Prevalence of Blood Types and Alloantibodies of the AB Blood Group System in Non-Pedigree Cats from Northern (Lombardy) and Southern (Sicily) Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Population
2.2. Blood Typing
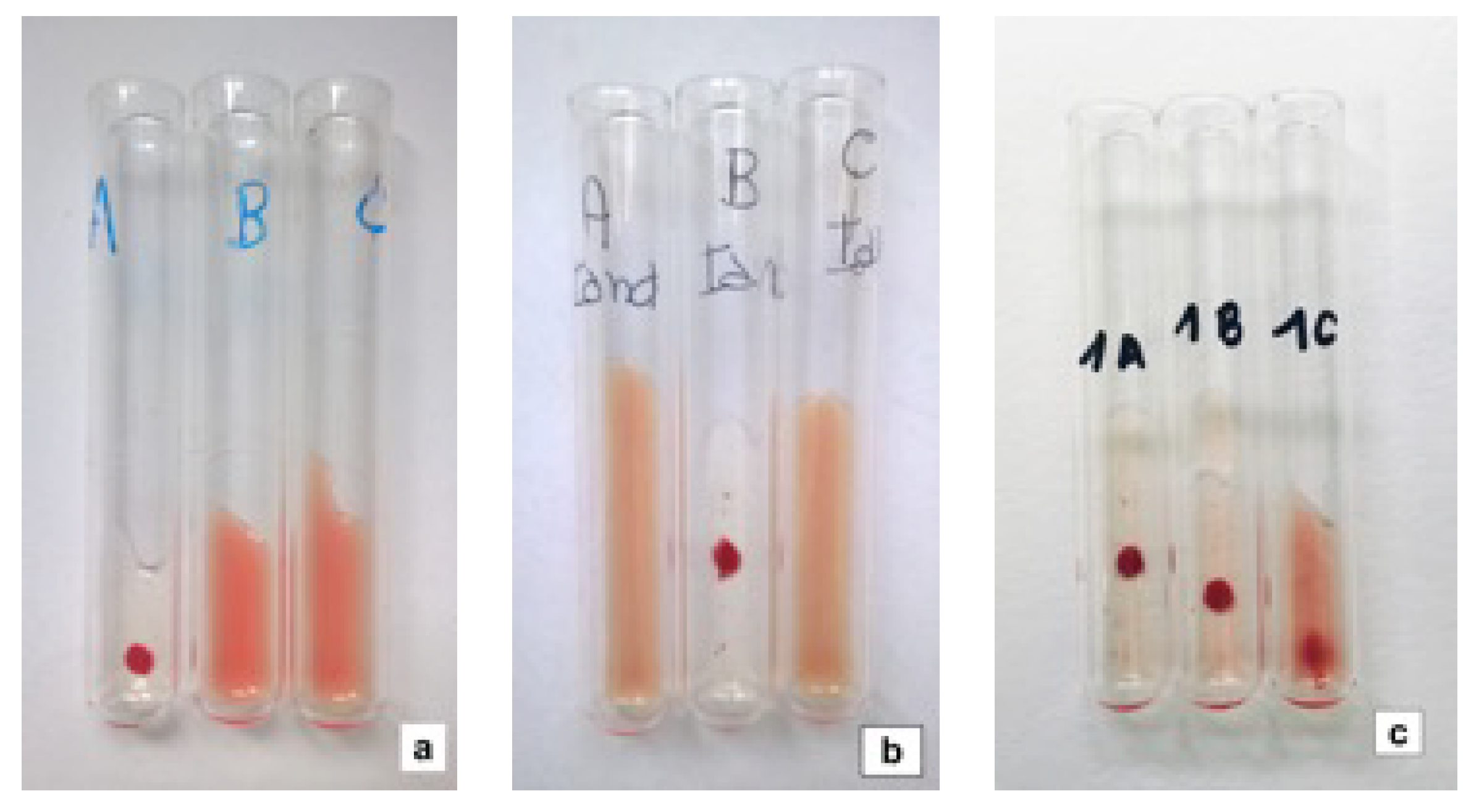
2.3. Alloantibody Screening
2.4. Statistical Analysis
3. Results
3.1. Demographic Data and Blood Type
3.2. Alloantibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andrews, G.A.; Chavey, P.S.; Smith, J.E.; Rich, L. N-glycolylneuraminic acid and N-acetylneuraminic acid define feline blood group A and B antigens. Blood 1992, 79, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Ferreira, A.C.; Masso, O.; Pastor, J. High-performance liquid chromatography ganglioside pattern of the AB feline blood group. Comp. Clin. Pathol. 2011, 20, 597–605. [Google Scholar] [CrossRef]
- Bücheler, J.; Giger, U. Alloantibodies against A and B blood types in cats. Vet. Immunol. Immunopathol. 1993, 38, 283–295. [Google Scholar] [CrossRef]
- Auer, L.; Bell, K. Transfusion reactions in cats due to AB blood group incompatibility. Res. Vet. Sci. 1983, 35, 145–152. [Google Scholar] [CrossRef]
- Giger, U.; Akol, K.G. Acute Hemolytic Transfusion Reaction in an Abyssinian Cat With Blood Type B. J. Vet. Intern. Med. 1990, 4, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Bucheler, J. Transfusion of type-A and type-B blood to cats. J. Am. Vet. Med. Assoc. 1991, 198, 411–418. [Google Scholar]
- Casal, M.; Jezyk, P.; Giger, U. Transfer of colostral antibodies from queens to their kittens. Am. J. Vet. Res. 1996, 57, 1653–1658. [Google Scholar]
- Griot-Wenk, M.; Callan, M.; Casal, L.; Chisholm-Chait, A.; Spitalnik, S.; Patterson, D.; Giger, U. Blood type AB in the feline AB blood group system. Am. J. Vet. Res. 1996, 57, 1438–1442. [Google Scholar]
- Barrot, A.C.; Buttin, R.; Linsart, A.; Bachy, V.; Guidetti, M.; Blais, M.C. Frequency of feline blood types in non-pedigree cats in France. Rev. Med. Vet. 2017, 168, 235–240. [Google Scholar]
- Fosset, F.T.; Blais, M.C. Prevalence of feline blood groups in the Montreal area of Quebec, Canada. Can. Vet. J. 2014, 55, 1225–1228. [Google Scholar]
- Vieira, S.M.; Ferreira, R.R.F.; de Matos, A.J.; Cardoso, I.M.; Graça, R.M.C.; Soares, A.R.; Blasi-Brugué, C.; Sánchez, I.M.; Gopegui, R.R. Distribution of feline AB blood types: A review of frequencies and its implications in the Iberian Peninsula. J. Feline Med. Surg. Open Rep. 2017, 3, 2055116917727693. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, D.; Spada, E.; Baggiani, L.; Perego, R.; Milici, A.; Ferro, E. Comparison of gel column agglutination with monoclonal antibodies and card agglutination methods for assessing the feline AB group system and a frequency study of feline blood types in northern Italy. Vet. Clin. Pathol. 2011, 40, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Arikan, S.; Guzel, M.; Ozturk, A.S.; Simsek, O. Frequencies of blood type A, B and AB in cats from the mediterranean sea coast of the Turkey. Rev. Med. Vet. 2010, 161, 322–325. [Google Scholar]
- Marques, C.; Ferreira, M.; Gomes, J.F.; Leitão, N.; Costa, M.; Serra, P.; Duarte Correia, J.H.; Pomba, C.F. Frequency of blood type A, B, and AB in 515 domestic shorthair cats from the Lisbon area. Vet. Clin. Pathol. 2011, 40, 185–187. [Google Scholar] [CrossRef]
- Forcada, Y.; Guitian, J.; Gibson, G. Frequencies of feline blood types at a referral hospital in the south east of England. J. Small Anim. Pract. 2007, 48, 570–573. [Google Scholar] [CrossRef]
- Arikan, S.; Akkan, H.A. Titres of naturally occurring alloantibodies against feline blood group antigens in Turkish Van cats. J. Small Anim. Pract. 2004, 45, 289–292. [Google Scholar] [CrossRef]
- Knottenbelt, C.M.; Addie, D.D.; Day, M.J.; Mackin, A.J. Determination of the prevalence of feline blood types in the UK. J. Small Anim. Pract. 1999, 40, 115–118. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Koutinas, A.F.; Saridomichelakis, M.; Leontidis, L.; Papadogiannakis, E.; Plevraki, K. Determination of the prevalence of blood types in the non-pedigree feline population in Greece. Vet. Rec. 2001, 149, 213–214. [Google Scholar] [CrossRef]
- Bighignoli, B.; Niini, T.; Grahn, R.A.; Pedersen, N.C.; Millon, L.V.; Polli, M.; Longeri, M.; Lyons, L.A. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet. 2007, 8, 27. [Google Scholar] [CrossRef]
- Omi, T.; Nakazawa, S.; Udagawa, C.; Tada, N.; Ochiai, K.; Chong, Y.H.; Kato, Y.; Mitsui, H.; Gin, A.; Oda, H.; et al. Molecular Characterization of the Cytidine Monophosphate-N-Acetylneuraminic Acid Hydroxylase (CMAH) Gene Associated with the Feline AB Blood Group System. PLoS ONE 2016, 11, e0165000. [Google Scholar] [CrossRef]
- Ejima, H.; Kurokawa, K.; Ikemoto, S. Feline red blood cell groups detected by naturally occurring isoantibody. Jpn. J. Vet. Sci. 1986, 48, 971–976. [Google Scholar] [CrossRef]
- Merbl, Y.; Hason, A.; Sethon, E.D.; Aroch, I. A survey of feline AB group blood types in Israel (2007 to 2009). Isr. J. Vet. Med. 2011, 66, 21–28. [Google Scholar]
- Giger, U.; Bucheler, J.; Diserens, D.; Hale, A.; Griot-Wenk, M. Geographical variation of the feline blood type frequencies in the United States. Feline Pract. 1991, 19, 21–26. [Google Scholar]
