Intake of Spineless Cladodes of Opuntia ficus-indica During Late Pregnancy Improves Progeny Performance in Underfed Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
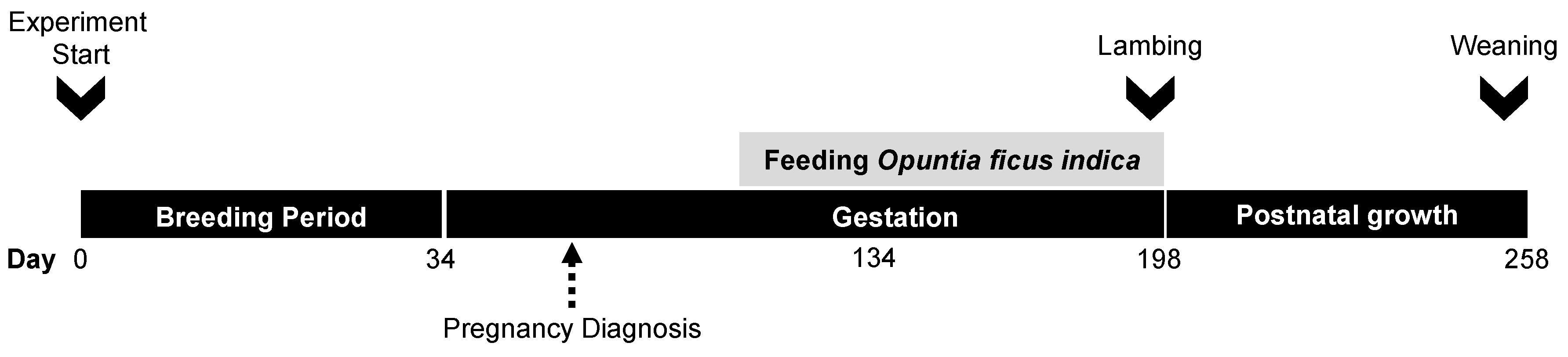
2.2. Animals and Experimental Procedure
2.3. Experimental Diets
2.4. Maternal Live Weight
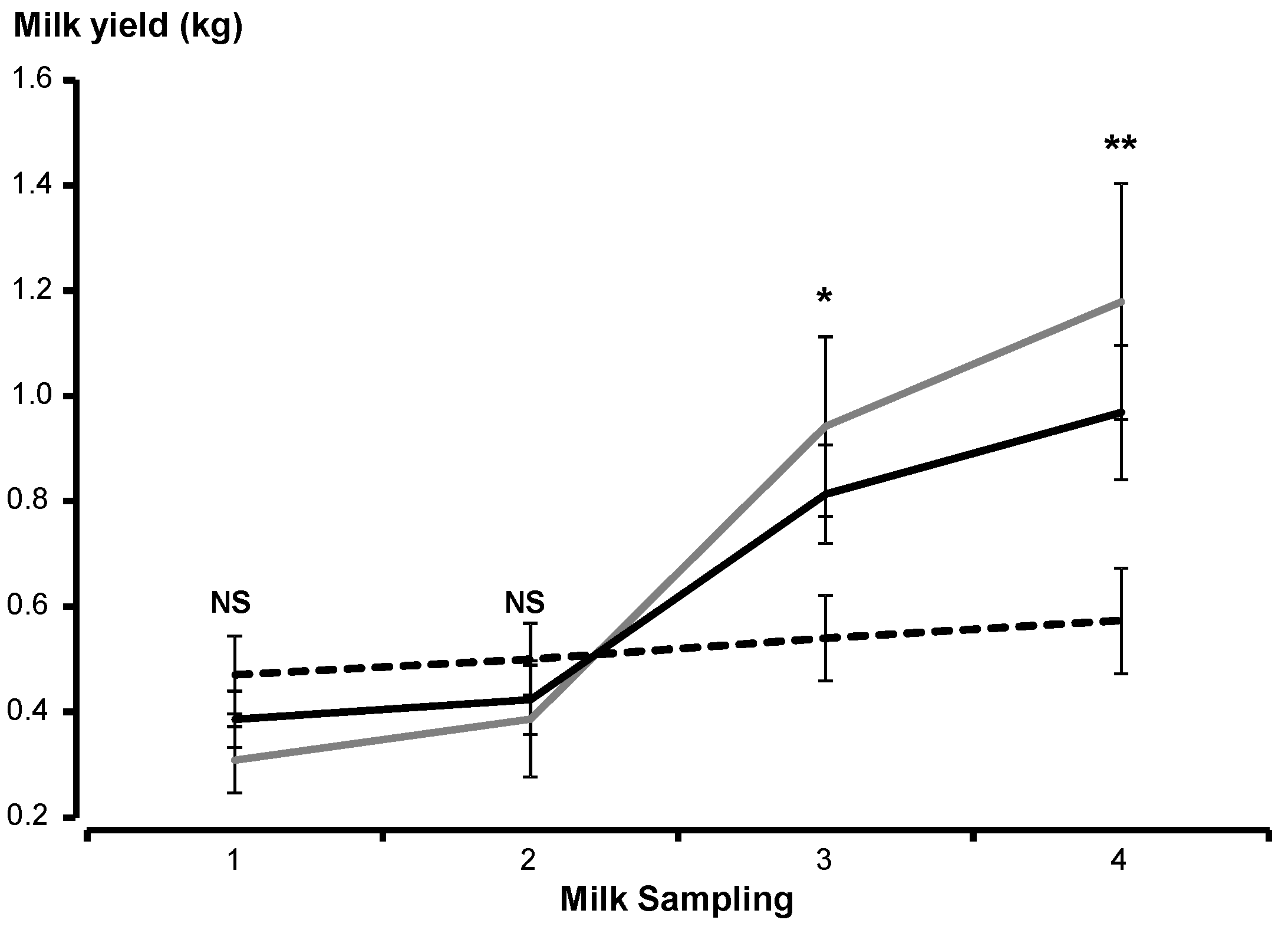
2.5. Milk Yield
2.6. Newborn Outcomes and Offspring Growth
2.7. Statistical Analysis
3. Results
3.1. Effects of Supplementation on Maternal Traits
3.2. Progeny Birth Weight and Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Repository Resources
References
- Tovar-Luna, I. Goat production in Mexico—Overview of the industry and its production practices. In Proceedings of the 24th Annual Goat Field Day, Langston, OK, USA, 25 April 2009. [Google Scholar]
- Escareño, L.; Salinas-Gonzalez, H.; Wurzinger, M.; Iñiguez, L.; Sölkner, J.; Meza-Herrera, C.A. Dairy goat production systems. Trop. Anim. Health Prod. 2012, 45, 17–34. [Google Scholar] [CrossRef]
- Cuevas-Reyes, V.; Rosales-Nieto, C.A. Characterization of the dual-purpose bovine system in northwest Mexico: Producers, resources and problematic. Rev. MVZ Córdoba 2018, 23, 6448–6460. [Google Scholar] [CrossRef]
- Ben Salem, H.; Smith, T. Feeding strategies to increase small ruminant production in dry environments. Small Rum. Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- Arellano-Rodriguez, G.; Meza-Herrera, C.A.; Rodriguez-Martinez, R.; Dionisio-Tapia, R.; Hallford, D.M.; Mellado, M.; Gonzalez-Bulnes, A. Short-term intake of β-carotene-supplemented diets enhances ovarian function and progesterone synthesis in goats. J. Anim. Physiol. Anim. Nutr. 2009, 93, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Gámez Vázquez, H.G.; Urrutia Morales, J.; Rosales Nieto, C.A.; Meza-Herrera, C.A.; Echavarría Chaires, F.G.; Beltrán López, S. Tillandsia recurvata and its chemical value as an alternative use for feeding ruminants in northern Mexico. J. Appl. Anim. Res. 2018, 46, 295–300. [Google Scholar] [CrossRef]
- Pinos-Rodríguez, J.M.; Duque-Briones, R.; Reyes-Agüero, J.A.; Aguirre-Rivera, J.R.; García-López, J.C.; González-Muñoz, S. Effect of species and age on nutrient content and in vitro digestibility of Opuntia spp. J. Appl. Anim. Res. 2006, 30, 13–17. [Google Scholar] [CrossRef][Green Version]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.A.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia Ficus Indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Pitacas, F.I.; Reis, C.M.G.; Blasco, M. Nutritional value of Opuntia ficus-indica cladodes from Portuguese ecotypes. Bulg. J. Agric. Sci. 2016, 22, 40–45. [Google Scholar]
