchTablePageState (accessed on 23 April 2020).
- Multidisciplinary Research Projects on Personalised Medicine—Pre-/Clinical Research, Big Data and ICT, Implementation and User’s Perspective. Available online: http://www.erapermed.eu/3211-2/ (accessed on 23 April 2020).
- IMI. Innovative Medicines Initiative. Available online: https://http://www.imi.europa.eu/ (accessed on 23 April 2020).
- EC. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015–2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1581689520921&uri=CELEX:52020DC0016 (accessed on 23 April 2020).
- WHO. Ageing and Health. Available online: https://http://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 23 April 2020).
- Dementia Statistics. Available online: https://http://www.alz.co.uk/research/statistics (accessed on 23 April 2020).
- Wimo, A.; Jonsson, L.; Bond, J.; Prince, M.; Winblad, B.; Alzheimer Disease, I. The worldwide economic impact of dementia 2010. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 1–11 e13. [Google Scholar] [CrossRef] [PubMed]
- Early Onset Famlial, A.D. Available online: https://http://www.alzforum.org/early-onset-familial-ad/overview/what-early-onset-familial-alzheimer-disease-efad (accessed on 23 April 2020).
- Hunsberger, H.C.; Pinky, P.D.; Smith, W.; Suppiramaniam, V.; Reed, M.N. The role of APOE4 in Alzheimer’s disease: Strategies for future therapeutic interventions. Health Psychol. Behav. Med. 2019, 3, NS20180203. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Quintessential risk factors: Their role in promoting cognitive dysfunction and Alzheimer’s disease. Neurochem. Res. 2012, 37, 2627–2658. [Google Scholar] [CrossRef]
- World Alzheimer Report 2018. Available online: https://http://www.alz.co.uk/research/world-report-2018 (accessed on 23 April 2020).
- PCRM. Retiring the Amyloid Cascade Hypothesis as a Cause of Alzheimer’s. Available online: https://http://www.pcrm.org/news/good-science-digest/retiring-amyloid-cascade-hypothesis-cause-alzheimers (accessed on 23 April 2020).
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef]
- Makin, S. The amyloid hypothesis on trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef]
- Beach, T.G.; Monsell, S.E.; Phillips, L.E.; Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 2012, 71, 266–273. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Qian, J.; Monsell, S.E.; Blacker, D.; Gomez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Johnson, K.A.; Frosch, M.P.; Sperling, R.A.; et al. Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann. Neurol. 2014, 75, 597–601. [Google Scholar] [CrossRef]
- Pippin, J.J.; Cavanaugh, S.E.; Pistollato, F. Animal Research for Alzheimer Disease: Failures of Science and Ethics. In Animal Experimentation: Working Towards a Paradigm Change; Herrmann, K., Jayne, J., Eds.; Brill: Leiden, The Netherlands, 2019. [Google Scholar] [CrossRef]
- FDA-Approved Treatments for Alzheimer’s. Available online: https://http://www.alz.org/media/documents/fda-approved-treatments-alzheimers-ts.pdf (accessed on 23 April 2020).
- Birks, J. Cholinesterase Inhibitors for Alzheimer’s Disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, CD001190. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Banks, S.; Miller, J.B.; Ritter, A.; Bernick, C.; Lombardo, J.; Cummings, J.L. Statistical advances in clinical trials and clinical research. Alzheimer’s Dement. 2018, 4, 366–371. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; Van Der Graaf, P.H.; Arrowsmith, J.; Feltner, D.E.; Drummond, K.S.; Wegner, C.D.; Street, S.D. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov. Today 2012, 17, 419–424. [Google Scholar] [CrossRef]
- De Witte, W.E.A.; Danhof, M.; van der Graaf, P.H.; de Lange, E.C.M. The implications of target saturation for the use of drug-target residence time. Nat. Rev. Drug Discov. 2018, 18, 82–84. [Google Scholar] [CrossRef]
- Kleiman, R.J.; Ehlers, M.D. Data gaps limit the translational potential of preclinical research. Sci. Transl. Med. 2016, 8, 320–321. [Google Scholar] [CrossRef]
- Karran, E.; Hardy, J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol. 2014, 76, 185–205. [Google Scholar] [CrossRef]
- Gold, M. Phase II clinical trials of anti-amyloid beta antibodies: When is enough, enough? Alzheimer’s Dement. 2017, 3, 402–409. [Google Scholar] [CrossRef]
- Gray, J.A.; Fleet, D.; Winblad, B. The need for thorough phase II studies in medicines development for Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 67. [Google Scholar] [CrossRef]
- Pagliarulo, N.; Gardner, J. 7 Questions on Biogen’s Revival of a Failed Alzheimer’s Drug. Available online: https://http://www.biopharmadive.com/news/biogen-alzheimers-aducanumab-revival-7-questions/565609/ (accessed on 23 April 2020).
