Seroprevalence and Risk Factors of Toxoplasma gondii in Ruminant Meats from Wet Markets in Klang Valley and Abattoirs in Selangor, Malaysia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
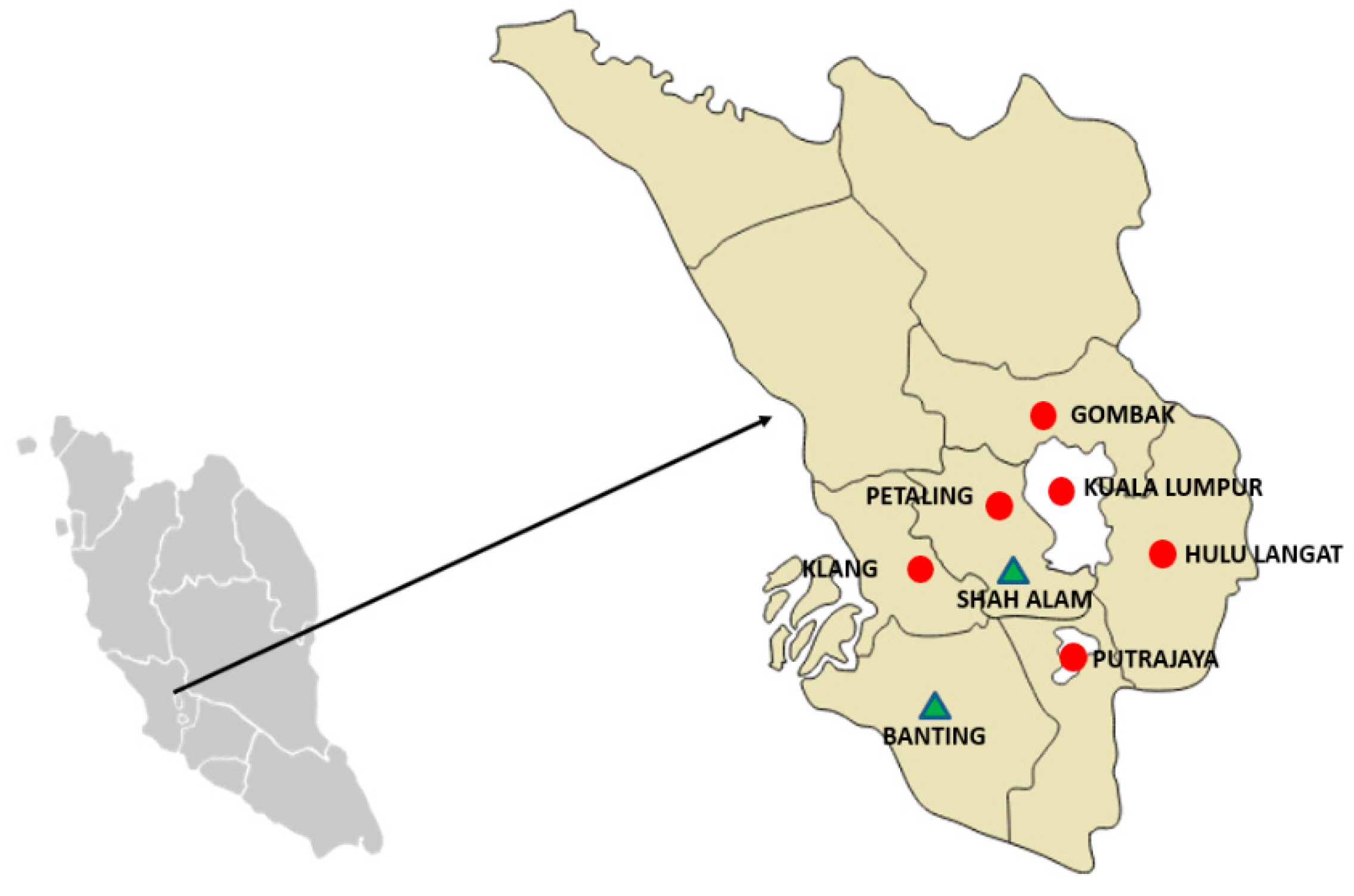
2.1. Study Design, Study Area and Selection of Sample Sites
2.2. Meat Sampling
2.3. Serology
2.4. Detection of T. gondii DNA in Meat Samples
2.4.1. DNA Extraction
2.4.2. Polymerase Chain Reaction
2.5. Statistical Analysis
3. Results
3.1. Descriptive Results
3.2. Seroprevalence of T. gondii in Wet Markets and Abattoirs Meat Samples
3.3. Risk Factors Associated with T. gondii Seropositivity
3.4. Detection of T. gondii DNA in Goat and Meat Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cook, A.J.C.; Holliman, R.; Gilbert, R.E.; Buffolano, W.; Zufferey, J.; Petersen, E.; Dunn, D.T. Sources of Toxoplasma Infection in Pregnant Women: European Multicentre Case-Control Study Commentary: Congenital Toxoplasmosis—Further Thought for Food; BMJ Publisher: London, UK, 2000; Volume 321, pp. 142–147. [Google Scholar]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Stillwaggon, E.; Carrier, C.S.; Sautter, M.; McLeod, R. Maternal serologic screening to prevent congenital toxoplasmosis: A decision-analytic economic model. PLoS Neglect. Trop. Dis. 2011, 5, e1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, C. New Study Estimates Toxoplasmosis Costs Sheep Industry $70 Million per Year in South Australia. 2017. Available online: https://ab.co/2Mgd5px (accessed on 5 May 2020).
- Moskwa, B.; Kornacka, A.; Cybulska, A.; Cabaj, W.; Reiterova, K.; Bogdaszewski, M.; Bień, J. Seroprevalence of Toxoplasma gondii and Neospora caninum infection in sheep, goats, and fallow deer farmed on the same area. J. Anim. Sci. 2018, 96, 2468–2473. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, S.; Sevinc, F.; Sevinc, M.; Ceylan, O.; Liu, M.; Xuan, X. Enzyme-linked immunosorbent assays using recombinant TgSAG2 and NcSAG1 to detect Toxoplasma gondii and Neospora caninum-specific antibodies in domestic animals in Turkey. J. Vet. Med. Sci. 2017, 78, 1877–1881. [Google Scholar] [CrossRef] [Green Version]
- Al-Kappany, Y.M.; Abbas, I.E.; Devleesschauwer, B.; Dorny, P.; Jennes, M.; Cox, E. Seroprevalence of anti-Toxoplasma gondii antibodies in Egyptian sheep and goats. BMC Vet. Res. 2018, 14, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreoletti, O.; Budka, H.; Buncic, S.; Colin, P.; Collins, J.D.; De, A.; Vågsholm, I. Surveillance and monitoring of Toxoplasma in humans, food and animals scientific opinion of the panel on biological hazards. EFSA J. 2007, 583, 1–64. [Google Scholar]
