Age-Related Changes in Serum Lipid Levels, Hepatic Morphology, Antioxidant Status, Lipid Metabolism Related Gene Expression and Enzyme Activities of Domestic Pigeon Squabs (Columba livia)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Birds and Conditions
2.2. Sample Collection
2.3. Serum Lipid Level Analyses
2.4. Hepatic Antioxidant Index Analyses
2.5. Lipid Metabolism-Related Enzyme Activity Measurement
2.6. RNA Extraction and Quantitative PCR Analyses
2.7. Hepatic Histological Analyses
2.8. Statistical Analyses
3. Results
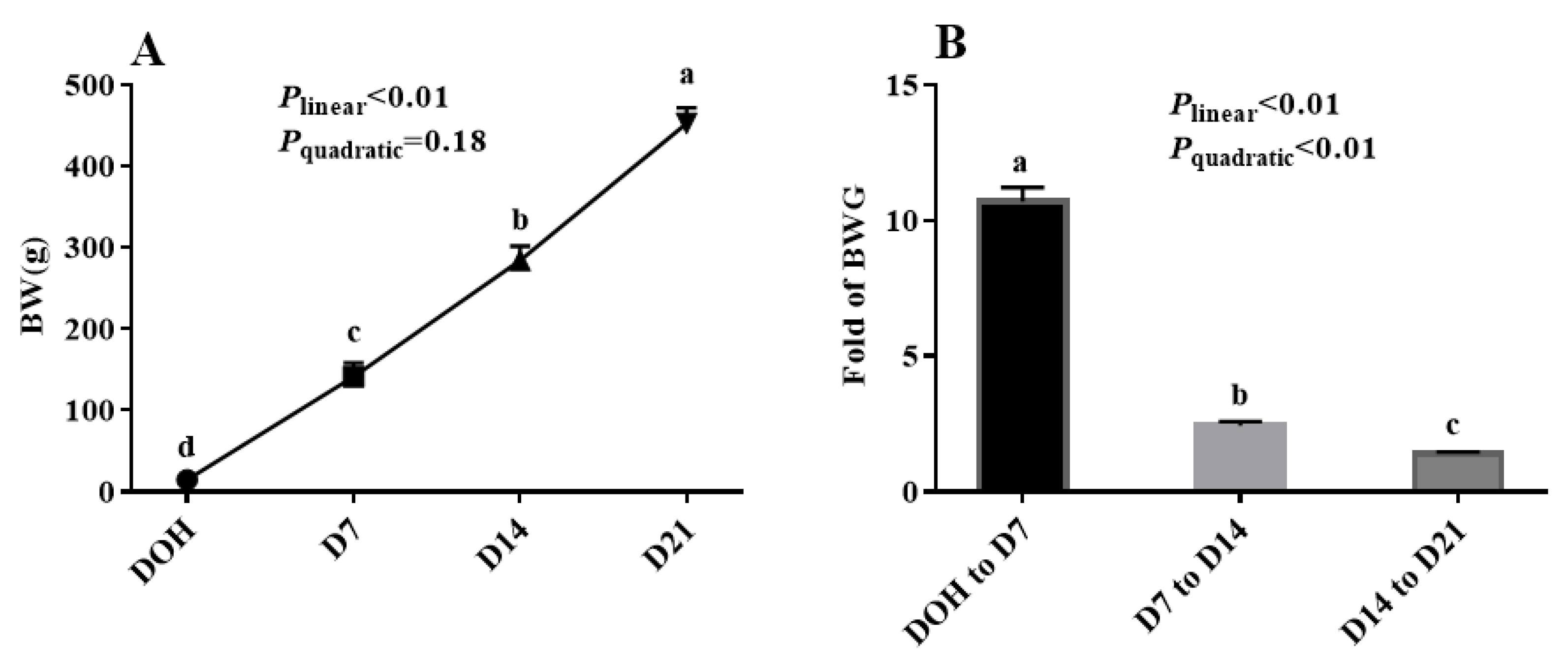
3.1. Body Weight
3.2. Serum Lipid Levels
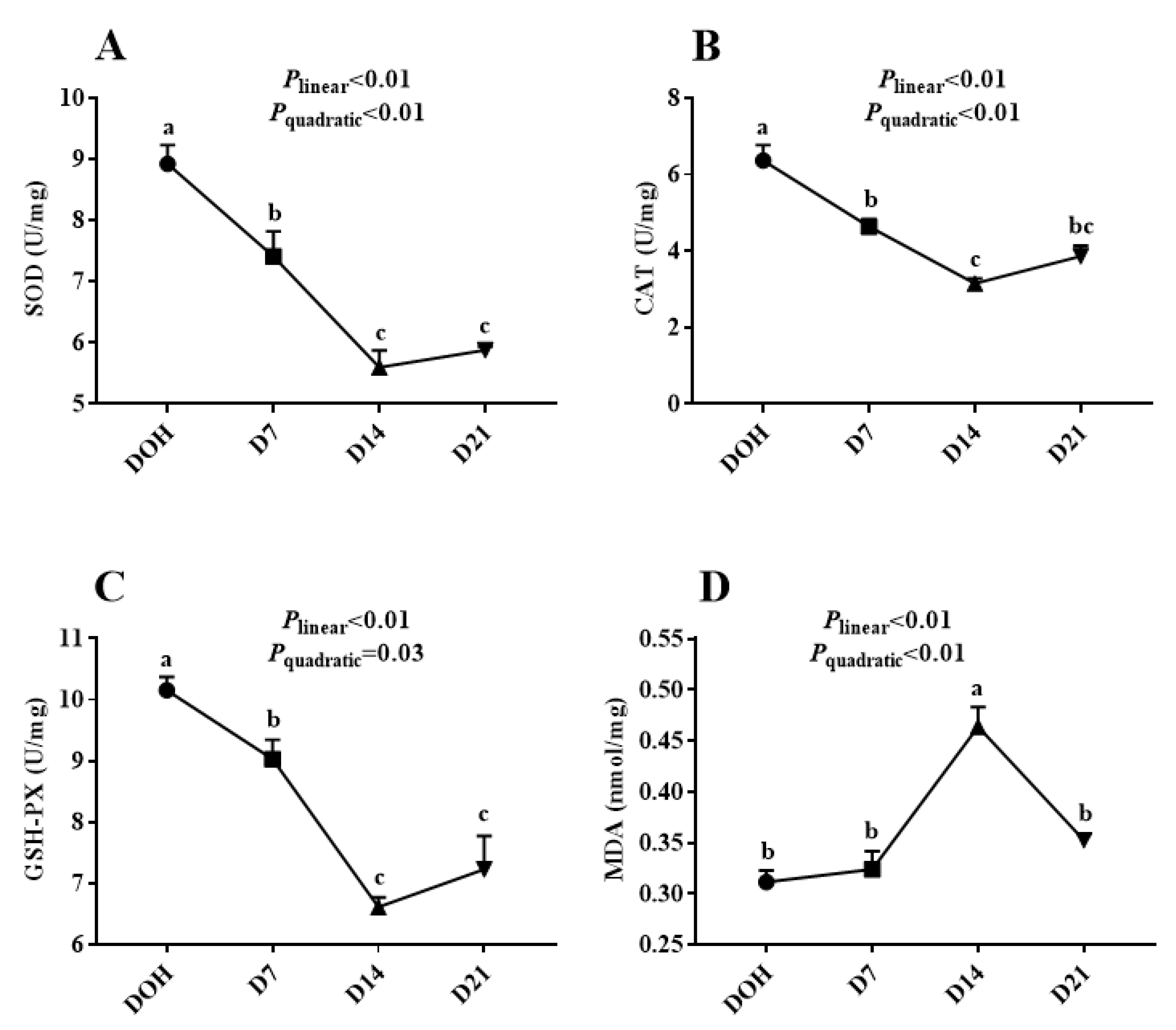
3.3. Hepatic Antioxidant Capacity
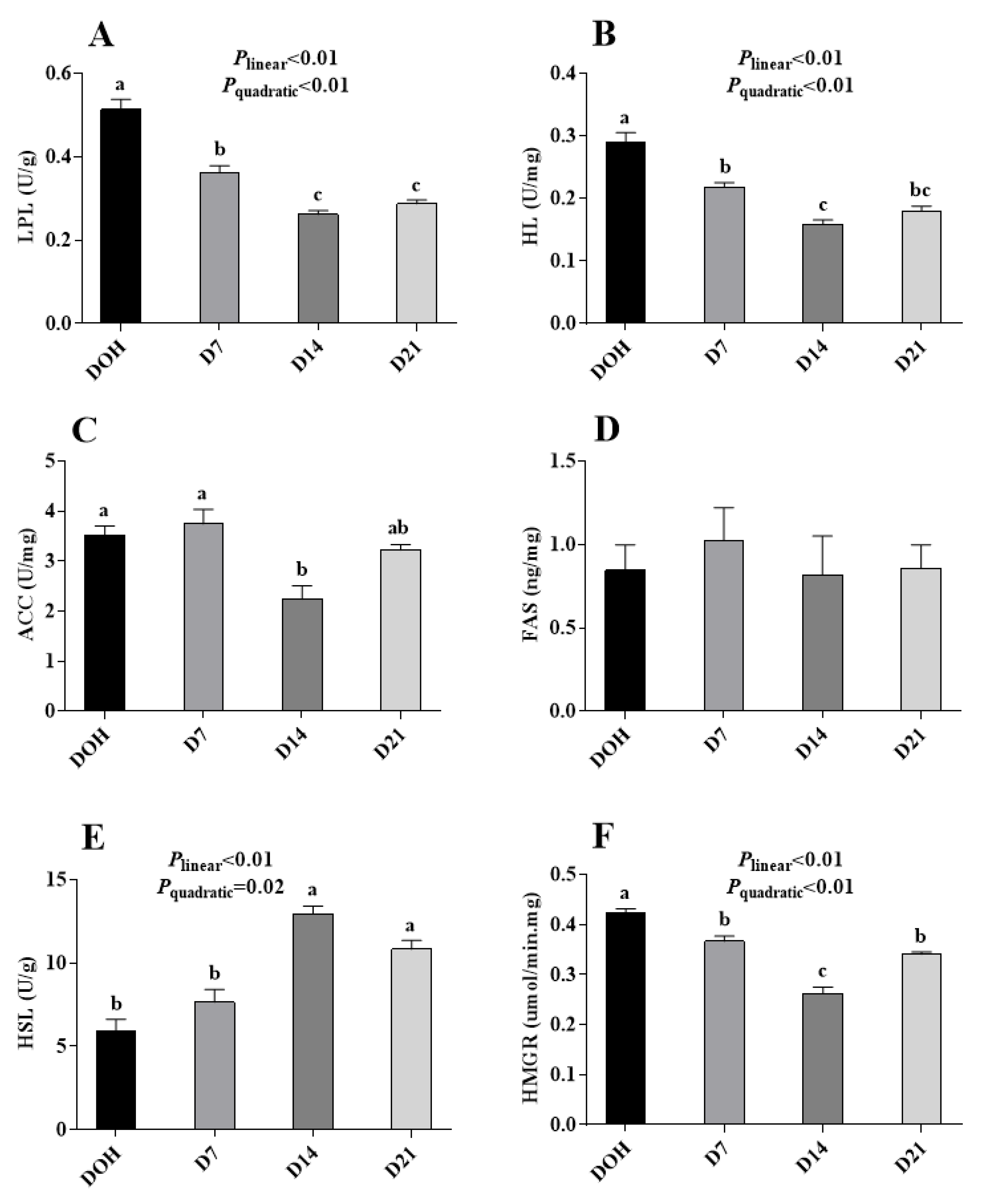
3.4. Hepatic Lipid Metabolism-Related Enzyme Activities
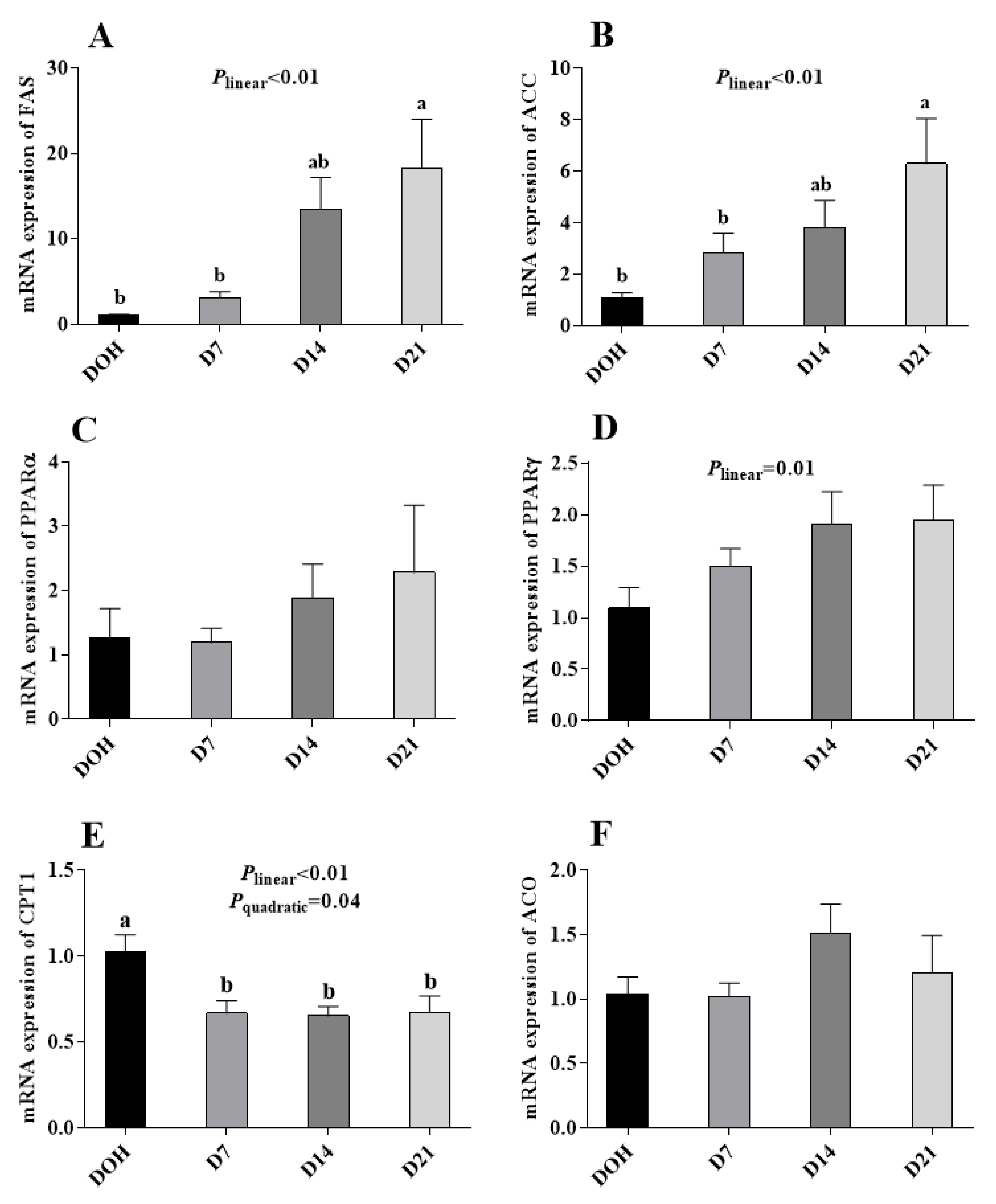
3.5. Hepatic Lipid Metabolism-Related Gene Expression
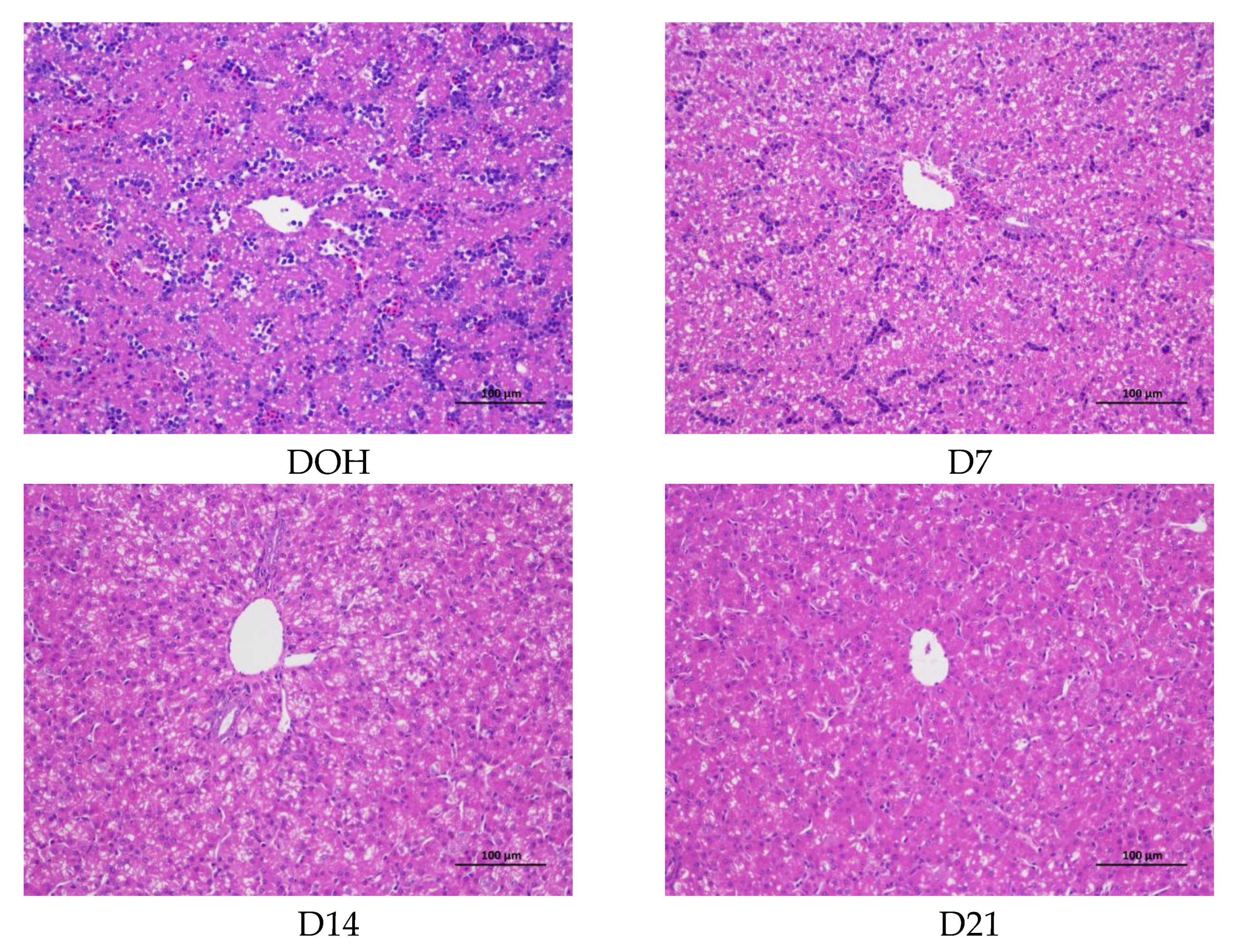
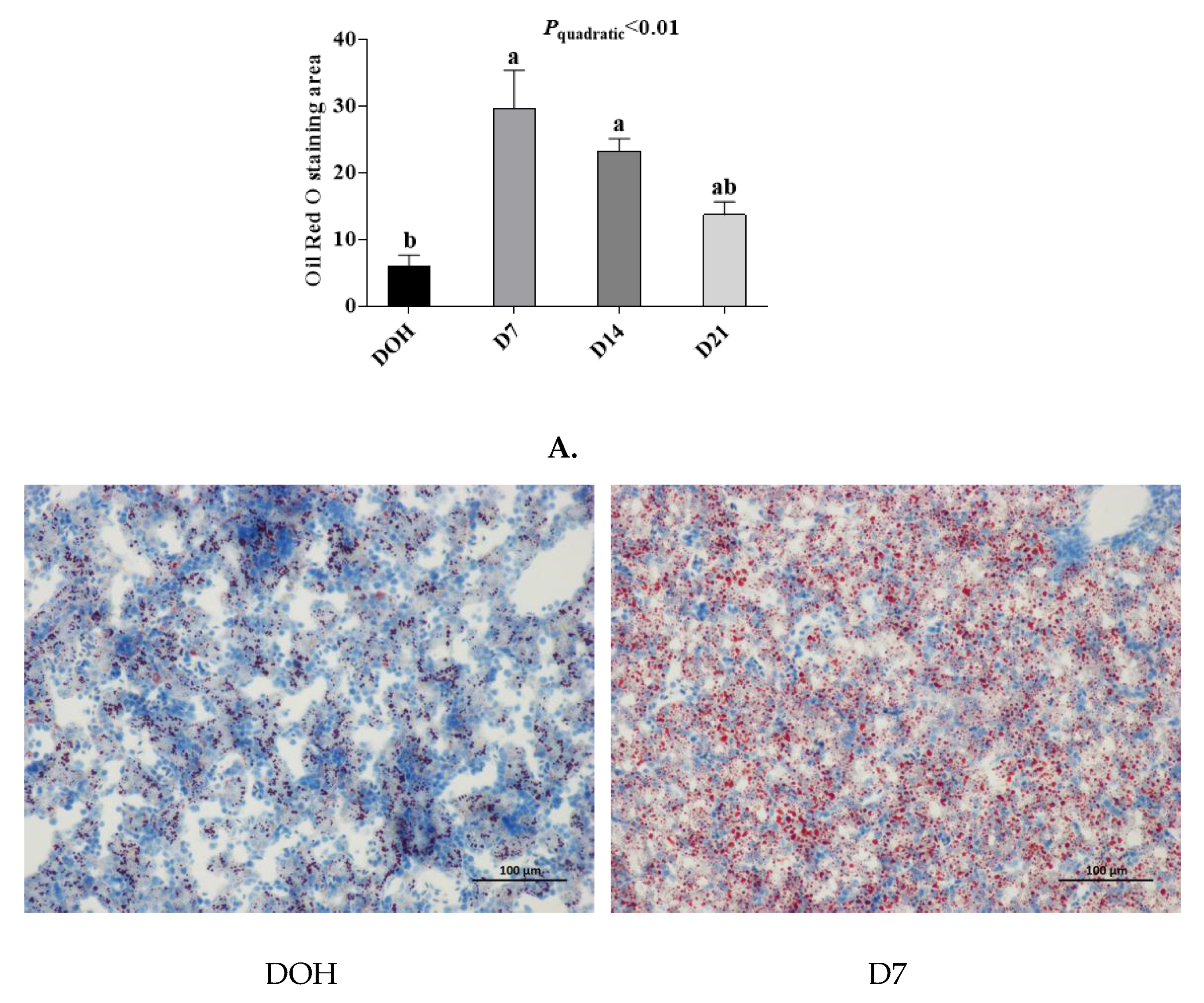
3.6. Hepatic histology and Lipid Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Q.Q.; Zhang, X.Y.; Zou, X.T.; Dong, X.Y. Effects of in ovo injection of L-histidine on hatch performance and post-hatch development in domestic pigeons (Columba livia). Poult. Sci. 2019, 98, 3194–3203. [Google Scholar] [CrossRef]
- Horseman, N.D.; Buntin, J.D. Regulation of pigeon cropmilk secretion and parental behaviors by prolactin. Annu. Rev. Nutr. 1995, 15, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, L.; Shenoy, K.B.; Mojamdar, M.; Hegde, S.N. Studies on the growth-stimulatory activity of pigeon milk--comparison and synergistic effects with serum. J. Comp. Physiol. B 1993, 163, 332–336. [Google Scholar] [CrossRef]