- WHO. Risk Reduction of Cognitive Decline and Dementia; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Barrett, J.E.; McGonigle, P. Rodent Models for Alzheimer’s Disease in Drug Discovery. In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders; Academic Press: San Diego, CA, USA, 2017; pp. 235–247. [Google Scholar] [CrossRef]
- Newman, M.; Kretzschmar, D.; Khan, I.; Chen, M.; Verdile, G.; Lardelli, M. Animal models of Alzheimer’s Disease. In Animal Models for the Study of Human Disease, 2nd ed.; Michael Conn, P., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 1031–1085. [Google Scholar]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Research Models. Alzheimer’s Disease. Available online: https://http://www.alzforum.org/research-models/alzheimers-disease (accessed on 23 April 2020).
- King, A. The search for better animal models of Alzheimer’s disease. Nature 2018, 559, S13–S15. [Google Scholar] [CrossRef] [PubMed]
- Pallàs, M. Senescence-Accelerated Mice P8: A Tool to Study Brain Aging and Alzheimer’s Disease in a Mouse Model. Int. Sch. Res. Not. Cell Biol. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Cheng, X.R.; Zhou, W.X.; Zhang, Y.X. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res. Rev. 2014, 13, 13–37. [Google Scholar] [CrossRef]
- Lok, K.; Zhao, H.; Zhang, C.; He, N.; Shen, H.; Wang, Z.; Zhao, W.; Yin, M. Effects of accelerated senescence on learning and memory, locomotion and anxiety-like behavior in APP/PS1 mouse model of Alzheimer’s disease. J. Neurol. Sci. 2013, 335, 145–154. [Google Scholar] [CrossRef] [PubMed]
- App KO/APOE4/Trem2*R47H. Available online: https://http://www.jax.org/strain/031722 (accessed on 23 April 2020).
- Dong, J.; Zhou, M.; Wu, X.; Du, M.; Wang, X. Memantine combined with environmental enrichment improves spatial memory and alleviates Alzheimer’s disease-like pathology in senescence-accelerated prone-8 (SAMP8) mice. J. Biomed. Res. 2012, 26, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, G.; Shi, S.; Li, Y.; Li, Z. Effects of manual acupuncture combined with donepezil in a mouse model of Alzheimer’s disease. Acupunct. Med. J. Br. Med Acupunct. Soc. 2019, 37, 64–71. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sanchez-Lopez, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesus, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on its Current and Future Applications. J. Alzheimer’s Dis. JAD 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Grossberg, G.T.; Manes, F.; Allegri, R.F.; Gutierrez-Robledo, L.M.; Gloger, S.; Xie, L.; Jia, X.D.; Pejovic, V.; Miller, M.L.; Perhach, J.L.; et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): A multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs 2013, 27, 469–478. [Google Scholar] [CrossRef]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) trial: Secondary and post-hoc analyses. Lancet. Neurol. 2015, 14, 1171–1181. [Google Scholar] [CrossRef]
- Porquet, D.; Andres-Benito, P.; Grinan-Ferre, C.; Camins, A.; Ferrer, I.; Canudas, A.M.; Del Valle, J.; Pallas, M. Amyloid and tau pathology of familial Alzheimer’s disease APP/PS1 mouse model in a senescence phenotype background (SAMP8). Age 2015, 37, 9747. [Google Scholar] [CrossRef]
- Morris, G.P.; Clark, I.A.; Vissel, B. Questions concerning the role of amyloid-beta in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 2018, 136, 663–689. [Google Scholar] [CrossRef]
- Veening-Griffioen, D.H.; Ferreira, G.S.; van Meer, P.J.K.; Boon, W.P.C.; Gispen-de Wied, C.C.; Moors, E.H.M.; Schellekens, H. Are some animal models more equal than others? A case study on the translational value of animal models of efficacy for Alzheimer’s disease. Eur. J. Pharmacol. 2019, 859, 172524. [Google Scholar] [CrossRef] [PubMed]
- Rae, E.A.; Brown, R.E. The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci. Biobehav. Rev. 2015, 57, 238–251. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. Mouse Models in Aging Research. In The Mouse in Biomedical Research, 2nd ed.; Medicine, A., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume III, pp. 637–672. [Google Scholar]
- Yuan, R.; Peters, L.L.; Paigen, B. Mice as a mammalian model for research on the genetics of aging. Ilar J. 2011, 52, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer: Statistics. Available online: https://http://www.cancer.net/cancer-types/breast-cancer/statistics (accessed on 24 April 2020).
- cancer.org. Survival Rates for Breast Cancer. Available online: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stage (accessed on 24 April 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- NIH. Drugs Approved for Breast Cancer. Available online: https://http://www.cancer.gov/about-cancer/treatment/drugs/breast (accessed on 23 April 2020).