- Brandon-Mong, G.J.; Che Mat Seri, N.A.; Sharma, R.S.; Andiappan, H.; Tan, T.C.; Lim, Y.A.; Nissapatorn, V. Seroepidemiology of toxoplasmosis among people having close contact with animals. Front. Immunol. 2015, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Nissapatorn, V.; Kamarulzaman, A.; Init, I.; Tan, L.H.; Rohela, M.; Norliza, A.; Quek, K.F. Seroepidemiology of toxoplasmosis among HIV-infected patients and healthy blood donors. Med. J. Malays. 2002, 57, 304–310. [Google Scholar]
- Andiappan, H.; Nissapatorn, V.; Sawangjaroen, N.; Nyunt, M.H.; Lau, Y.L.; Khaing, S.L.; bin Mat Adenan, N.A. Comparative study on Toxoplasma infection between Malaysian and Myanmar pregnant women. Parasites Vectors 2014, 7. [Google Scholar] [CrossRef]
- Nissapatorn, V.; Suwanrath, C.; Sawangjaroen, N.; Ling, L.Y.; Chandeying, V. Toxoplasmosis-serological evidence and associated risk factors among pregnant women in southern Thailand. Am. J. Trop. Med. Hyg. 2011, 85, 243–247. [Google Scholar] [CrossRef]
- Salibay, C.; Dungca, J.; Claveria, F.G. Serological survey of Toxoplasma gondii infection amoug Urban (Manila) and Suburban (Dasmariñas, Cavite) Residents, Philippines. J. Protozool. Res. 2008, 18, 26–33. [Google Scholar]
- Fazly, Z.A.; Nurulaini, R.; Shafarin, M.S.; Fariza, N.J.; Zawida, Z.; Muhamad, H.Y.; Adnan, M.; Premaalatha, B.; Erwanas, A.I.; Zaini, C.M.; et al. Zoonotic parasites from exotic meat in Malaysia. Trop. Biomed. 2013, 30, 535–542. [Google Scholar] [PubMed]
- Puvanesuaran, V.R.; Noordin, R.; Balakrishnan, V. Isolation and genotyping of Toxoplasma gondii from free-range ducks in Malaysia. Avian Dis. 2013, 57, 128–132. [Google Scholar] [CrossRef]
- Puvanesuaran, V.R.; Noordin, R.; Balakrishnan, V. Genotyping of Toxoplasma gondii isolates from wild boars in Peninsular Malaysia. PLoS ONE 2013, 8, e61730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrawathani, P.; Nurulaini, R.; Zanin, C.M.; Premaalatha, B.; Adnan, M.; Jamnah, O.; Zatil, S.A. Seroprevalence of Toxoplasma gondii antibodies in pigs, goats, cattle, dogs and cats in peninsular Malaysia. Trop. Biomed. 2009, 25, 257–258. [Google Scholar]
- Department of Veterinary Services (DVS) Malaysia. Livestock Statistic. 2017. Available online: http://www.dvs.gov.my/index.php/pages/view/2234 (accessed on 5 November 2019).
- Jones, C.D.; Okhravi, N.; Adamson, P.; Tasker, S.; Lightman, S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T. gondii in aqueous humor. Investigative Ophthalmol. Vis. Sci. 2000, 41, 634–644. [Google Scholar]
- Dubey, J.P.; Rajendran, C.; Ferreira, L.R.; Martins, J.; Kwok, O.C.; Hill, D.E.; Jones, J.L. High prevalence and genotypes of Toxoplasma gondii isolated from goats, from a retail meat store, destined for human consumption in the USA. Inter. J. Parasitol. 2011, 41, 827–833. [Google Scholar] [CrossRef]