- Russell, C.E.; Felker, P. The prickly-pears (Opuntia spp., Cactaceae): A source of human and animal food in semiarid regions. Econ. Bot. 1987, 41, 433–445. [Google Scholar] [CrossRef]
- Torres-Ponce, R.L.; Morales-Corral, D.; Ballinas-Casarrubias, M.L.; Nevárez-Moorillón, G.V. El nopal: Planta del semidesierto con aplicaciones en farmacia, alimentos y nutrición animal. Rev. Mex. Cien. Agríc. 2015, 6, 1129–1142. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological actions of Opuntia ficus indica: A review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Ben Salem, H.; Nefzaoui, A.; Ben Salem, L. Spineless cactus (Opuntia ficus indica f. inermis) and oldman saltbush (Atriplex nummularia L.) as alternative supplements for growing Barbarine lambs given straw-based diets. Small Rum. Res. 2004, 51, 65–73. [Google Scholar] [CrossRef]
- Rekik, M.; Ben Salem, H.; Lassoued, N.; Chalouati, H.; Ben Salem, I. Supplementation of Barbarine ewes with spineless cactus (Opuntia ficus-indica f. inermis) cladodes during late gestation-early suckling: Effects on mammary secretions, blood metabolites, lamb growth and postpartum ovarian activity. Small Rum. Res. 2010, 90, 53–57. [Google Scholar] [CrossRef]
- Rekik, M.; Gonzalez-Bulnes, A.; Lassoued, N.; Ben Salem, H.; Tounsi, A.; Ben Salem, I. The cactus effect: An alternative to the lupin effect for increasing ovulation rate in sheep reared in semi-arid regions? J. Anim. Physiol. Anim. Nutr. 2012, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Cano-Villegas, O.; Flores-Hernandez, A.; Veliz-Deras, F.G.; Calderon-Leyva, G.; Guillen-Muñoz, J.M.; García de la Peña, C.; Rosales-Nieto, C.A.; Macias-Cruz, U.; Avendaño-Reyes, L. Reproductive outcomes of anestrous goats supplemented with spineless Opuntia megacantha Salm-Dyck protein-enriched cladodes and exposed to the male effect. Trop. Anim. Health Prod. 2017, 49, 1511–1516. [Google Scholar] [CrossRef]
- Sadjadian, R.; Seifi, H.A.; Mohri, M.; Naserian, A.A.; Farzaneh, N. Variations of energy biochemical metabolites in periparturient dairy Saanen goats. Comp. Clin. Pathol. 2013, 22, 449–456. [Google Scholar] [CrossRef]
- Araújo, L.F.; Medeiros, A.N.; Perazzo Neto, A.; Oliveira, L.S.C.; Silva, F.L.H. Protein enrichment of cactus pear (Opuntia ficus-indica Mill) using Saccharomyces cerevisiae in solid-state fermentation. Braz. Arch. Biol. Technol. 2005, 48, 161–168. [Google Scholar] [CrossRef]
- Herrera, E.; Murillo, M.; Berumen, L.; Soto-Cruz, N.O.; Páez-Lerma, J.B. Protein enrichment of Opuntia ficus-indica using Kluyveromyces marxianus in solid-state fermentation. Cienc. Investig. Agrar. 2017, 44, 113–120. [Google Scholar] [CrossRef]