- Davies, W.L. The composition of the crop milk of pigeons. Biochem. J. 1939, 33, 898–901. [Google Scholar] [CrossRef]
- Gillespie, M.J.; Haring, V.R.; McColl, K.A.; Monaghan, P.; Donald, J.A.; Nicholas, K.R. Histological and global gene expression analysis of the ‘lactating’ pigeon crop. BMC Genomics. 2011, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.; Janssens, G.P.J. Nutrition of the domestic pigeon (Columba livia domestica). Worlds Poult. Sci. J. 2003, 59, 221–232. [Google Scholar] [CrossRef]
- Peng, M.; Li, L.; Yu, L.; Ge, C.; Ma, H. Effects of (−)-hydroxycitric acid on lipid droplet accumulation in chicken embryos. Anim. Sci. J. 2018, 89, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J. Animal lipoproteins: Chemistry, structure, and comparative aspects. J. Lipid. Res. 1980, 21, 789–853. [Google Scholar]
- Langelier, M.; Connelly, P.; Subbiah, M.T.R. Plasma lipoprotein profile and composition in white carneau and show racer breeds of pigeons. Can. J. Biochem. 1976, 54, 27–31. [Google Scholar] [CrossRef]
- Jensen, P.F.; Jensen, G.L.; Smith, S.C. Serum lipoprotein profiles of young atherosclerosis-susceptible white carneau and atherosclerosis-resistant show racer pigeons. Comp. Biochem. Phys. B 1978, 60, 67–69. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, J.; Ma, H.; Dai, B.; Zheng, L.; Miao, J.; Zhang, Y. The influence of dietary taurine and reduced housing density on hepatic functions in laying hens. Poult. Sci. 2014, 93, 1724–1736. [Google Scholar] [CrossRef]