- Leo, C.P.; Leo, C.; Szucs, T.D. Breast cancer drug approvals by the US FDA from 1949 to 2018. Nat. Rev. Drug Discov. 2020, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, L.; Kirk, R. High drug attrition rates--where are we going wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef]
- Ju, J.; Zhu, A.J.; Yuan, P. Progress in targeted therapy for breast cancer. Chronic Dis. Transl. Med. 2018, 4, 164–175. [Google Scholar] [CrossRef]
- Fan, P.; Jordan, V.C. New insights into acquired endocrine resistance of breast cancer. Cancer Drug Resist. 2019, 2, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Guo, J.; Shen, B.Q.; Yadav, D.B.; Sliwkowski, M.X.; Crocker, L.M.; Lacap, J.A.; Phillips, G.D.L. Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells. Mol. Cancer Ther. 2018, 17, 1441–1453. [Google Scholar] [CrossRef]
- Luque-Cabal, M.; Garcia-Teijido, P.; Fernandez-Perez, Y.; Sanchez-Lorenzo, L.; Palacio-Vazquez, I. Mechanisms Behind the Resistance to Trastuzumab in HER2-Amplified Breast Cancer and Strategies to Overcome It. Clin. Med. Insights Oncol. 2016, 10, 21–30. [Google Scholar] [CrossRef]
- Moiseenko, F.; Volkov, N.; Bogdanov, A.; Dubina, M.; Moiseyenko, V. Resistance mechanisms to drug therapy in breast cancer and other solid tumors: An opinion. F1000Research 2017, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Osborne, C.; Pippen, J.E.; Yoffe, M.; Patt, D.; Rocha, C.; Koo, I.C.; Sherman, B.M.; Bradley, C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. New Engl. J. Med. 2011, 364, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Mateo, J.; Ong, M.; Tan, D.S.; Gonzalez, M.A.; de Bono, J.S. Appraising iniparib, the PARP inhibitor that never was—What must we learn? Nat. Rev. Clin. Oncol. 2013, 10, 688–696. [Google Scholar] [CrossRef]
- Whittle, J.R.; Lewis, M.T.; Lindeman, G.J.; Visvader, J.E. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. BCR 2015, 17, 17. [Google Scholar] [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12. [Google Scholar] [CrossRef]
- NIH. The Cancer Genome Atlas Program. Available online: https://http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 24 April 2020).
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: Progress, challenges and future directions. Dis. Models Mech. 2017, 10, 359–371. [Google Scholar] [CrossRef]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.P.; Brennan, K.; Brown, N.J.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. BCR 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Gisselsson, D.; Lichtenzstejn, D.; Kachko, P.; Karlsson, J.; Manor, E.; Mai, S. Clonal evolution through genetic bottlenecks and telomere attrition: Potential threats to in vitro data reproducibility. GenesChromosomes Cancer 2019, 58, 452–461. [Google Scholar] [CrossRef]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef]
- Fleming, J.M.; Miller, T.C.; Meyer, M.J.; Ginsburg, E.; Vonderhaar, B.K. Local regulation of human breast xenograft models. J. Cell. Physiol. 2010, 224, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.S.; Lee, C.; Yu, M.; Tannock, I.F. The effect of chemotherapeutic agents on tumor vasculature in subcutaneous and orthotopic human tumor xenografts. BMC Cancer 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Cell Line-Derived Xenograft—CDX Model Studies in Mice. Available online: https://http://www.criver.com/products-services/discovery-services/pharmacology-studies/oncology-immuno-oncology-studies/oncology-models/cell-line-derived-xenograft-cdx-mouse-models?region=3696 (accessed on 29 June 2020).
- Cell-Line-Derived Xenograft Models. Available online: https://http://www.creative-animodel.com/animal-model-development/cell-line-derived-xenograft-models.html (accessed on 29 June 2020).
- DeRose, Y.S.; Wang, G.; Lin, Y.C.; Bernard, P.S.; Buys, S.S.; Ebbert, M.T.; Factor, R.; Matsen, C.; Milash, B.A.; Nelson, E.; et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011, 17, 1514–1520. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinska, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Maelandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, A.; Wang, L. Bridging the divide: Preclinical research discrepancies between triple-negative breast cancer cell lines and patient tumors. Oncotarget 2017, 8, 113269–113281. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, K.; Kim, S.H.; Chung, Y.J.; Lee, C. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp. Mol. Med. 2018, 50, 99. [Google Scholar] [CrossRef]
- Brehm, M.A.; Shultz, L.D.; Luban, J.; Greiner, D.L. Overcoming current limitations in humanized mouse research. J. Infect. Dis. 2013, 208 (Suppl. 2), S125–S130. [Google Scholar] [CrossRef]
- Garcia, S.; Freitas, A.A. Humanized mice: Current states and perspectives. Immunol. Lett. 2012, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, K.; Stentz, M.; DiMeglio, M.; Furey, W.; Steinberg, T.; Patel, A. Potential Pitfalls of the Humanized Mice in Modeling Sepsis. Int. J. Inflamm. 2018, 2018, 6563454. [Google Scholar] [CrossRef] [PubMed]
- Yeadon, J. Immunodeficient Mice for Cancer Studies: Which Host Strain Should I Use? Available online: https://http://www.jax.org/news-and-insights/jax-blog/2013/july/which-host-strain-should-i-use (accessed on 29 June 2020).