- da Conceição, MG.; de Andrade Ferreira, M.; Silva, J.L.; Ferreira Costa, C.T.; Chagas, J.C.C.; Monteiro, C.C.F. Can cactus (Opuntia stricta [Haw.] Haw) cladodes plus urea replace wheat bran in steers’ diet? Asian Australas J. Anim. Sci. 2018, 31, 1627–1634. [Google Scholar] [CrossRef]
- Ben Salem, H.; Abdouli, H.; Nefzaoui, A.; El-Mastouri, A.; Ben Salem, L. Nutritive value, behaviour, and growth of Barbarine lambs fed on oldman saltbush (Atriplex Nummularia L.) and supplemented or not with barley grains or spineless cactus (Opuntia ficus-indica f. inermis) pads. Small Rum. Res. 2005, 59, 229–237. [Google Scholar] [CrossRef]
- Misra, A.K.; Mishra, A.S.; Tripathi, M.K.; Chaturvedi, O.H.; Vaithiyanathan, S.; Prasad, R.; Jakhmola, R.C. Intake, digestion and microbial protein synthesis in sheep on hay supplemented with prickly pear cactus [Opuntia ficus-indica (L.) Mill.] with or without Groundnut Meal. Small Rum. Res. 2006, 63, 125–134. [Google Scholar] [CrossRef]
- Banchero, G.E.; Clariget, R.P.; Bencini, R.; Lindsay, D.R.; Milton, J.T.B.; Martin, G.B. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reprod. Nutr. Dev. 2006, 46, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Federation Animal Science Society. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- NAM-National Academy of Medicine. Guide for the Care and Use of Laboratory Animals. Co-Produced by the National Academy of Medicine–Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2010. [Google Scholar]
- González de Bulnes, A.; Santiago Moreno, J.; López Sebastián, A. Estimation of fetal development in manchega dairy ewes by transrectal ultrasonographic measurements. Small Rum. Res. 1998, 27, 243–250. [Google Scholar] [CrossRef]
- Nuclear Regulatory Commission. Nutrient Requirements of Small Ruminants, Sheep, Goats, Cervids, and New World Camelids, 1st ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Rosales Nieto, C.A.; Meza-Herrera, C.A.; Morón Cedillo, F.J.; Flores Najera, M.J.; Gámez Vázquez, H.G.; Cuevas Reyes, V.; Liu, S.M. Effects of vitamin E supply during late gestation and early lactation upon colostrum composition, milk production and quality in nutritional restricted ewes. Small Rum. Res. 2015, 133, 77–81. [Google Scholar] [CrossRef]
- Rosales Nieto, C.A.; Ferguson, M.B.; Macleay, C.A.; Briegel, J.R.; Wood, D.A.; Martin, G.B.; Bencini, R.; Thompson, A.N. Milk production and composition, and progeny performance in young ewes with high merit for rapid growth and muscle and fat accumulation. Animal 2018, 12, 2292–2299. [Google Scholar] [CrossRef]
- SAS Institute. SAS/Stat User’s Guide, Version 9.3; SAS Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- McNeill, D.M.; Slepetis, R.; Ehrhardt, R.A.; Smith, D.M.; Bell, A.W. Protein requirements of sheep in late pregnancy: Partitioning of nitrogen between gravid uterus and maternal tissues. J. Anim. Sci. 1997, 75, 809–816. [Google Scholar] [CrossRef]