- Yu, C.Y.; Chen, G.W.; Cline, D.; Zhang, H.; Zong, Y.; Wang, R. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. oxidants and antioxidant in diseases: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Jia, R.; Bao, Y.H.; Zhang, Y.; Ji, C.; Zhao, L.H.; Zhang, J.Y.; Gao, C.Q.; Ma, Q.G. Effects of dietary α-lipoic acid, acetyl-L-carnitine, and sex on antioxidative ability, energy, and lipid metabolism in broilers. Poult. Sci. 2014, 93, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y. Research on Early Development Regulation of Pigeon Squabs by Cationic Amino Acids. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2018. [Google Scholar]
- Schmittgen, T.D. Real-time quantitative PCR. Methods 2001, 25, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Hünigen, H.; Mainzer, K.; Hirschberg, R.M.; Custodis, P.; Gemeinhardt, O.; Al-Masri, S.; Richardson, K.C.; Hafez, H.M.; Plendl, J. Structure and age-dependent development of the turkey liver: A comparative study of a highly selected meat-type and a wild-type turkey line. Poult. Sci. 2016, 95, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Lynn, E.; Anne, G.; Anja, B.; Martin, W.; Andreas, W.; Arndt, R.; Kuhla, A. Evaluation of two liver treatment strategies in a mouse model of niemann–pick-disease type c1. Int. J. Mol. Sci. 2018, 19, 972. [Google Scholar]
- Gao, C.Q.; Yang, J.X.; Chen, M.X.; Yan, H.C.; Wang, X.Q. Growth curves and age-related changes in carcass characteristics, organs, serum parameters, and intestinal transporter gene, expression in domestic pigeon (columba livia). Poult. Sci. 2016, 95, 867. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wan, X.P.; Miao, L.P.; Zou, X.T.; Dong, X.Y. Effects of in ovo injection of l-arginine on hatchability, hatching time, early posthatch development, and carcass traits in domestic pigeons (Columba livia). J. Anim. Sci. 2017, 95, 4462–4471. [Google Scholar] [CrossRef]
- Vandeputte-Poma, J. Feeding, growth and metabolism of the pigeon, columba livia domestica: Duration and role of crop milk feeding. J. Comp. Physiol. 1980, 135, 97–99. [Google Scholar] [CrossRef]
- Leash, A.M.; Liebman, J.; Taylor, A.; Limbert, R. An analysis of the crop contents of white carneau pigeons (columba livia), days one through twenty-seven. Lab. Anim. Sci. 1971, 21, 86–90. [Google Scholar] [PubMed]
- Levi, W.M. The Pigeon; Oscar Riddle: Sumter, SC, USA, 1963. [Google Scholar]
- Smet, K.; Raes, K.; Huyghebaert, G.; Haak, L.; Arnouts, S.; De Smet, S. Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poult. Sci. 2008, 87, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I. Nutritional modulation of the antioxidant capacities in poultry: The case of selenium. Poult. Sci. 2018, 98, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortega, J.; Goeger, D.E.; Cherian, G. Egg yolk omega-6 and omega-3 fatty acids modify tissue lipid components, antioxidant status, and ex vivo eicosanoid production in chick cardiac tissue. Poult. Sci. 2009, 88, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; Bailey, D.M.; Hullin, D.; Young, I.; Davies, B. Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. Eur. J. Appl. Physiol. 2004, 92, 321–327. [Google Scholar] [CrossRef]