- Arikan, S.; Gurkan, M.; Ozaytekim, E.; Dodurka, T.; Giger, U. Frequencies of blood types A, B, and AB in non-pedigree domestic cats in Turkey. J. Small Anim. Pract. 2006, 47, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Ferreira, A.C.; Pastor, J.; Almeida, O.; Montoya, A. Frequencies of feline blood types in northern Portugal. Vet. Clin. Pathol. 2004, 33, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Giger, U.; Gorman, N.T.; Hubler, M.; Leidinger, J.I.; Leidinger, E.F.; Lubas, G.; Niini, T.; Slappendel, R.J. Frequencies of Feline A and B Blood Types in Europe. In Proceedings of the 23rd International Society of Animal Genetics Conference, Interlaken, Switzerland, 3–7 August 1992; pp. 17–18. [Google Scholar]
- Auer, L.; Bell, K. The AB blood group system of cats. Anim. Blood Groups Biochem. Genet. 1981, 12, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Griffin, D.L.; White, J.D.; Rozmanec, M.; Tisdall, P.L.C.; Fosters, S.F.; Bell, K.; Nicholas, F.W. The prevalence of feline A/B blood types in the Sydney region. Aust. Vet. J. 2005, 83, 38–44. [Google Scholar] [CrossRef] [PubMed]
- McDermott, F.M.; Maloney, S.; McMillan, C.; Snead, E. The prevalence of blood groups in Domestic cats in the Saskatoon and Calgary areas of Saskatchewan and Alberta, Canada. Front. Vet. Sci. 2020, 7, 160. [Google Scholar] [CrossRef]
- Gurkan, M.; Arikan, Ş.; Ozaytekin, E.; Dodurka, T. Titres of alloantibodies against A and B blood types in non-pedigree domestic cats in Turkey: Assessing the transfusion reaction risk. J. Feline Med. Surg. 2005, 7, 301–305. [Google Scholar] [CrossRef]
- Silvestre-Ferreira, A.C.; Pastor, J.; Sousa, A.P.; Pires, M.J.; Morales, M.; Abreu, Z.; Montoya, J.A. Blood types in the non-pedigree cat population of Gran Canaria. Vet. Rec. 2004, 155, 778–779. [Google Scholar]
- Cavana, P.; Picco, S.; Bellino, C.; Farca, A.M. Distribuzione dei gruppi sanguigni nei gatti della regione Piemonte (Italia). In Proceedings of the 55° Congresso Nazionale SCIVAC-SIMEF. Medicina Felina. Le Nuove Acquisizioni Nella Pratica Clinica, Milan, Italy, 2–4 March 2007; pp. 438–440. [Google Scholar]
- Continanza, R.; Lubas, G.; Gugliucci, B. Indagini Preliminari sul Sistema di Gruppo Sanguigno AB nel Gatto Allevato in Italia. In Proceedings of the XLVI Congresso SISVet, Venice, Italy, 30 September–3 October 1992; pp. 1473–1477. [Google Scholar]
