Predicting Disease in Transition Dairy Cattle Based on Behaviors Measured Before Calving
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Health Assessment
2.2. Behavioral Data Collection
2.3. Statistical Analysis
2.3.1. Data Preparation
2.3.2. Descriptive Analysis
2.3.3. Model Development
3. Results
3.1. Descriptive Statistics
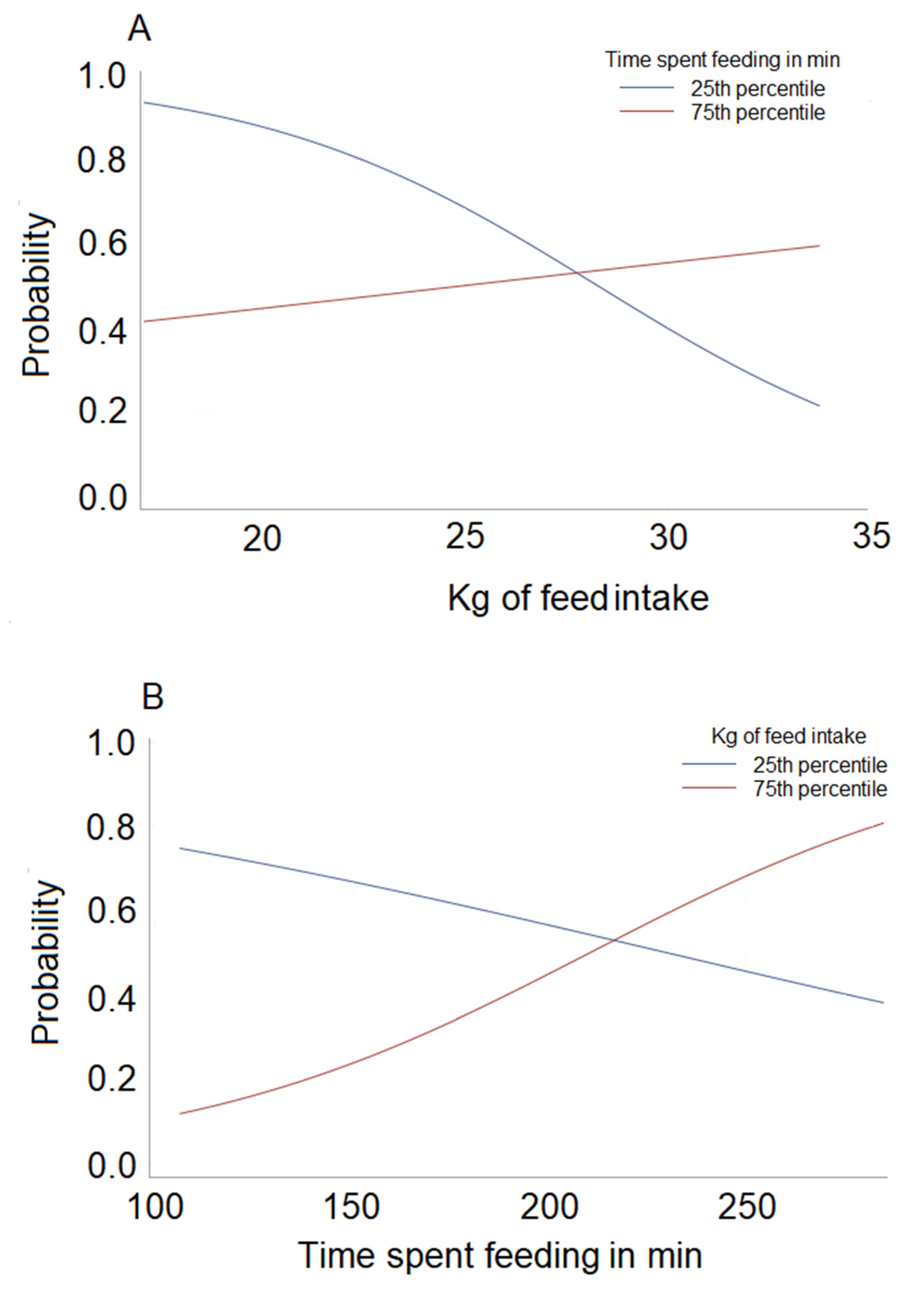
3.2. Model Results
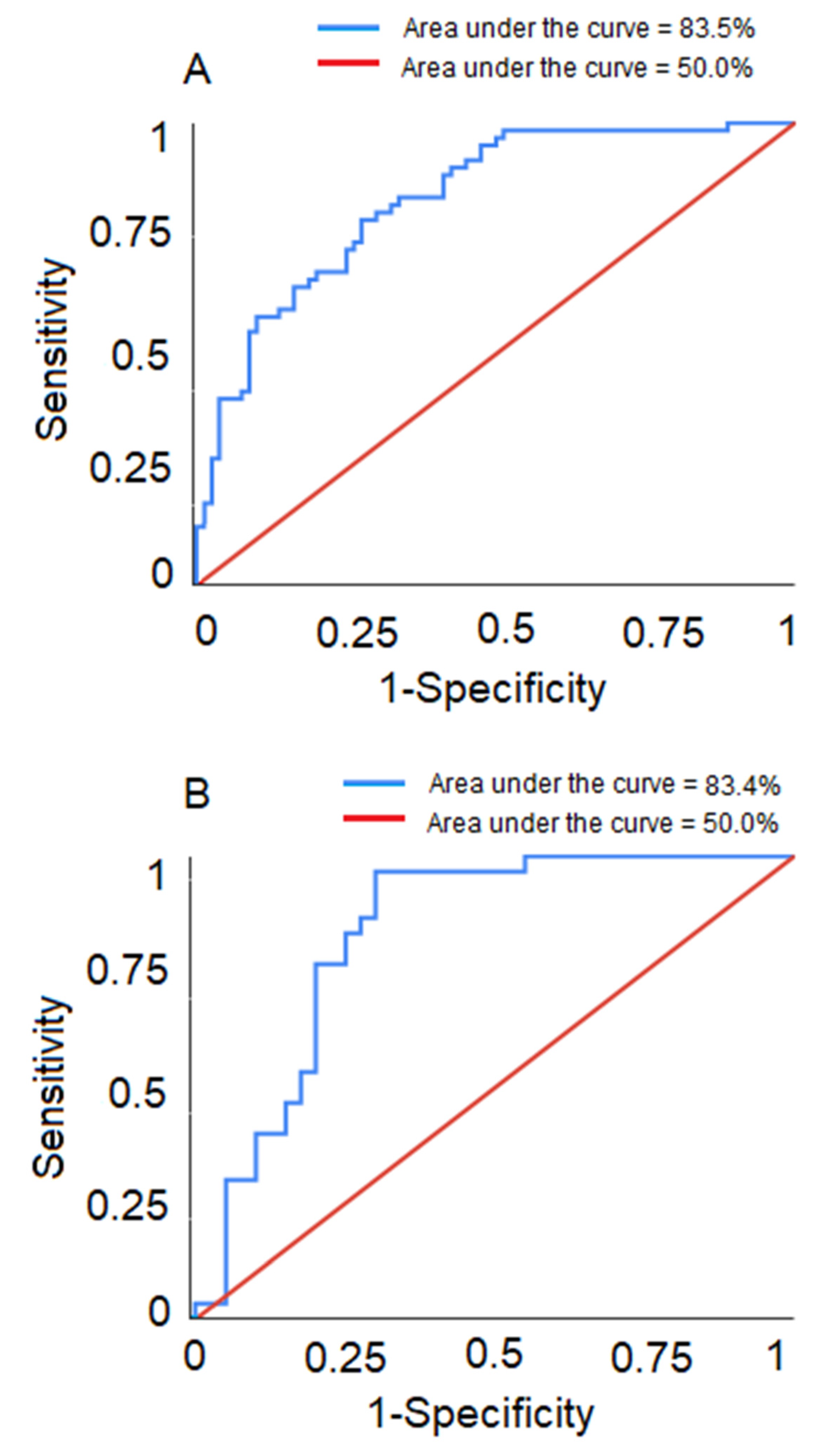
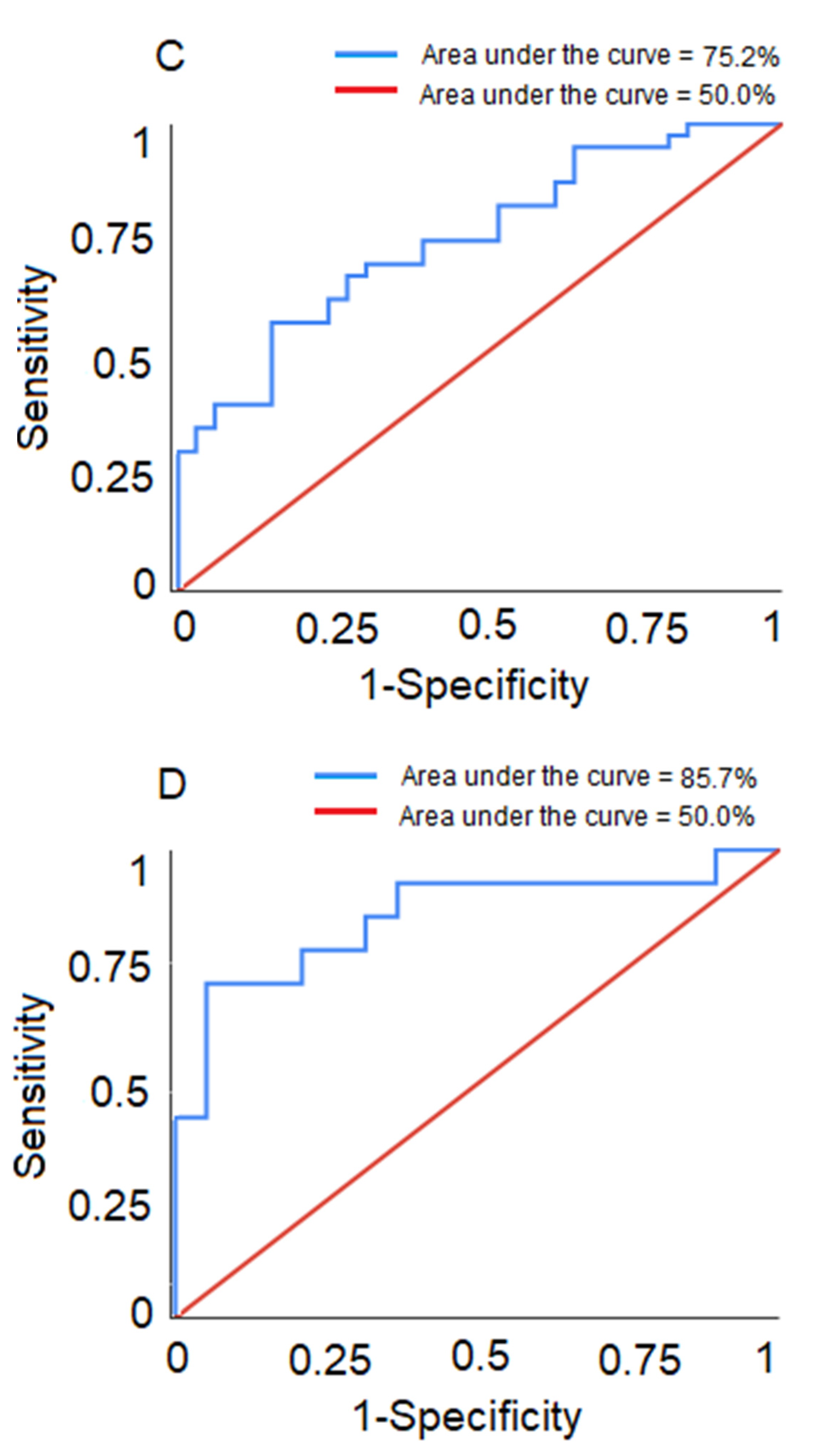
Predictive Ability of the Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Develop. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.C.; Vertenten, G. A field study to determine the prevalence, dairy herd management systems, and fresh cow clinical conditions associated with ketosis in western European dairy herds. J. Dairy Sci. 2014, 97, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; McArt, J.; Abuelo, A. Production-related metabolic disorders of cattle: Ketosis, milk fever and grass staggers. In Practice 2019, 41, 205–219. [Google Scholar] [CrossRef]
- Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. N AM-Food A 2000, 16, 231–253. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef]
- Vanholder, T.; Papen, J.; Bemers, R.; Vertenten, G.; Berge, A.C.B. Risk factors for subclinical and clinical ketosis and association with production parameters in dairy cows in the Netherlands. J. Dairy Sci. 2015, 98, 880–888. [Google Scholar] [CrossRef]
- Genís, S.; Cerri, R.L.; Bach, À.; Silper, B.F.; Baylao, M.; Denis-Robichaud, J.; Arís, A. Pre-calving intravaginal administration of lactic acid bacteria reduces metritis prevalence and regulates blood neutrophil gene expression after calving in dairy cattle. Frontiers Vet. Sci. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Thibier, M. Reproductive disorders in dairy cattle: I. Respective influence of herds, seasons, milk yield and parity. Theriogenology 1984, 21, 569–581. [Google Scholar] [CrossRef]