- Doni, N.Y.; Simsek, Z.; Gurses, G.; Zeyrek, F.Y.; Demir, C. Prevalence and associated risk factors of Toxoplasma gondii in female farmworkers of southeastern Turkey. J. Infect. Dev. Ctries. 2015, 9, 087–093. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, K.; Kul, O.; Gökpinar, S.; Atmaca, H.T.; Gencay, Y.E.; Gazyağci, A.N.; Gürcan, İ.S. The relationship between seropositivity and tissue cysts in sheep naturally infected with Toxoplasma gondii. Turk. Turk. J. Vet. Anim. Sci. 2014, 38, 169–175. [Google Scholar] [CrossRef]
- Bahrami, S.; Zarei, M.; Ghorbanpour, M.; Karami, S. Toxoplasma gondii in sheep and goat livers: Risks for human consumption. J. Hell. Vet. Med. Soc. 2000, 70, 1387–1392. [Google Scholar] [CrossRef]
- Ahmed, H.; Malik, A.; Arshad, M.; Mustafa, I.; Khan, M.R.; Afzal, M.S.; Simsek, S. Seroprevalence and spatial distribution of toxoplasmosis in sheep and goats in North-Eastern region of Pakistan. Korean J. Parasitol. 2016, 54, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Bawm, S.; Maung, W.Y.; Win, M.Y.; Thu, M.J.; Chel, H.M.; Khaing, T.A.; Tiwananthagorn, S. Serological survey and factors associated with Toxoplasma gondii infection in domestic goats in Myanmar. Scientifica 2016, 2016, 4794318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzonis, A.L.; Zanzani, S.A.; Villa, L.; Manfredi, M.T. Toxoplasma gondii in naturally infected goats: Monitoring of specific IgG levels in serum and milk during lactation and parasitic DNA detection in milk. Prev. Vet. Med. 2019, 170, 104738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarado-Esquivel, C.; Silva-Aguilar, D.; Villena, I.; Dubey, J.P. Seroprevalence and correlates of Toxoplasma gondii infection in domestic sheep in Michoacan State, Mexico. Prev. Vet. Med. 2013, 112, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Dubey, J.P.; Hill, D.; Buchanan, R.L.; Gamble, H.R.; Jones, J.L.; Pradhan, A.K. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J. Food. Prod. 2015, 78, 457–476. [Google Scholar] [CrossRef]
- Rajamanickam, C.; Cheah, T.; Paramasvaran, S. Antibodies to Toxoplasma gondii from domestic animals in Malaysia. Trop. Anim. Health Prod. 1990, 22, 61–62. [Google Scholar] [CrossRef]
- Rahman, W.; Manimegalai, V.; Chandrawathani, P.; Nurulaini, R.; Zaini, C.; Premaalatha, B. Seroprevalence of Toxoplasma gondii in Malaysian cattle. Malays. J. Vet. Res. 2011, 2, 51–56. [Google Scholar]
- Berger-Schoch, A.E.; Herrmann, D.C.; Schares, G.; Muller, N.; Bernet, D.; Gottstein, B.; Frey, C.F. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet. Parasitol. 2011, 177, 290–297. [Google Scholar] [CrossRef]
- Kamani, J.; Mani, A.U.; Egwu, G.O. Seroprevalence of Toxoplasma gondii infection in domestic sheep and goats in Borno state, Nigeria. Trop. Anim. Health Prod. 2010, 42, 793–797. [Google Scholar] [CrossRef]