- Spada, E.; Miglio, A.; Proverbio, D.; Antognoni, M.T.; Bagnagatti De Giorgi, G.; Ferro, E.; Mangili, V. Signalment and blood types in cats being evaluated as blood donors at two Italian university blood banks. Vet. Med. Int. 2014, 2014, 704836. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Antognoni, M.T.; Proverbio, D.; Ferro, E.; Mangili, V.; Miglio, A. Haematological and biochemical reference intervals in adult Maine Coon cat blood donors. J. Feline Med. Surg. 2015, 17, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Andrews, G.A.; Smith, J.E. Reactivity of lectins with feline erythrocytes. Comp. Haematol. Int. 1991, 1, 217–219. [Google Scholar] [CrossRef]
- Giger, U.; Kilrain, C.G.; Filippich, L.J.; Bell, K. Frequencies of feline blood groups in the United States. J. Am. Vet. Med. Assoc. 1989, 195, 1230–1232. [Google Scholar] [PubMed]
- Proverbio, D.; Spada, E.; Perego, R.; Della Pepa, A.; Bagnagatti De Giorgi, G.; Baggiani, L. Assessment of blood types of Ragdoll cats for transfusion purposes. Vet. Clin. Pathol. 2013, 42, 157–162. [Google Scholar] [CrossRef]
- Seth, M.; Jackson, K.V.; Giger, U. Comparison of five blood-typing methods for the feline AB blood group system. Am. J. Vet. Res. 2011, 72, 203–209. [Google Scholar] [CrossRef]
- Stieger, K.; Palos, H.; Giger, U. Comparison of various blood-typing methods for the feline AB blood group system. Am. J. Vet. Res. 2005, 66, 1393–1399. [Google Scholar] [CrossRef]
- Juvet, F.; Brennan, S.; Mooney, C.T. Assessment of feline blood for transfusion purposes in the Dublin area of Ireland. Vet. Rec. 2011, 168, 352. [Google Scholar] [CrossRef] [PubMed]
- Bagdi, N.; Magdus, M.; Leidinger, E.; Leidinger, J.; Vörös, K. Frequencies of feline blood types in Hungary. Acta Vet. Hung. 2001, 49, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Karadjole, T.; Kovačević, I.; Samardžija, M.; Babić, T.; Kreszinger, M.; Radišić, B.; Harapin, I.; Bedrica, L. Blood groups in cats in the city of Zagreb. Vet. Arh. 2016, 86, 209–216. [Google Scholar]
- Marenzoni, M.L.; Lauzi, S.; Miglio, A.; Coletti, M.; Arbia, A.; Paltrinieri, S.; Antognoni, M.T. Comparison of three blood transfusion guidelines applied to 31 feline donors to minimise the risk of transfusion-transmissible infections. J. Feline Med. Surg. 2018, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Sorgatto, S.; Brito De Oliveira, B.; Cristina, K.; Godoy, S.; Antunes, T.R.; Almeida Lacerda, L.; De Souza, A.I. Frequência dos tipos sanguíneos de gatos domésticos mestiços no município de Campo Grande, Mato Grosso do Sul, Brasil. Med. Vet. 2017, 11, 172–178. [Google Scholar] [CrossRef]
- Thengchaisri, N.; Tongme, S.; Chotrattanasiri, B.; Chokchoowattanalert, H.; Poopuak, P.; Nanhawatakarn, C.; Sattasathichana, P. Development of Feline Blood Typing Test Kit in Thailand. Kasetsart Vet. 2008, 18, 23–31. [Google Scholar]