- Zwald, N.R.; Weigel, K.A.; Chang, Y.M.; Welper, R.D.; Clay, J.S. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates, and sire breeding values. J. Dairy Sci. 2004, 87, 4287–4294. [Google Scholar] [CrossRef]
- Bruun, J.; Ersbøll, A.K.; Alban, L. Risk factors for metritis in Danish dairy cows. Prev. Vet. Med. 2002, 54, 179–190. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk factors for postpartum uterine diseases in dairy cows. J. Dairy Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Saei, H.D. Staphylococcal species associated with bovine mastitis in the North West of Iran: Emerging of coagulase-negative staphylococci. Int. J. Vet. Sci. Med. 2014, 2, 27–34. [Google Scholar] [CrossRef]
- Olde Riekerink, R.; Barkema, H.W.; Kelton, D.F.; Scholl, D.T. Incidence rate of clinical mastitis on Canadian dairy farms. J. Dairy Sci. 2008, 91, 1366–1377. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Repro. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Quart. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; Gibbons, J.; Wagner, S.; de Passillé, A.M.; Rushen, J. Behavioral changes in dairy cows with mastitis. J. Dairy Sci. 2012, 95, 6994–7002. [Google Scholar] [CrossRef]
- Stojkov, J.; von Keyserlingk, M.A.G.; Marchant-Forde, J.N.; Weary, D.M. Assessment of visceral pain associated with metritis in dairy cows. J. Dairy Sci. 2015, 98, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Huzzey, J.M.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J. Dairy Sci. 2007, 90, 3220–3233. [Google Scholar] [CrossRef] [PubMed]
- Goldhawk, C.; Chapinal, N.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Prepartum feeding behavior is an early indicator of subclinical ketosis. J. Dairy Sci. 2009, 92, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Varas, P.; Proudfoot, K.L.; Weary, D.M.; von Keyserlingk, M.A.G. Changes in behaviour of dairy cows with clinical mastitis. Appl. Anim. Behav. Sci. 2016, 175, 8–13. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Dewhurst, R.J.; Friggens, N.C. On the relationship between lactational performance and health: Is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Lives. Prod. Sci. 2003, 83, 277–308. [Google Scholar] [CrossRef]