- Rosales Nieto, C.A.; Meza-Herrera, C.A.; Moron Cedillo, F.J.; Flores Najera, M.J.; Gámez Vázquez, H.G.; Ventura Pérez, F.J.; Liu, S.M. Vitamin E supplementation of undernourished ewes pre- and post-lambing reduces weight loss of ewes and increases weight of lambs. Trop. Anim. Health Prod. 2016, 48, 613–618. [Google Scholar] [CrossRef]
- Bromley, C.M.; Snowder, G.D.; Van Vleck, L.D. Genetic parameters among weight, prolificacy, and wool traits of Columbia, Polypay, Rambouillet, and Targhee sheep. J. Anim. Sci. 2000, 78, 846–858. [Google Scholar] [CrossRef]
- Hanford, K.J.; Van Vleck, L.D.; Snowder, G.D. Estimates of genetic parameters and genetic change for reproduction, weight, and wool characteristics of Rambouillet sheep. Small Rumin. Res. 2005, 57, 175–186. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef]
- Nolan, J.V.; Stachiw, S. Fermentation and nitrogen dynamics in Merino sheep given a low-quality-roughage diet. Br. J. Nutr. 2007, 42, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Denek, N.; Yazgan, K. Effect of urea and molasses supplementation on nutrient intake and digestibility of sheep fed with straw. J. Anim. Vet. Adv. 2004, 3, 466–469. [Google Scholar]
- Caldeira, R.M.; Belo, A.T.; Santos, C.C.; Vazques, M.I.; Portugal, A.V. The effect of long-term feed restriction and over-nutrition on body condition score, blood metabolites and hormonal profiles in ewes. Small Rumin. Res. 2007, 68, 242–255. [Google Scholar] [CrossRef]
- Tygesen, M.P.; Nielsen, M.O.; Nørgaard, P.; Ranvig, H.; Harrison, A.P.; Tauson, A.-H. Late gestational nutrient restriction: Effects on ewes’ metabolic and homeorhetic adaptation, consequences for lamb birth weight and lactation performance. Arch. Anim. Nutr. 2008, 62, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.A.; Williams, A.H.; Campbell, I.P.; Witcombe, G.F.; Roberts, A.M. Low nutrition of ewes in early pregnancy and the residual effect on the offspring. J. Agric. Sci. 1986, 106, 81–87. [Google Scholar] [CrossRef]
- Vincent, I.C.; Williams, H.L.; Hill, R. The influence of a low-nutrient intake after mating on gestation and perinatal survival of lambs. Br. J. Nutr. 1985, 141, 611–617. [Google Scholar] [CrossRef]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The short-chain fatty acid propionate increases glucagon and fabp4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019, 11, eaav0120. [Google Scholar] [CrossRef]
- Gaccioli, F.; Lager, S.; Powell, T.L.; Jansson, T. Placental transport in response to altered maternal nutrition. J. Dev. Orig. Health Dis. 2012, 4, 101–115. [Google Scholar] [CrossRef]