- Zheng, L.; Zhong, Y.; Shi, Z.; Giger, U. Frequencies of blood types A, B, and AB in non-pedigree domestic cats in Beijing. Vet. Clin. Pathol. 2011, 40, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.M.; Shin, J.H.; Kim, J.Y.; Hyun, C.B.; Kim, D.; Pak, S. Il Prevalence of feline blood types in Seoul and Kangwon area of Korea. J. Vet. Clin. 2008, 25, 227–230. [Google Scholar]
- Nectoux, A.; Guidetti, M.; Barthélemy, A.; Pouzot-Nevoret, C.; Hoareau, G.L.; Goy-Thollot, I. Assessment of risks of feline mismatched transfusion and neonatal isoerythrolysis in the Lyon (France) area. J. Feline Med. Surg. Open Rep. 2019, 5, 205511691986317. [Google Scholar] [CrossRef]
- Cattin, R. Distribution of blood types in a sample of 245 New Zealand non-purebred cats. N. Z. Vet. J. 2016, 64, 154–157. [Google Scholar] [CrossRef]
- Giger, U.; Bucheler, J.; Patterson, D.F. Frequency and inheritance of A and B blood types in feline breeds of the United States. J. Hered. 1991, 82, 15–20. [Google Scholar] [CrossRef]
- Mattucci, F.; Oliveira, R.; Lyons, L.A.; Alves, P.C.; Randi, E. European wildcat populations are subdivided into five main biogeographic groups: Consequences of Pleistocene climate changes or recent anthropogenic fragmentation? Ecol. Evol. 2016, 6, 3–22. [Google Scholar] [CrossRef]
- Spada, E.; Proverbio, D.; Baggiani, L.; Bagnagatti De Giorgi, G.; Perego, R.; Ferro, E. Evaluation of an immunochromatographic test for feline AB system blood typing. J. Vet. Emerg. Crit. Care 2016, 26, 137–141. [Google Scholar] [CrossRef]
- Knottenbelt, C.M.; Day, M.J.; Cripps, P.; Mackin, A.J. Measurement of titres of naturally occurring alloantibodies against feline blood group antigens in the UK. J. Small Anim. Pract. 1999, 40, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Goy-Thollot, I.; Nectoux, A.; Guidetti, M.; Chaprier, B.; Bourgeois, S.; Boisvineau, C.; Barthélemy, A.; Pouzot-Nevoret, C.; Giger, U. Detection of naturally occurring alloantibody by an in-clinic antiglobulin-enhanced and standard crossmatch gel column test in non-transfused domestic shorthair cats. J. Vet. Intern. Med. 2019, 33, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, N.M.; Blais, M.-C.; Harris, K.; Oakley, D.A.; Aronson, L.R.; Giger, U. A newly recognized blood group in domestic shorthair cats: The Mik red cell antigen. J. Vet. Intern. Med. 2007, 21, 287–292. [Google Scholar] [CrossRef] [PubMed]
| Variables | n Positive/n Tested (Percentage: 95% Confidence Interval) | p Value | |||
|---|---|---|---|---|---|
| Whole Population | Northern Italy | Southern Italy | |||
| Blood type | A | 378/448 (84.4%: 76.0–93.3) | 212/233 (91.0%: 79.1–104.9) | 166/215 (77.2%: 65.9–89.8) | 0.0001 |