- Skoie, I.M.; Dalen, I.; Omdal, R.; Jonsson, G. Malondialdehyde and advanced oxidation protein products are not increased in psoriasis: A controlled study. Arch. Dermatol. Res. 2019, 311, 299–308. [Google Scholar] [CrossRef]
- Zhou, M.; Zeng, D.; Ni, X.; Tu, T.; Yin, Z.; Pan, K.; Jing, B. Effects of bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with clostridium perfringens-induced necrotic enteritis. Lipids. Health. Dis. 2016, 15, 48. [Google Scholar] [CrossRef]
- Wnuk, A.; Mroczeksosnowska, N.M.; Łukasiewicz, M.; Batorska, M.; Niemiec, J. Influence of the system of rearing on cholesterol level and its fraction in blood serum of slow-growing chickens. Ann. Warsaw Univ. Life Sci. SGGW Anim. Sci. 2013, 52, 219–225. [Google Scholar]
- Du, X.; Liu, Y.; Lu, L.; Wang, W.; Zeng, T.; Tian, Y.; Lu, Y. Effects of dietary fats on egg quality and lipid parameters in serum and yolks of shan partridge duck. Poult. Sci. 2016, 96, 348. [Google Scholar] [CrossRef]
- Badinga, L.; Selberg, K.; Dinges, A.; Corner, C.; Miles, R. Dietary conjugated linoleic acid alters hepatic lipid content and fatty acid composition in broiler chickens. Poult. Sci. 2003, 82, 111–116. [Google Scholar] [CrossRef]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver x receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.Y.; Liu, F.Z.; Min, Y.N.; Li, W.C. Effects of dietary dihydropyridine supplementation on growth performance and lipid metabolism of broiler chickens. Czech. J. Anim. Sci. 2010, 55, 116–122. [Google Scholar] [CrossRef]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2014, 