- Eyre, R.; Alferez, D.G.; Spence, K.; Kamal, M.; Shaw, F.L.; Simoes, B.M.; Santiago-Gomez, A.; Sarmiento-Castro, A.; Bramley, M.; Absar, M.; et al. Patient-derived Mammosphere and Xenograft Tumour Initiation Correlates with Progression to Metastasis. J. Mammary Gland Biol. Neoplasia 2016, 21, 99–109. [Google Scholar] [CrossRef] [PubMed]
- STOCK Trp53tm1Brd Brca1tm1Aash Tg(LGB-cre)74Acl/J. Available online: https://http://www.jax.org/strain/012620 (accessed on 29 June 2020).
- Menezes, M.E.; Das, S.K.; Emdad, L.; Windle, J.J.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. Genetically engineered mice as experimental tools to dissect the critical events in breast cancer. Adv. Cancer Res. 2014, 121, 331–382. [Google Scholar] [CrossRef] [PubMed]
- Dabydeen, S.A.; Furth, P.A. Genetically engineered ERalpha-positive breast cancer mouse models. Endocr. -Relat. Cancer 2014, 21, R195–R208. [Google Scholar] [CrossRef] [PubMed]
- Greenow, K.R.; Smalley, M.J. Overview of Genetically Engineered Mouse Models of Breast Cancer Used in Translational Biology and Drug Development. Curr. Protoc. Pharmacol. 2015, 70, 14.36. 1–14.36. 14. [Google Scholar] [CrossRef]
- Ben-David, U.; Ha, G.; Khadka, P.; Jin, X.; Wong, B.; Franke, L.; Golub, T.R. The landscape of chromosomal aberrations in breast cancer mouse models reveals driver-specific routes to tumorigenesis. Nat. Commun. 2016, 7, 12160. [Google Scholar] [CrossRef]
- Fry, E.A.; Taneja, P.; Inoue, K. Oncogenic and tumor-suppressive mouse models for breast cancer engaging HER2/neu. Int. J. Cancer 2017, 140, 495–503. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr. Curing Metastatic Breast Cancer. J. Oncol. Pract. 2016, 12, 6–10. [Google Scholar] [CrossRef]
- Estimates for Funding for Research for Metastatic Disease: LOW. Available online: http://mbcn.org/research-funding/ (accessed on 12 May 2020).
- EC. CORDIS. Available online: https://cordis.europa.eu/projects/en (accessed on 23 April 2020).
- Milosevic, M.; Jankovic, D.; Milenkovic, A.; Stojanov, D. Early diagnosis and detection of breast cancer. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2018, 26, 729–759. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, J.; Sun, Y. Circulating Tumor DNA as Biomarkers for Cancer Detection. Genom. Proteom. Bioinform. 2017, 15, 59–72. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Men’s Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Abate-Shen, C.; Agus, D.B.; Attar, R.M.; Chung, L.W.; Greenberg, N.M.; Hahn, W.C.; Isaacs, J.T.; Navone, N.M.; Peehl, D.M.; et al. The current state of preclinical prostate cancer animal models. Prostate 2008, 68, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ittmann, M.; Huang, J.; Radaelli, E.; Martin, P.; Signoretti, S.; Sullivan, R.; Simons, B.W.; Ward, J.M.; Robinson, B.D.; Chu, G.C.; et al. Animal models of human prostate cancer: The consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013, 73, 2718–2736. [Google Scholar] [CrossRef]
- Cho, H.; Herzka, T.; Zheng, W.; Qi, J.; Wilkinson, J.E.; Bradner, J.E.; Robinson, B.D.; Castillo-Martin, M.; Cordon-Cardo, C.; Trotman, L.C. RapidCaP, a novel GEM model for metastatic prostate cancer analysis and therapy, reveals myc as a driver of Pten-mutant metastasis. Cancer Discov. 2014, 4, 318–333. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Xie, Z.; Liu, Z.Y.; Green, J.E.; Martin, W.D.; Datta, M.W.; Yeung, F.; Pan, D.; Chung, L.W. A luciferase transgenic mouse model: Visualization of prostate development and its androgen responsiveness in live animals. J. Mol. Endocrinol. 2005, 35, 293–304. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ricklis, R.M.; Williams, S.A.; Denmeade, S.R. Comparative study of PSMA expression in the prostate of mouse, dog, monkey, and human. Prostate 2006, 66, 903–910. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; Pienta, K.J. Drug discovery in prostate cancer mouse models. Expert Opin. Drug Discov. 2015, 10, 1011–1024. [Google Scholar] [CrossRef]