| B | 38/448 (8.5%: 6.0–11.6) | 12/233 (5.2%: 2.6–8.9) | 26/215 (12.1%: 7.9–17.7) | 0.0085 | |
| AB | 32/448 (7.1%: 4.8–10.0) | 9/233 (3.8%: 1.7–7.3) | 23/215 (10.7%: 6.7–16.0) | 0.0051 | |
| Country | Region | Blood Type | Blood Typing Method | ||
|---|---|---|---|---|---|
| A | B | AB | |||
| Northern Italy | Lombardy (n = 140) [12] | 90.7% | 7.1% | 2.1% | Gel column agglutination |
| Lombardy (n = 233) (current study) | 91.0% | 5.2% | 3.8% | Tube agglutination | |
| Piedmont (n = 122) [32] | 86.9% | 7.4% | 5.7% | Card agglutination | |
| Central Italy | Tuscany (n = 401) [26] | 88.8% | 11.2% | 0.0% | Microplate agglutination |
| Southern Italy | Sicily (n = 215) (current study) | 77.2% | 12.1% | 10.7% | Tube agglutination |
| Whole Population (n = 327) | Origin | p Value | |||
|---|---|---|---|---|---|
| Northern Italy (n = 196) | Southern Italy (n = 131) | ||||
| Alloantibodies | Presence | 62 (19.0%) | 30 (15.3%) | 32 (24.4%) | 0.7015 |
| Absence | 265 (81.0%) | 166 (84.7%) | 99 (75.6%) | ||
| Alloantibodies by blood type | A | 30/268 (11.2%) | 19/177 (10.7%) | 11/91 (12.1%) | 0.7398 |
| B | 32/34 (94.1%) | 11/12 (91.7%) | 21/22 (95.5%) | 0.6585 | |
| AB | 0/25 (0.0%) | 0/7 (0.0%) | 0/18 (0.0%) | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spada, E.; Perego, R.; Baggiani, L.; Salatino, E.; Priolo, V.; Mangano, C.; Pennisi, M.G.; Proverbio, D. Prevalence of Blood Types and Alloantibodies of the AB Blood Group System in Non-Pedigree Cats from Northern (Lombardy) and Southern (Sicily) Italy. Animals 2020, 10, 1129. https://doi.org/10.3390/ani10071129
Spada E, Perego R, Baggiani L, Salatino E, Priolo V, Mangano C, Pennisi MG, Proverbio D. Prevalence of Blood Types and Alloantibodies of the AB Blood Group System in Non-Pedigree Cats from Northern (Lombardy) and Southern (Sicily) Italy. Animals. 2020; 10(7):1129. https://doi.org/10.3390/ani10071129
Chicago/Turabian StyleSpada, Eva, Roberta Perego, Luciana Baggiani, Elisabetta Salatino, Vito Priolo, Cyndi Mangano, Maria Grazia Pennisi, and Daniela Proverbio. 2020. "Prevalence of Blood Types and Alloantibodies of the AB Blood Group System in Non-Pedigree Cats from Northern (Lombardy) and Southern (Sicily) Italy" Animals 10, no. 7: 1129. https://doi.org/10.3390/ani10071129
APA StyleSpada, E., Perego, R., Baggiani, L., Salatino, E., Priolo, V., Mangano, C., Pennisi, M. G., & Proverbio, D. (2020). Prevalence of Blood Types and Alloantibodies of the AB Blood Group System in Non-Pedigree Cats from Northern (Lombardy) and Southern (Sicily) Italy. Animals, 10(7), 1129. https://doi.org/10.3390/ani10071129