- Opsteegh, M.; Spano, F.; Aubert, D.; Balea, A.; Burrells, A.; Cherchi, S.; Györke, A. The relationship between the presence of antibodies and direct detection of Toxoplasma gondii in slaughtered calves and cattle in four European countries. Int. J. Parasitol. 2011, 49, 515–522. [Google Scholar] [CrossRef]
- Amdouni, Y.; Rjeibi, M.R.; Rouatbi, M.; Amairia, S.; Awadi, S.; Gharbi, M. Molecular detection of Toxoplasma gondii infection in slaughtered ruminants (sheep, goats and cattle) in Northwest Tunisia. Meat Sci. 2017, 133, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.M.; Katzer, F.; Innes, E.A.; Kelly, P.J. Seroprevalence of Toxoplasma gondii in small ruminants from four Caribbean islands. Parasites Vectors 2014, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armand, B.; Solhjoo, K.; Shabani-Kordshooli, M.; Davami, M.H.; Sadeghi, M. Toxoplasma infection in sheep from south of Iran monitored by serological and molecular methods; risk assessment to meat consumers. Vet. World 2016, 9, 850–855. [Google Scholar] [CrossRef] [Green Version]
- Bartova, E.; Kobedova, K.; Lamka, J.; Kotrba, R.; Vodicka, R.; Sedlak, K. Seroprevalence of Neospora caninum and Toxoplasma gondii in exotic ruminants and camelids in the Czech Republic. Parasitol. Res. 2017, 116, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Berger-Schoch, A.E.; Bernet, D.; Doherr, M.G.; Gottstein, B.; Frey, C.F. Toxoplasma gondii in Switzerland: A serosurvey based on meat juice analysis of slaughtered pigs, wild boar, sheep and cattle. Zoonoses Public Health 2011, 58, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Vismarra, A.; Barilli, E.; Miceli, M.; Mangia, C.; Genchi, M.; Brindani, F.; Bacci, C. Toxoplasma gondii in the Cornigliese sheep breed in Italy: Meat juice serology, in vitro isolation and genotyping. Vet. Parasitol. 2017, 243, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bayarri, S.; Gracia, M.J.; Perez-Arquillue, C.; Lazaro, R.; Herrera, A. Toxoplasma gondii in commercially available pork meat and cured ham: A contribution to risk assessment for consumers. J. Food Protect. 2012, 75, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Wahab, T.; Edvinsson, B.; Palm, D.; Lindh, J. Comparison of the AF146527 and B1 repeated elements, two real-time PCR targets used for detection of Toxoplasma gondii. J. Clin. Microbiol. 2010, 48, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.C.; Melo, H.R.; Costa, V.M. Influence of neurotoxoplasmosis characteristics on real-time PCR sensitivity among AIDS patients in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 24–28. [Google Scholar] [CrossRef]