- Louacini, B.K.; Dellal, A.; Halbouche, M.; Ghazi, K. Effect of incorporation of the spineless Opuntia Ficus Indica in diets on biochemical parameters and its impact on the average weight of ewes during the maintenance. Glob. Vet. 2012, 8, 352–359. [Google Scholar]
- Gardner, D.S.; Buttery, P.J.; Daniel, Z.; Symonds, M.E. Factors affecting birth weight in sheep: Maternal environment. Reproduction 2007, 133, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Clifton, V.L. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 2010, 31, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Rosales Nieto, C.A.; Mantey, A.; Makela, B.; Byrem, T.; Ehrhardt, R.; Veiga-Lopez, A. Shearing during late pregnancy increases size at birth but does not alter placental endocrine responses in sheep. Animal 2020, 14, 799–806. [Google Scholar] [CrossRef]
- Oldham, C.M.; Thompson, A.N.; Ferguson, M.B.; Gordon, D.J.; Kearney, G.A.; Paganoni, B.L. The birthweight and survival of merino lambs can be predicted from the profile of liveweight change of their mothers during pregnancy. Anim. Prod. Sci. 2011, 51, 776–783. [Google Scholar] [CrossRef]
- Paganoni, B.L.; Ferguson, M.B.; Kearney, G.A.; Thompson, A.N. Increasing weight gain during pregnancy results in similar increases in lamb birthweights and weaning weights in merino and non-merino ewes regardless of sire type. Anim. Prod. Sci. 2014, 54, 727–735. [Google Scholar] [CrossRef]
- Lane, M.A.; Baldwin, R.L.; Jesse, B.W. Sheep rumen metabolic development in response to age and dietary treatments. J. Anim. Sci. 2000, 78, 1990–1996. [Google Scholar] [CrossRef]
- Ochoa Cordero, M.; Meza Herrera, C.A.; Vázquez García, J.M.; Stewart, C.A.; Rosales Nieto, C.A.; Ochoa Alfaro, A.E.; Purvis, I.A.; Cuevas Reyes, V.; Lee, H.; Martin, G.B. Pregnancy and litter size, but not lamb sex, affect feed intake and wool production by Merino-type ewes. Animals 2019, 9, 214. [Google Scholar] [CrossRef]
- Bencini, R.; Pulina, G. The quality of sheep milk: A review. Aust. J. Exp. Agric. 1997, 37, 485–504. [Google Scholar] [CrossRef]
- Pulina, G.; Macciotta, N.; Nudda, A. Milk composition and feeding in the Italian dairy sheep. Ital. J. Anim. Sci. 2004, 4, 5–14. [Google Scholar] [CrossRef]
- Costa, R.G.; Beltrão Filho, E.M.; Nunes de Medeiros, A.; Naves Givisiez, P.E.; Ramos do Egypto Queiroga, R.C.; Silva Melo, A.A. Effects of increasing levels of cactus pear (Opuntia ficus-indica L. Miller) in the diet of dairy goats and its contribution as a source of water. Small Rumin. Res. 2009, 82, 62–65. [Google Scholar] [CrossRef]