1841, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Perret, B.; Mabile, L.; Martinez, L.; Tercé, F.; Barbaras, R.; Collet, X. Hepatic lipase: Structure/function relationship, synthesis, and regulation. J. Lipid. Res. 2002, 43, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; von Eynatten, M.; Schiekofer, S.; Nawroth, P.P.; Dugi, K.A. Low plasma adiponectin levels are associated with increased hepatic lipase activity in Vivo. Diabetes Care 2005, 28, 2181–2186. [Google Scholar] [CrossRef]
- Lindberg, A.; Olivecrona, G. Lipase evolution: Trout, Xenopus and chicken have lipoprotein lipase and apolipoprotein C-II-like activity but lack hepatic lipase-like activity. Biochim. Biophys. Acta, Lipids. Lipid. Metab. 1995, 1255, 205–211. [Google Scholar] [CrossRef]
- Liu, W.M.; Zhang, J.; Lu, L.Z.; Shi, F.X.; Niu, D.; Wang, D.L. Effects of perilla extract on productive performance, serum values and hepatic expression of lipid-related genes in shaoxing ducks. Bri. Poult. Sci. 2011, 52, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bloomgarden, Z.T. Concepts of Insulin Resistance. Metab. Syndr. Relat. D 2005, 3, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Nikkilä, E.A. Lipoprotein lipase of adipose tissue and skeletal muscle in human obesity: Response to glucose and to semistarvation. Metab. Clin. Exp. 1981, 30, 810–817. [Google Scholar] [CrossRef]
- Merkel, M.; Eckel, R.H.; Goldberg, I.J. Lipoprotein lipase. J. Lipid. Res. 2002, 43, 1997–2006. [Google Scholar] [CrossRef]
- Ferraris, R.P.; Diamond, J. Regulation of intestinal sugar transport. Physiol. Rev. 1997, 77, 257–302. [Google Scholar] [CrossRef] [PubMed]