- Franco-Hernandez, E.N.; Acosta, A.; Cortes-Vecino, J.; Gomez-Marin, J.E. Survey for Toxoplasma gondii by PCR detection in meat for human consumption in Colombia. Parasitol. Res. 2016, 115, 691–695. [Google Scholar] [CrossRef]
- Mahami-Oskouei, M.; Moradi, M.; Fallah, E.; Hamidi, F.; Asl Rahnamaye Akbari, N. Molecular detection and genotyping of Toxoplasma gondii in chicken, beef, and lamb meat consumed in Northwestern Iran. Iran. J. Parasitol. 2017, 12, 38–45. [Google Scholar] [PubMed]
- Galván-Ramirez, M.L.; Madriz Elisondo, A.L.; Rico Torres, C.P.; Luna-Pastén, H.; Rodríguez Pérez, L.R.; Rincón-Sánchez, A.R.; Correa, D. Frequency of Toxoplasma gondii in pork meat in Ocotlán, Jalisco, Mexico. J. Food Prot. 2010, 73, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P.; Lunney, J.K.; Gamble, H.R. Comparison of detection methods for Toxoplasma gondii in naturally and experimentally infected swine. Vet. Parasitol. 2006, 141, 9–17. [Google Scholar] [CrossRef]
- Burrells, A.; Taroda, A.; Opsteegh, M.; Schares, G.; Benavides, J.; Dam-Deisz, C.; Bartley, P.M.; Chianini, F.; Villena, I.; van der Giessen, J.; et al. Detection and dissemination of Toxoplasma gondii in experimentally infected calves, a single test does not tell the whole story. Parasites Vectors 2018, 11, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opsteegh, M.; Teunis, P.; Mensink, M.; Zuchner, L.; Titilincu, A.; Langelaar, M.; van der Giessen, J. Evaluation of ELISA test characteristics and estimation of Toxoplasma gondii seroprevalence in Dutch sheep using mixture models. Prev. Vet. Med. 2010, 96, 232–240. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in sheep—The last 20 years. Vet. Parasitol. 2009, 163, 1–14. [Google Scholar] [CrossRef]
- Gutierrez, J.; O’Donovan, J.; Proctor, A.; Brady, C.; Marques, P.X.; Worrall, S.; Maley, S. Application of quantitative real-time polymerase chain reaction for the diagnosis of toxoplasmosis and enzootic abortion of ewes. J. Vet. Diag. Investig. 2012, 24, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Gazzonis, A.L.; Veronesi, F.; Di Cerbo, A.R.; Zanzani, S.A.; Molineri, G.; Moretta, I.; Manfredi, M.T. Toxoplasma gondii in small ruminants in Northern Italy—Prevalence and risk factors. Ann. Agric. Environ. Med. 2015, 22, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Tilahun, B.; Tolossa, Y.H.; Tilahun, G.; Ashenafi, H.; Shimelis, S. Seroprevalence and risk factors of Toxoplasma gondii infection among domestic ruminants in East Hararghe Zone of Oromia Region, Ethiopia. Vet. Med. Int. 2018, 2018, 4263470. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, A.C.; Carneiro, M.; Gouveia, A.M.G.; Vilas-Boas, L.S.; Vitor, R.W.A. Seroprevalence and risk factors of sheep toxoplasmosis in Minas Gerais, Brazil. Rev. Med. Vet. 2009, 160, 527–531. [Google Scholar]