- Mahouachi, M.; Atti, N.; Hajji, H. Use of Spineless Cactus (Opuntia ficus indica F. Inermis) for dairy goats and growing kids: Impacts on milk production, kid’s growth, and meat quality. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.G.; Beltrão Filho, E.M.; Ramos do Egypto Queiroga, R.C.; Suely Madruga, M.; Nunes de Medeiros, A.; Bruno de Oliveira, C.J. Chemical composition of milk from goats fed with cactus pear (Opuntia ficus-indica L. Miller) in substitution to corn meal. Small Rumin. Res. 2010, 94, 214–217. [Google Scholar] [CrossRef]
- Fleming, T.P.; Lucasm, E.S.; Watkins, A.J.; Eckert, J.J. Adaptive responses of the embryo to maternal diet and consequences for post-implantation development. Reprod. Fertil. Dev. 2011, 24, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M.; Fletcher, J.M.; Stickland, N.C. Muscle cellularity and postnatal growth in the pig. J. Anim. Sci. 1993, 71, 3339–3343. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Fiedler, I.; Dietl, G.; Ender, K. Myogenesis and postnatal skeletal muscle cell growth as influenced by selection. Livest. Prod. Sci. 2000, 66, 177–188. [Google Scholar] [CrossRef]
- Gnanalingham, M.G.; Mostyn, A.; Symonds, M.E.; Stephenson, T. Ontogeny and nutritional programming of adiposity in sheep: Potential role of glucocorticoid action and uncoupling protein-2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1407–R1415. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Duffield, J.A.; McMillen, I.C. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology. 2007, 148, 878–885. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Hunt, A.S.; Hermanson, J.W.; Bell, A.W. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J. Anim. Sci. 2000, 78, 50–61. [Google Scholar] [CrossRef]
- Ford, S.P.; Hess, B.W.; Schwope, M.M.; Nijland, M.J.; Gilbert, J.S.; Vonnahme, K.A.; Means, W.J.; Han, H.; Nathanielsz, P.W. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 2007, 85, 1285–1294. [Google Scholar] [CrossRef]
- Taylor, P.D.; Poston, L. Developmental programming of obesity in mammals. Exp. Physiol. 2007, 92, 287–298. [Google Scholar] [CrossRef]
- Thompson, A.N.; Ferguson, M.B.; Campbell, A.J.D.; Gordon, D.J.; Kearney, G.A.; Oldham, C.M.; Paganoni, B.L. Improving the nutrition of Merino ewes during pregnancy and lactation increases weaning weight and survival of progeny but does not affect their mature size. Anim. Prod. Sci. 2011, 51, 784–793. [Google Scholar] [CrossRef]
- Gatford, K.L.; Fletcher, T.P.; Clarke, I.J.; Owens, P.C.; Quinn, K.J.; Walton, P.E.; Grant, P.A.; Hosking, B.J.; Egan, A.R.; Ponnampalam, E.N. Sexual dimorphism of circulating somatotropin, insulin-like growth factor I and II, insulin-like growth factor binding proteins, and insulin: Relationships to growth rate and carcass characteristics in growing lambs. J. Anim. Sci. 1996, 74, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Quinn, K.J.; Walton, P.E.; Grant, P.A.; Hosking, B.J.; Egan, A.R.; Owens, P.C. Ontogenic and nutritional changes in circulating insulin-like growth factor (IGF)-I, IGF-II and IGF-binding proteins in growing ewe and ram lambs. J. Endocrinol. 1997, 155, 47–54. [Google Scholar] [CrossRef] [PubMed]