| Items | Content |
|---|---|
| Ingredients of a whole-grain form feed (%) | |
| Corn | 56.27 |
| Pea | 28.12 |
| Wheat | 5.63 |
| Sorghum | 5.63 |
| Rapeseed oil | 4.35 |
| Total | 100.00 |
| Calculated nutrients 2 (%) | |
| Metabolizable energy (MJ/kg) 3 | 13.84 |
| Crude protein | 12.63 |
| Ingredients of grit meal (%) | |
| Limestone | 52.93 |
| Shell meal | 28.10 |
| Yellow mud | 14.05 |
| Salt | 1.41 |
| Ferrous sulfate (monohydrate) | 0.23 |
| Premix 4 | 3.28 |
| Total | 100.00 |
| Calculated nutrients (%) | |
| Calcium | 27.79 |
| Phosphorus | 0.01 |
| Sodium | 0.03 |
| Chlorine | 0.59 |
| Ferrum | 0.30 |
| Genes 1 | Accession Number 2 | Forward (5′ ➔ 3′) | Reverse (5′ ➔ 3′) |
|---|---|---|---|
| β-actin | AB618546.1 | CCCATCTACGAAGGCTACGC | CTTGATGTCACGCACAATTTC |
| FAS | XM_005515764.1 | AAACTGAAGGCTGCTGATAAGT | CCTCCAATAAGGTGCGGTGAT |
| ACC | XM_013367232.1 | CTCATGGTCTTCGCCAACTGGA | CACGATGTAGGCACCGAACTT |
| ACO | XM_005503118.2 | GGCATTGAGGAGTGTCGGA | GCACAGTCACAGATGGAGCA |
| CPT1 | XM_013369225.1 | TCGTCTTGCCATGACTGGTG | GCTGTGGTGTCTGACTCGTT |
| PPARα | XM_021297326.1 | AGAATAAGGAAGCCGAAGTTC | GGAGAAGCCAGGGATAGATTTG |
| PPARγ | XM_021288013.1 | CCAGCGACATCGACCAGTT | GGTGATTTGTCTGTCGTCTTTCC |
| Items 2 | TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | HDL/LDL | vLDL (nmol/L) | FFA (nmol/L) |
|---|---|---|---|---|---|---|---|
| DOH | 1.20b | 8.57 | 2.04c | 6.27a | 0.35b | 309.06a | 242.99c |
| D7 | 3.25a | 5.62 | 2.08bc | 2.73b | 0.70ab | 249.15ab | 383.48b |
| D14 | 3.81a | 7.26 | 3.13ab | 3.07b | 1.07a | 199.81ab | 447.89a |