- Lashari, M.H.; Tasawar, Z. Seroprevalence of toxoplasmosis in sheep in Southern Punjab, Pakistan. Pak. Vet. J. 2010, 30, 91–94. [Google Scholar]
| Oligonucleotide Primer | Sequence | Sequence Position | PCR Product |
|---|---|---|---|
| Outer primer (forward) | 5′-GGAACTGCATCCGTTCATGAG-3′ | 694–714 | 193 bp |
| Outer primer (reverse) | 5′-TCTTTAAAGCGTTCGTGGTC-3′ | 887–868 | |
| Inner primer (forward) | 5′-TGCATAGGTTGCAGTCACTG-3′ | 757–776 | 96 bp |
| Inner primer (reverse) | 5′-TGCATAGGTTGCAGTCACTG-3′ | 853–831 |
| Wet Market Samples (Meat) | Abattoir Samples (Diaphragm) | ||||
|---|---|---|---|---|---|
| Category | Number of Samples (n) | Percentage (%) | Category | Number of Samples (n) | Percentage (%) |
| Meat | Species | ||||
| Beef | 108 | 56.3 | Cattle | 100 | 50.0 |
| Chevron | 35 | 18.2 | Goat | 40 | 20.0 |
| Mutton | 49 | 25.5 | Sheep | 60 | 30.0 |
| Location | Location | ||||
| Gombak | 13 | 6.7 | Banting (local) | 50 | 25.0 |
| Hulu Langat | 42 | 21.8 | Shah Alam (imported) | 150 | 75.0 |
| Klang | 25 | 13.0 | |||
| Kuala Lumpur | 24 | 12.5 | |||
| Petaling | 79 | 41.4 | |||
| Putrajaya | 9 | 4.7 | |||
| Type of wet markets | Sex | ||||
| Pasar Besar | 151 | 78.6 | Male | 53 | 26.5 |
| Pasar Tani | 41 | 21.4 | Female | 147 | 73.5 |
| Meat source | |||||
| Licensed abattoir | 118 | 61.4 | |||
| Non-licensed abattoir | 74 | 38.5 | |||
| Location | Beef | Chevon | Mutton | Total | Seropositive (%) |
|---|---|---|---|---|---|
| Wet Market | |||||
| District | |||||
| Gombak | 0/11 | 0/0 | 1/2 | 1/13 | 7.7 |
| Hulu Langat | 0/13 | 10/14 | 4/15 | 14/42 | 33.3 |
| Klang | 0/7 | 5/8 | 6/10 | 11/25 | 44.0 |
| Kuala Lumpur | 1/21 | 0/0 | 1/3 | 2/24 | 9.1 |
| Petaling | 3/47 | 9/13 | 5/19 | 17/79 | 21.5 |
| Putrajaya | 2/9 | 0/0 | 0/0 | 2/9 | 22.2 |
| Total for Wet Markets | 6/108 (5.6%) | 24/35 (68.6%) | 17/49 (34.7%) | 47/192 | 24.5 |
| Abattoir | |||||
| Shah Alam (imported) | 5/50 | 17/40 | 21/60 | 43/150 | 28.6 |
| Banting (local) | 8/50 | 0 | 0 | 8/50 | 1.6 |
| Total for Abattoirs | 13/100 (13%) | 17/40 (42.5%) | 21/60 (35%) | 51/200 | 25.5 |
| Total for Wet Markets + Abattoirs | 19/208 | 41/75 | 38/109 | 98/392 | |
| Overall Seropositive (%) | 9.1 | 54.7 | 34.9 | 25.0 |
| Seropositive Samples (n) | Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Category | Samples (n) | Positive (n) | Prevalence (%) | Exact 95% (CI) | Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
| Meat | ||||||||
| Cattle | 108 | 6 | 5.6 | 2.1,11.7 | 1.0 | - | 1.0 | - |
| Goats | 35 | 24 | 68.6 | 50.7, 83.1 | 37.1(12.5,110.3) | <0.001 | 26.0(12.5,94.6) | <0.001 |
| Sheep | 49 | 17 | 34.7 | 21.7,49.6 | 9.1(3.3-24.8) | <0.001 | 7.5(2.5,20.8) | <0.001 |