| Treatment | DM (%) | CP (%) | EE (%) | CARB (%) | NDF (%) | ADF (%) | ME (Mcal/kg) |
|---|---|---|---|---|---|---|---|
| E-Opuntia | 26.4 | 7.3 | 1.5 | 41.6 | 35.8 | 17.9 | 2.1 |
| Opuntia | 26.7 | 4.0 | 2.9 | 47.1 | 32.0 | 21.5 | 2.2 |
| Control (alfalfa) | 93.5 | 13.9 | 1.0 | 19.0 | 53.0 | 39.0 | 1.5 |
| NRC requirement | 10.9 | 3.4 |
| Variable/Sex type | Female | Male | SEM 1 | p Value |
| n | 33 | 21 | ||
| Birth weight (kg) | 3.7 | 3.8 | 0.26 | 0.2 |
| Live weight gain (g day−1) | 96 | 130 | 21 | 0.01 |
| Weaning weight (kg) | 9.5 | 11.5 | 1.3 | 0.03 |
| Variable/Birth type | Singleton | Twin | SEM1 | p Value |
| n | 31 | 22 | ||
| Birth weight (kg) | 3.9 | 3.4 | 0.27 | 0.002 |
| Live weight gain (g day−1) | 125 | 98 | 21 | 0.09 |
| Weaning weight (kg) | 11.2 | 9.6 | 1.3 | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas Reyes, V.; Santiago Hernandez, F.; Flores Najera, M.d.J.; Vazquez Garcia, J.M.; Urrutia Morales, J.; Hosseini-Ghaffari, M.; Chay-Canul, A.; Meza-Herrera, C.A.; Gonzalez-Bulnes, A.; Martin, G.B.; et al. Intake of Spineless Cladodes of Opuntia ficus-indica During Late Pregnancy Improves Progeny Performance in Underfed Sheep. Animals 2020, 10, 995. https://doi.org/10.3390/ani10060995
Cuevas Reyes V, Santiago Hernandez F, Flores Najera MdJ, Vazquez Garcia JM, Urrutia Morales J, Hosseini-Ghaffari M, Chay-Canul A, Meza-Herrera CA, Gonzalez-Bulnes A, Martin GB, et al. Intake of Spineless Cladodes of Opuntia ficus-indica During Late Pregnancy Improves Progeny Performance in Underfed Sheep. Animals. 2020; 10(6):995. https://doi.org/10.3390/ani10060995
Chicago/Turabian StyleCuevas Reyes, Venancio, Francisco Santiago Hernandez, Manuel de Jesus Flores Najera, Juan Manuel Vazquez Garcia, Jorge Urrutia Morales, Morteza Hosseini-Ghaffari, Alfonso Chay-Canul, César A. Meza-Herrera, Antonio Gonzalez-Bulnes, Graeme B. Martin, and et al. 2020. "Intake of Spineless Cladodes of Opuntia ficus-indica During Late Pregnancy Improves Progeny Performance in Underfed Sheep" Animals 10, no. 6: 995. https://doi.org/10.3390/ani10060995
APA StyleCuevas Reyes, V., Santiago Hernandez, F., Flores Najera, M. d. J., Vazquez Garcia, J. M., Urrutia Morales, J., Hosseini-Ghaffari, M., Chay-Canul, A., Meza-Herrera, C. A., Gonzalez-Bulnes, A., Martin, G. B., & Rosales Nieto, C. A. (2020). Intake of Spineless Cladodes of Opuntia ficus-indica During Late Pregnancy Improves Progeny Performance in Underfed Sheep. Animals, 10(6), 995. https://doi.org/10.3390/ani10060995