- Wei, C.; Willis, R.A.; Tilton, B.R.; Looney, R.J.; Lord, E.M.; Barth, R.K.; Frelinger, J.G. Tissue-specific expression of the human prostate-specific antigen gene in transgenic mice: Implications for tolerance and immunotherapy. Proc. Natl. Acad. Sci. USA. 1997, 94, 6369–6374. [Google Scholar] [CrossRef] [PubMed]
- Bullock, L.P. Brief overview of selected aspects of testicular hormone action. Environ. Health Perspect. 1981, 38, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.S.; Dzinic, S.; Bonfil, A.I.; Saliganan, A.D.; Sheng, S.; Bonfil, R.D. The mouse prostate: A basic anatomical and histological guideline. Bosn. J. Basic Med Sci. 2016, 16, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Michiel Sedelaar, J.P.; Dalrymple, S.S.; Isaacs, J.T. Of mice and men—warning: Intact versus castrated adult male mice as xenograft hosts are equivalent to hypogonadal versus abiraterone treated aging human males, respectively. Prostate 2013, 73, 1316–1325. [Google Scholar] [CrossRef]
- Jeet, V.; Russell, P.J.; Khatri, A. Modeling prostate cancer: A perspective on transgenic mouse models. Cancer Metastasis Rev. 2010, 29, 123–142. [Google Scholar] [CrossRef]
- Kasper, S. Survey of genetically engineered mouse models for prostate cancer: Analyzing the molecular basis of prostate cancer development, progression, and metastasis. J. Cell. Biochem. 2005, 94, 279–297. [Google Scholar] [CrossRef]
- Kido, L.A.; de Almeida Lamas, C.; Marostica, M.R., Jr.; Cagnon, V.H.A. Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model: A good alternative to study PCa progression and chemoprevention approaches. Life Sci. 2019, 217, 141–147. [Google Scholar] [CrossRef]
- Civenni, G.; Carbone, G.M.; Catapano, C.V. Overview of Genetically Engineered Mouse Models of Prostate Cancer and Their Applications in Drug Discovery. Curr. Protoc. Pharmacol. 2018, 81, e39. [Google Scholar] [CrossRef]
- Irshad, S.; Abate-Shen, C. Modeling prostate cancer in mice: Something old, something new, something premalignant, something metastatic. Cancer Metastasis Rev. 2013, 32, 109–122. [Google Scholar] [CrossRef]
- Lin, D.; Wyatt, A.W.; Xue, H.; Wang, Y.; Dong, X.; Haegert, A.; Wu, R.; Brahmbhatt, S.; Mo, F.; Jong, L.; et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014, 74, 1272–1283. [Google Scholar] [CrossRef]
- Hara, T.; Kouno, J.; Kaku, T.; Takeuchi, T.; Kusaka, M.; Tasaka, A.; Yamaoka, M. Effect of a novel 17,20-lyase inhibitor, orteronel (TAK-700), on androgen synthesis in male rats. J. Steroid Biochem. Mol. Biol. 2013, 134, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, M.; Hara, T.; Hitaka, T.; Kaku, T.; Takeuchi, T.; Takahashi, J.; Asahi, S.; Miki, H.; Tasaka, A.; Kusaka, M. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: Effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J. Steroid Biochem. Mol. Biol. 2012, 129, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Van Hook, K.; Huang, T.; Alumkal, J.J. Orteronel for the treatment of prostate cancer. Future Oncol. 2014, 10, 803–811. [Google Scholar] [CrossRef] [PubMed]
- NIH. Study to Investigate the Effects of Orteronel on the QT/QTc Interval in Patients with Metastatic Castration-Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01549951?view=results (accessed on 23 April 2020).
- NIH. Study Comparing Orteronel Plus Prednisone in Participants with Metastatic Castration-Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01193257 (accessed on 23 April 2020).