- Luchterhand, K.M.; Silva, P.R.B.; Chebel, R.C.; Endres, M.I. Association between prepartum feeding behavior and periparturient health disorders in dairy cows. Front. Vet. Sci. 2016, 3, 65. [Google Scholar] [CrossRef]
- Patbandha, T.K.; Mohanty, T.K.; Layek, S.S.; Kumaresan, A.; Behera, K. Application of pre-partum feeding and social behaviour in predicting risk of developing metritis in crossbred cows. Appl. Anim. Behav. Sci. 2012, 139, 10–17. [Google Scholar] [CrossRef]
- Sepúlveda-Varas, P.; Weary, D.M.; von Keyserlingk, M.A.G. Lying behavior and postpartum health status in grazing dairy cows. J. Dairy Sci. 2014, 97, 6334–6343. [Google Scholar] [CrossRef]
- Lomb, J.; Neave, H.W.; Weary, D.M.; LeBlanc, S.J.; Huzzey, J.M.; von Keyserlingk, M.A.G. Changes in feeding, social, and lying behaviors in dairy cows with metritis following treatment with a nonsteroidal anti-inflammatory drug as adjunctive treatment to an antimicrobial. J. Dairy Sci. 2018a, 101, 4400–4411. [Google Scholar] [CrossRef]
- Lomb, J.; Weary, D.M.; Mills, K.E.; von Keyserlingk, M.A.G. Effects of metritis on stall use and social behavior at the lying stall. J. Dairy Sci. 2018b, 101, 7471–7479. [Google Scholar] [CrossRef]
- Neave, H.W.; Lomb, J.; Weary, D.M.; LeBlanc, S.J.; Huzzey, J.M.; von Keyserlingk, M.A.G. Behavioral changes before metritis diagnosis in dairy cows. J. Dairy Sci. 2018, 101, 4388–4399. [Google Scholar] [CrossRef] [PubMed]
- Neave, H.W.; Lomb, J.; von Keyserlingk, M.A.G.; Behnam-Shabahang, A.; Weary, D.M. Parity differences in the behavior of transition dairy cows. J. Dairy Sci. 2017, 100, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Flower, F.C.; Weary, D.M. Effect of hoof pathologies on subjective assessments of dairy cow gait. J. Dairy Sci. 2006, 89, 139–146. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th rev. ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Proudfoot, K.L.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Competition at the feed bunk changes the feeding, standing, and social behavior of transition dairy cows. J. Dairy Sci. 2009, 92, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Huzzey, J.M.; Weary, D.M.; Tiau, B.Y.F.; von Keyserlingk, M.A.G. Automatic detection of social competition using an electronic feeding system. J. Dairy Sci. 2014, 97, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.V.; von Keyserlingk, M.A.G.; Weary, D.M. Hot weather increases competition between dairy cows at the drinker. J. Dairy Sci. 2020, 103, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Liong, C.Y.; Jemain, A.A. Validity of the best practice in splitting data for hold-out validation strategy as performed on the ink strokes in the context of forensic science. Microchem. J. 2018, 139, 125–133. [Google Scholar] [CrossRef]
- Sahar, M.W.; Beaver, A.; Weary, D.M.; von Keyserlingk, M.A.G. Feeding behavior and agonistic interactions at the feed bunk are associated with hyperketonemia and metritis diagnosis in dairy cattle. J. Dairy Sci. 2020, 103, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Hammon, D.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunop. 2006, 113, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Veenhuizen, J.J.; Drackley, J.K.; Richard, M.J.; Sanderson, T.P.; Miller, L.D.; Young, J.W. Metabolic changes in blood and liver during development and early treatment of experimental fatty liver and ketosis in cows1. J. Dairy Sci. 1991, 74, 4238–4253. [Google Scholar] [CrossRef]
- Siivonen, J.; Taponen, S.; Hovinen, M.; Pastell, M.; Lensink, B.J.; Pyörälä, S.; Hänninen, L. Impact of acute clinical mastitis on cow behaviour. Appl. Anim. Behav. Sci. 2011, 132, 101–106. [Google Scholar] [CrossRef]