| Location | ||||||||
| Gombak | 13 | 1 | 7.7 | 0.2,36.0 | - | - | - | - |
| Hulu Langat | 42 | 14 | 33.3 | 19.6,49.5 | - | - | - | - |
| Klang | 25 | 11 | 44.0 | 24.4, 65.1 | - | - | - | - |
| Kuala Lumpur | 24 | 2 | 8.3 | 1.0, 27.0 | - | - | - | - |
| Petaling | 79 | 17 | 21.5 | 13.1, 32.2 | - | - | - | - |
| Putrajaya | 9 | 2 | 22.2 | 2.8, 60.0 | - | - | - | - |
| Meat Source | ||||||||
| Licensed abattoir | 118 | 14 | 11.9 | 6.6,19.1 | 1.0 | - | - | 0.57 |
| Non-licensed abattoir | 74 | 29 | 49.2 | 35.9,62.5 | 5.9(2.9,12.3) | <0.001 | - | - |
| Seropositive Samples (n) | Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Category | Sample (n) | Positive (n) | Prevalence (%) | Exact 95% (CI) | Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
| Location | ||||||||
| Banting | 50 | 8 | 16.0 | 7.2–29.1 | 1.00 | |||
| Shah Alam | 150 | 55 | 36.0 | 29.0–44.9 | 2.11 | 0.080 | - | 0.372 |
| Species | ||||||||
| Cattle | 100 | 19 | 19.0 | 11.8–28.1 | 1.00 | - | 1.00 | - |
| Goat | 40 | 17 | 42.5 | 27.0–59.1 | 4.9(2.1,11.6) | <0.001 | 4.2(1.9,10.4) | <0.001 |
| Sheep | 60 | 21 | 33.3 | 22.9–45.2 | 3.6(1.6–7.9) | <0.001 | 3.1(1.3,6.4) | <0.001 |
| Sex | ||||||||
| Male | 53 | 3 | 5.6 | 1.2–15.7 | 1.00 | - | - | - |
| Female | 147 | 48 | 32.6 | 25.2–40.9 | 6.7(2.0–22.7) | 0.002 | 4.4(0.84,18.4) | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Hamid, N.; Sadiq, M.B.; Ramanoon, S.Z.; Mansor, R.; Watanabe, M.; Md Isa, N.M.; Kamaludeen, J.; Syed-Hussain, S.S. Seroprevalence and Risk Factors of Toxoplasma gondii in Ruminant Meats from Wet Markets in Klang Valley and Abattoirs in Selangor, Malaysia. Animals 2020, 10, 1139. https://doi.org/10.3390/ani10071139
Abdul Hamid N, Sadiq MB, Ramanoon SZ, Mansor R, Watanabe M, Md Isa NM, Kamaludeen J, Syed-Hussain SS. Seroprevalence and Risk Factors of Toxoplasma gondii in Ruminant Meats from Wet Markets in Klang Valley and Abattoirs in Selangor, Malaysia. Animals. 2020; 10(7):1139. https://doi.org/10.3390/ani10071139
Chicago/Turabian StyleAbdul Hamid, Norhamizah, Mohammed Babatunde Sadiq, Siti Zubaidah Ramanoon, Rozaihan Mansor, Malaika Watanabe, Nur Mahiza Md Isa, Juriah Kamaludeen, and Sharifah Salmah Syed-Hussain. 2020. "Seroprevalence and Risk Factors of Toxoplasma gondii in Ruminant Meats from Wet Markets in Klang Valley and Abattoirs in Selangor, Malaysia" Animals 10, no. 7: 1139. https://doi.org/10.3390/ani10071139
APA StyleAbdul Hamid, N., Sadiq, M. B., Ramanoon, S. Z., Mansor, R., Watanabe, M., Md Isa, N. M., Kamaludeen, J., & Syed-Hussain, S. S. (2020). Seroprevalence and Risk Factors of Toxoplasma gondii in Ruminant Meats from Wet Markets in Klang Valley and Abattoirs in Selangor, Malaysia. Animals, 10(7), 1139. https://doi.org/10.3390/ani10071139