- Loddick, S.A.; Ross, S.J.; Thomason, A.G.; Robinson, D.M.; Walker, G.E.; Dunkley, T.P.; Brave, S.R.; Broadbent, N.; Stratton, N.C.; Trueman, D.; et al. AZD3514: A small molecule that modulates androgen receptor signaling and function in vitro and in vivo. Mol. Cancer Ther. 2013, 12, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Wadosky, K.M.; Koochekpour, S. Therapeutic Rationales, Progresses, Failures, and Future Directions for Advanced Prostate Cancer. Int. J. Biol. Sci. 2016, 12, 409–426. [Google Scholar] [CrossRef]
- Suzman, D.L.; Antonarakis, E.S. Castration-resistant prostate cancer: Latest evidence and therapeutic implications. Ther. Adv. Med Oncol. 2014, 6, 167–179. [Google Scholar] [CrossRef]
- NIH. Drugs Approved for Prostate Cancer. Available online: https://http://www.cancer.gov/about-cancer/treatment/drugs/prostate (accessed on 23 April 2020).
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Natarajan, R.; Aljaber, D.; Au, D.; Thai, C.; Sanchez, A.; Nunez, A.; Resto, C.; Chavez, T.; Jankowska, M.M.; Benmarhnia, T.; et al. Environmental Exposures during Puberty: Window of Breast Cancer Risk and Epigenetic Damage. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef]
- Theodoratou, E.; Timofeeva, M.; Li, X.; Meng, X.; Ioannidis, J.P.A. Nature, Nurture, and Cancer Risks: Genetic and Nutritional Contributions to Cancer. Annu. Rev. Nutr. 2017, 37, 293–320. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, X.; Miao, Z.; Ling, Z.; Wei, X.; Pu, J.; Hou, J.; Shen, B. Data-driven translational prostate cancer research: From biomarker discovery to clinical decision. J. Transl. Med. 2020, 18, 119. [Google Scholar] [CrossRef]
- EC. Horizon 2020 indicators. Assessing the Results and Impact of Horizon. Available online: https://ec.europa.eu/programmes/horizon2020/en/news/horizon-2020-indicators-assessing-results-and-impact-horizon (accessed on 23 April 2020).
- EC. Evaluation, Impact Assessment and Monitoring of EU Research and Innovation Programmes. Available online: https://ec.europa.eu/info/research-and-innovation/strategy/support-policy-making/shaping-eu-research-and-innovation-policy/evaluation-impact-assessment-and-monitoring_en (accessed on 23 April 2020).
- OECD; EC. Handbook on Constructing Composite Indicators—Methodology and User Guide; OECD: Paris, France, 2008. [Google Scholar]
- Okubo, Y. Bibliometric Indicators and Analysis of Research Systems—Methods and Examples; OECD: Paris, France, 1997. [Google Scholar]
- Aksnes, D.W.; Langfeldt, L.; Wouters, P. Citations, Citation Indicators, and Research Quality: An Overview of Basic Concepts and Theories. SAGE 2019, 9. [Google Scholar] [CrossRef]
- EC. JRC Launches Online Survey on Innovation and Impact of Biomedical Research. Available online: https://ec.europa.eu/jrc/en/science-update/jrc-online-survey-innovation-and-impact-biomedical-research (accessed on 23 April 2020).
- EC. A Survey on Monitoring Innovation and Societal Impact of EU-funded Research—Factual Summary Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Kasunic, M. Designing an Effective Survey; Carnegie Mellon, Software Engineering Institute: Pittsburgh, PA, USA, 2005. [Google Scholar]
- EC. Bridging Across Methods in the Biosciences—BeAMS; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Breast Cancer Prevention: How to Reduce Your Risk. Available online: https://http://www.mayoclinic.org/healthy-lifestyle/womens-health/in-depth/breast-cancer-prevention/art-20044676 (accessed on 15 May 2020).
- Prostate Cancer Prevention: Ways to Reduce your Risk. Available online: https://http://www.mayoclinic.org/diseases-conditions/prostate-cancer/in-depth/prostate-cancer-prevention/art-20045641 (accessed on 15 May 2020).
- Prevention. Available online: https://http://www.alz.org/alzheimers-dementia/research_progress/prevention (accessed on 15 May 2020).
- Arrowsmith, J. Trial watch: Phase II failures: 2008–2010. Nat. Rev. Drug Discov. 2011, 10, 328–329. [Google Scholar] [CrossRef]
- Medical Publishing Insights and Practices (MPIP). Available online: http://www.mpip-initiative.org/ (accessed on 29 June 2020).
- International Society for Medical Publication Professionals (ISMPP). Available online: https://http://www.ismpp.org/ (accessed on 29 June 2020).
- Journal of Pharmaceutical Negative Results. Available online: http://www.pnrjournal.com/ (accessed on 29 June 2020).
- Journal of Negative Results in BioMedicine. Available online: https://jnrbm.biomedcentral.com/ (accessed on 29 June 2020).
- BMJ. Available online: https://http://www.bmj.com/ (accessed on 29 June 2020).
- PLoS ONE. Available online: https://journals.plos.org/plosone/ (accessed on 29 June 2020).