- González, L.A.; Tolkamp, B.J.; Coffey, M.P.; Ferret, A.; Kyriazakis, I. Changes in feeding behavior as possible indicators for the automatic monitoring of health disorders in dairy cows. J. Dairy Sci. 2008, 91, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Urton, G.; von Keyserlingk, M.A.G.; Weary, D.M. Feeding behavior identifies dairy cows at risk for metritis. J. Dairy Sci. 2005, 88, 2843–2849. [Google Scholar] [CrossRef]
- Bouissou, M.F. Influence of body weight and presence of horns on social rank in domestic cattle. Anim. Behav. 1972, 20, 474–477. [Google Scholar] [CrossRef]
- Barber, S.R.; Davies, M.J.; Khunti, K.; Gray, L.J. Risk assessment tools for detecting those with pre-diabetes: A systematic review. Diabetes Res. Clin. Pr. 2014, 105, 1–13. [Google Scholar] [CrossRef]
- Jie, M.A.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar]
- Grimm, K.; Haidn, B.; Erhard, M.; Tremblay, M.; Döpfer, D. New insights into the association between lameness, behavior, and performance in Simmental cows. J. Dairy Sci. 2019, 102, 2453–2468. [Google Scholar] [CrossRef]
- Machado, V.S.; Caixeta, L.S.; Bicalho, R.C. Use of data collected at cessation of lactation to predict incidence of sole ulcers and white line disease during the subsequent lactation in dairy cows. Am. J. Vet. Res. 2011, 72, 1338–1343. [Google Scholar] [CrossRef]
- Leslie, K.E.; Petersson-Wolfe, C.S. Assessment and management of pain in dairy cows with clinical mastitis. Vet. Clin. Food A 2012, 28, 289–305. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Overton, M.W. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J. Dairy Sci. 2015, 98, 2043–2054. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T.J.G.M. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
| Variable Name | Healthy | Sick | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Multiparous cows | ||||
| DMI (kg) | 33.0 | 4.26 | 30.0 | 5.47 |
| BW (kg) | 794.2 | 74.34 | 822.3 | 73.57 |
| Actor Behaviors during prepartum period | 15.9 | 7.03 | 17.0 | 6.26 |
| No. of different bins visited per day | 11.0 | 0.70 | 10.9 | 0.80 |
| No. of bins visited per meal | 4.3 | 1.08 | 4.4 | 1.02 |
| Primiparous cows | ||||