| D21 | 3.07ab | 6.9 | 3.23a | 3.09b | 1.08a | 161.63b | 334.09b |
| P value | <0.01 | 0.14 | <0.01 | <0.01 | 0.02 | 0.02 | <0.01 |
| SEM | 0.6 | 1.01 | 0.35 | 0.88 | 0.23 | 35.24 | 17.1 |
| Contrast | |||||||
| Linear | <0.01 | 0.17 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Quadratic | 0.01 | 0.24 | 0.97 | 0.04 | 0.35 | 0.71 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Li, H.; Zhou, W.; Zou, X.; Dong, X. Age-Related Changes in Serum Lipid Levels, Hepatic Morphology, Antioxidant Status, Lipid Metabolism Related Gene Expression and Enzyme Activities of Domestic Pigeon Squabs (Columba livia). Animals 2020, 10, 1121. https://doi.org/10.3390/ani10071121
Xu Q, Li H, Zhou W, Zou X, Dong X. Age-Related Changes in Serum Lipid Levels, Hepatic Morphology, Antioxidant Status, Lipid Metabolism Related Gene Expression and Enzyme Activities of Domestic Pigeon Squabs (Columba livia). Animals. 2020; 10(7):1121. https://doi.org/10.3390/ani10071121
Chicago/Turabian StyleXu, Qianqian, Huaiyu Li, Wenting Zhou, Xiaoting Zou, and Xinyang Dong. 2020. "Age-Related Changes in Serum Lipid Levels, Hepatic Morphology, Antioxidant Status, Lipid Metabolism Related Gene Expression and Enzyme Activities of Domestic Pigeon Squabs (Columba livia)" Animals 10, no. 7: 1121. https://doi.org/10.3390/ani10071121
APA StyleXu, Q., Li, H., Zhou, W., Zou, X., & Dong, X. (2020). Age-Related Changes in Serum Lipid Levels, Hepatic Morphology, Antioxidant Status, Lipid Metabolism Related Gene Expression and Enzyme Activities of Domestic Pigeon Squabs (Columba livia). Animals, 10(7), 1121. https://doi.org/10.3390/ani10071121