- Hayes, A.; Hunter, J. Why is publication of negative clinical trial data important? Br. J. Pharmacol. 2012, 167, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, I. The importance of publishing trials with negative results. Am. J. Obstet. Gynecol. 2017, 216, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Laurijssens, B.; Aujard, F.; Rahman, A. Animal models of Alzheimer’s disease and drug development. Drug Discov. Today. Technol. 2013, 10, e319–e327. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.; Buxton, M.J. Economic returns to medical research funding. BMJ Open 2018, 8, e022131. [Google Scholar] [CrossRef]
- Yao, L.; Li, Y.; Ghosh, S.; Evans, J.A.; Rzhetsky, A. Health ROI as a measure of misalignment of biomedical needs and resources. Nat. Biotechnol. 2015, 33, 807–811. [Google Scholar] [CrossRef]
- Hutchins, B.I.; Davis, M.T.; Meseroll, R.A.; Santangelo, G.M. Predicting translational progress in biomedical research. PLoS Biol. 2019, 17, e3000416. [Google Scholar] [CrossRef]
- Wooding, S.; Hanney, S.; Pollitt, A.; Buxton, M.; Grant, J. Project Retrosight—Understanding the Returns from Cardiovascular and Stroke Research: The Policy Report; RAND Corporation: Santa Monica, CA, USA, 2011; p. 64. [Google Scholar]
- Pistollato, F.; Ohayon, E.L.; Lam, A.; Langley, G.R.; Novak, T.J.; Pamies, D.; Perry, G.; Trushina, E.; Williams, R.S.; Roher, A.E.; et al. Alzheimer disease research in the 21st century: Past and current failures, new perspectives and funding priorities. Oncotarget 2016, 7, 38999–39016. [Google Scholar] [CrossRef]
- Rollin, B.E. The Ethics of Animal Use in Cancer Research: A Multidisciplinary Assessment and Strategies for Action. In Cancer and Society: A Multidisciplinary Assessment and Strategies for Action; Bernicker, E., Ed.; Springer: Cham, Switzerland, 2019; pp. 143–152. [Google Scholar] [CrossRef]
- Khoo, S.Y. Justifiability and Animal Research in Health: Can Democratisation Help Resolve Difficulties? Anim. Open Access J. 2018, 8. [Google Scholar] [CrossRef]
- Grimm, H.; Olsson, I.A.S.; Sandoe, P. Harm-benefit analysis—What is the added value? A review of alternative strategies for weighing harms and benefits as part of the assessment of animal research. Lab. Anim. 2019, 53, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Pistollato, F.; Stephens, M.L. Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. Altex 2019, 36, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Franco, N.H.; Sandoe, P.; Olsson, I.A.S. Researchers’ attitudes to the 3Rs-An upturned hierarchy? PLoS ONE 2018, 13, e0200895. [Google Scholar] [CrossRef] [PubMed]
- EC. Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=EN (accessed on 23 April 2020).
- Fisher, M.F.; Rao, S.S. Three dimensional culture models to study drug resistance in breast cancer. Biotechnol. Bioeng. 2020. [Google Scholar] [CrossRef]
- Brancato, V.; Gioiella, F.; Imparato, G.; Guarnieri, D.; Urciuolo, F.; Netti, P.A. 3D breast cancer microtissue reveals the role of tumor microenvironment on the transport and efficacy of free-doxorubicin in vitro. Acta Biomater. 2018, 75, 200–212. [Google Scholar] [CrossRef]
- Mosaad, E.O.; Chambers, K.F.; Futrega, K.; Clements, J.A.; Doran, M.R. The Microwell-mesh: A high-throughput 3D prostate cancer spheroid and drug-testing platform. Sci. Rep. 2018, 8, 253. [Google Scholar] [CrossRef]
- Antunes, J.; Gaspar, V.M.; Ferreira, L.; Monteiro, M.; Henrique, R.; Jeronimo, C.; Mano, J.F. In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening. Acta Biomater. 2019, 94, 392–409. [Google Scholar] [CrossRef]
- Mulholland, T.; McAllister, M.; Patek, S.; Flint, D.; Underwood, M.; Sim, A.; Edwards, J.; Zagnoni, M. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci. Rep. 2018, 8, 14672. [Google Scholar] [CrossRef]
- Sahlgren, C.; Meinander, A.; Zhang, H.; Cheng, F.; Preis, M.; Xu, C.; Salminen, T.A.; Toivola, D.; Abankwa, D.; Rosling, A.; et al. Tailored Approaches in Drug Development and Diagnostics: From Molecular Design to Biological Model Systems. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, K.; Zhang, Y.; Guo, Y.; Li, A.; Xiao, S.; Liu, Q.; Shen, L.; Ni, J. Identification of Blood Biomarkers for Alzheimer’s Disease Through Computational Prediction and Experimental Validation. Front. Neurol. 2018, 9, 1158. [Google Scholar] [CrossRef]
- Geerts, H.; Hofmann-Apitius, M.; Anastasio, T.J.; Brain Health Modeling, I. Knowledge-driven computational modeling in Alzheimer’s disease research: Current state and future trends. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- EC. Horizon Europe Programme Analysis. Available online: https://ec.europa.eu/info/research-and-innovation/strategy/support-policy-making/support-eu-research-and-innovation-policy-making/evaluation-impact-assessment-and-monitoring/horizon-europe_en (accessed on 23 April 2020).