| DMI (kg) | 28.8 | 2.75 | 27.2 | 3.54 |
| Time spent feeding (min) | 210.6 | 25.45 | 201.5 | 31.97 |
| No. of bins visited per meal | 4.8 | 0.87 | 5.2 | 0.76 |
| Variable Name | Slope | SE | P-Value | OR (95% CI) |
|---|---|---|---|---|
| Multiparous cows | ||||
| Kg of feed intake | −0.143 | 0.066 | 0.04 | 1 |
| BW prepartum | 0.011 | 0.003 | <0.01 | 1.31 (1.10–1.55) |
| Actor behavior | 0.151 | 0.043 | <0.01 | 2.47 (1.48–4.13) |
| Different bins visited per day | −3.354 | 0.930 | <0.01 | 1 |
| Bins visited per meal | 0.614 | 0.273 | 0.03 | 1 |
| Kg of feed intake * Different bins visited per day | 0.279 | 0.092 | <0.01 | 0.72 (0.61–0.85) 2 |
| Bins visited per meal * Different bins visited per day | −2.020 | 0.543 | <0.01 | 7.83(2.74–22.39) 3 |
| Primiparous cows | ||||
| Time spent feeding in min | −0.211 | 0.098 | 0.03 | 1 |
| Kg of feed intake | −1.641 | 0.738 | 0.03 | 1 |
| Bins visited per meal | 0.861 | 0.379 | 0.02 | 2.37 (1.13–4.97) |
| Time spent feeding in min * Kg of feed intake | 0.008 | 0.004 | 0.04 | 0.77 (0.60–0.99) 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahar, M.W.; Beaver, A.; von Keyserlingk, M.A.G.; Weary, D.M. Predicting Disease in Transition Dairy Cattle Based on Behaviors Measured Before Calving. Animals 2020, 10, 928. https://doi.org/10.3390/ani10060928
Sahar MW, Beaver A, von Keyserlingk MAG, Weary DM. Predicting Disease in Transition Dairy Cattle Based on Behaviors Measured Before Calving. Animals. 2020; 10(6):928. https://doi.org/10.3390/ani10060928
Chicago/Turabian StyleSahar, Mohammad W., Annabelle Beaver, Marina A. G. von Keyserlingk, and Daniel M. Weary. 2020. "Predicting Disease in Transition Dairy Cattle Based on Behaviors Measured Before Calving" Animals 10, no. 6: 928. https://doi.org/10.3390/ani10060928
APA StyleSahar, M. W., Beaver, A., von Keyserlingk, M. A. G., & Weary, D. M. (2020). Predicting Disease in Transition Dairy Cattle Based on Behaviors Measured Before Calving. Animals, 10(6), 928. https://doi.org/10.3390/ani10060928