| Ranking | Cancer Type | New Cases Diagnosed in 2018 (both Sexes) | % of All Cancers (Excluding Non-Melanoma Skin Cancer) |
|---|---|---|---|
| 1 | Lung | 2,093,876 | 12.3 |
| 2 | Breast | 2,088,849 | 12.3 |
| 3 | Colorectal | 1,800,977 | 10.6 |
| 4 | Prostate | 1,276,106 | 7.5 |
| Category | Indicator | |
|---|---|---|
| Funding/Economic | 1 | Number of projects within a certain EU framework programme (FP) |
| 2 | Value of projects within a certain EU FP | |
| 3 | Value of projects from other EU (but non-EC) funding bodies | |
| Dissemination | 4 | Number of publications (in the frame of a certain research FP) on new scientific insights (e.g., new biomarker, new signaling pathways or mode of action) and whether they were obtained using animal vs. non-animal approaches |
| 5 | Number of publications (in the frame of a certain research FP) on new methods, tools, and approaches (e.g., new diagnostic tool, new treatment approach, new preventive measure) and whether they were obtained using animal vs. non-animal methods and approaches | |
| 6 | Number of citations of papers above (i.e., describing either a new scientific insight, or new methods, tools, and approaches) | |
| Scientific and technological | 7 | Number of patents and whether they were based on animal vs. non-animal findings (e.g., suitable to study selected diseases and/or to test new drugs) |
| 8 | Number of diagnostic tools and whether they were based on animal vs. non-animal findings | |
| 9 | Number of approved drugs, treatments, or medical devices and whether they were based on animal vs. non-animal findings | |
| 10 | Number of clinical trials for new drugs and whether they were based on animal vs. non-animal findings | |
| 11 | Number of new preventive measures and whether they were based on animal vs. non-animal findings | |
| Regulatory and policy | 12 | Number of public health guidance values/options in regulatory medical-health sectors (e.g., by EMA, national governments, OECD, etc.) |
| 13 | Number of new regulatory policy actions | |
| 14 | Number of new non-regulatory targeted policy actions at the national and EU level | |
| Public and social engagement | 15 | Level of public/social engagement (to disseminate knowledge derived from EU-funded research) |
| 16 | Global indicator(s): Public health trends on selected diseases (e.g., disease prevalence, mortality rate, disease-associated risk factors) | |
| Education, training, and job opportunities | 17 | New job opportunities resulting from EU-funded research activities |
| 18 | New learning opportunities resulting from EU-funded research activities | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistollato, F.; Bernasconi, C.; McCarthy, J.; Campia, I.; Desaintes, C.; Wittwehr, C.; Deceuninck, P.; Whelan, M. Alzheimer’s Disease, and Breast and Prostate Cancer Research: Translational Failures and the Importance to Monitor Outputs and Impact of Funded Research. Animals 2020, 10, 1194. https://doi.org/10.3390/ani10071194
Pistollato F, Bernasconi C, McCarthy J, Campia I, Desaintes C, Wittwehr C, Deceuninck P, Whelan M. Alzheimer’s Disease, and Breast and Prostate Cancer Research: Translational Failures and the Importance to Monitor Outputs and Impact of Funded Research. Animals. 2020; 10(7):1194. https://doi.org/10.3390/ani10071194
Chicago/Turabian StylePistollato, Francesca, Camilla Bernasconi, Janine McCarthy, Ivana Campia, Christian Desaintes, Clemens Wittwehr, Pierre Deceuninck, and Maurice Whelan. 2020. "Alzheimer’s Disease, and Breast and Prostate Cancer Research: Translational Failures and the Importance to Monitor Outputs and Impact of Funded Research" Animals 10, no. 7: 1194. https://doi.org/10.3390/ani10071194
APA StylePistollato, F., Bernasconi, C., McCarthy, J., Campia, I., Desaintes, C., Wittwehr, C., Deceuninck, P., & Whelan, M. (2020). Alzheimer’s Disease, and Breast and Prostate Cancer Research: Translational Failures and the Importance to Monitor Outputs and Impact of Funded Research. Animals, 10(7), 1194. https://doi.org/10.3390/ani10071194






