Comparisons of Activity Budgets, Interactions, and Social Structures in Captive and Wild Chimpanzees (Pan troglodytes)
Simple Summary
Abstract
1. Introduction
1.1. Environmental Enrichment for Captive Chimpanzees
1.2. Purpose of Research and Predictions
2. Methods and Materials
2.1. Subjects and Observational Methods
2.2. Variables and Data Analysis
2.3. Statistical Analyses
2.4. Ethical Approval
3. Results
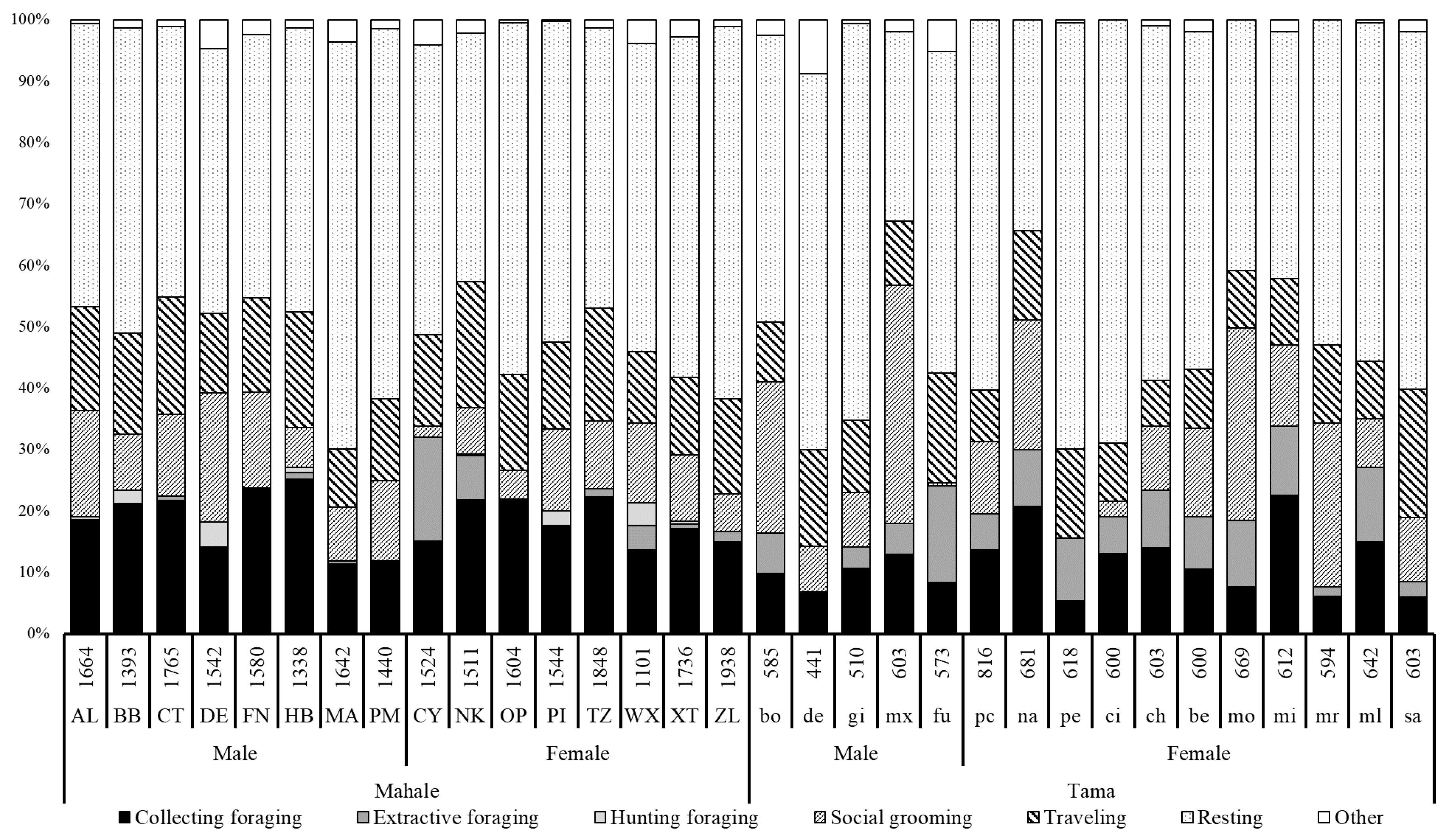
3.1. Activity Budget
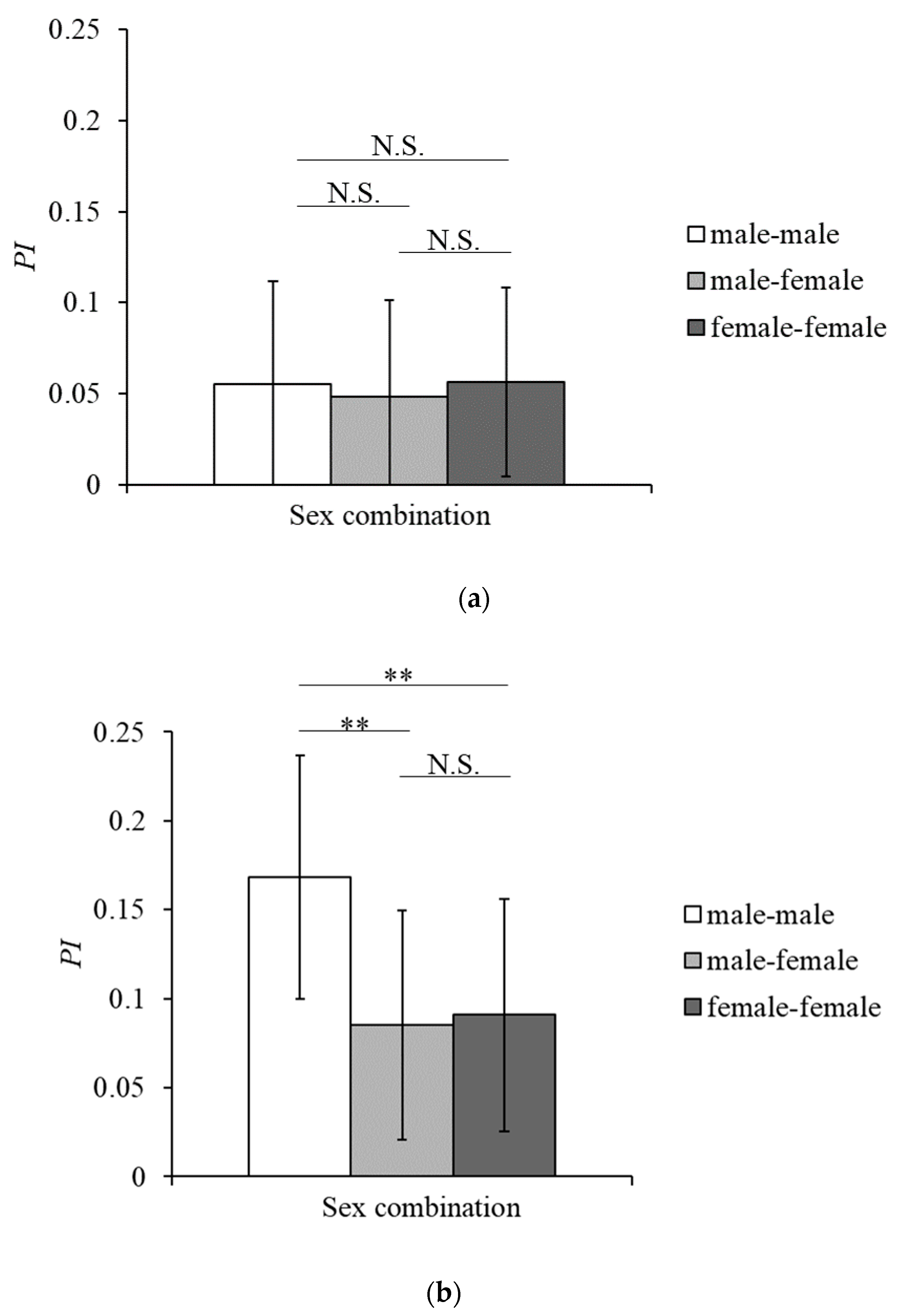
3.2. Social Grooming and Mutual Grooming
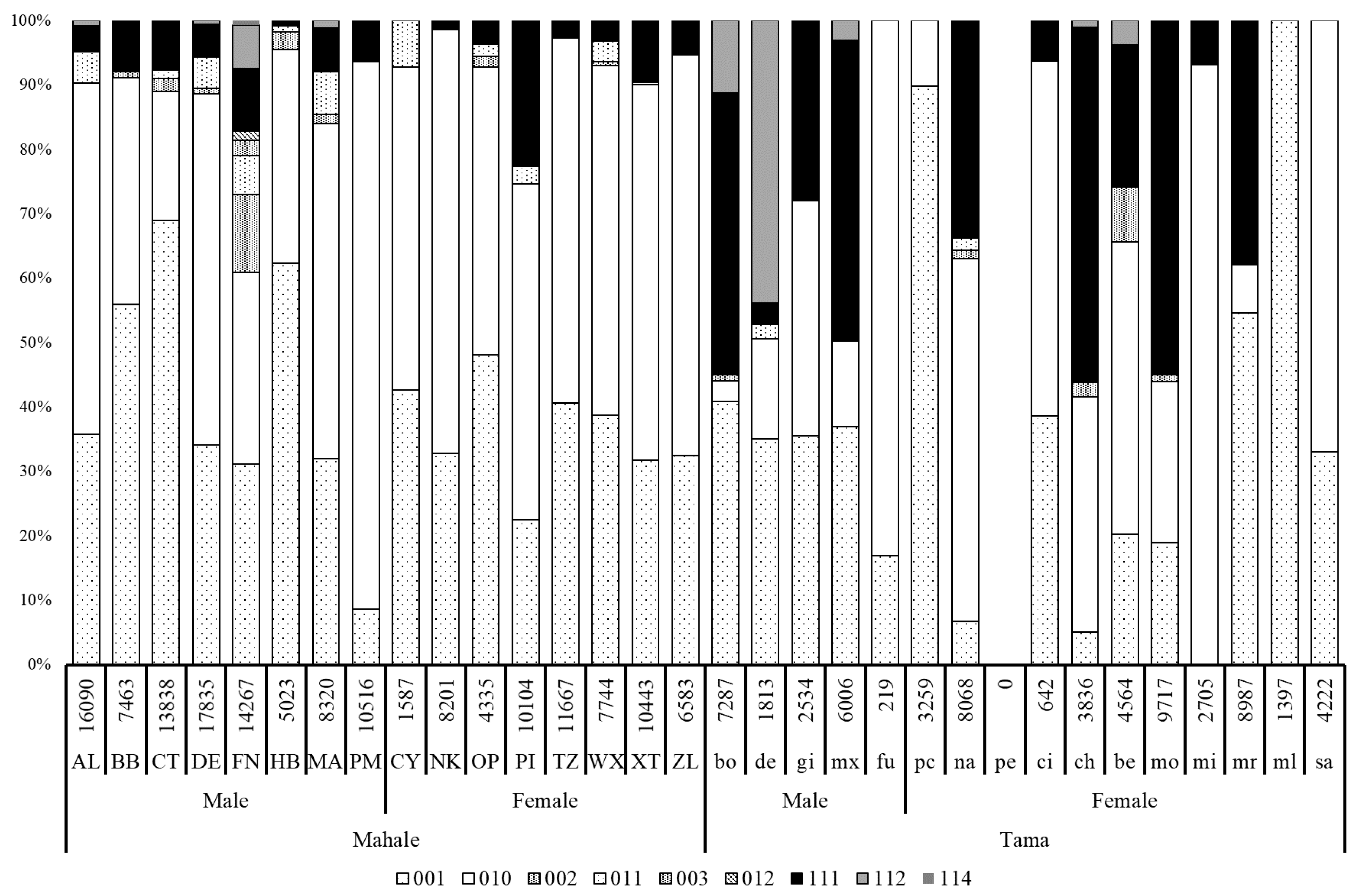
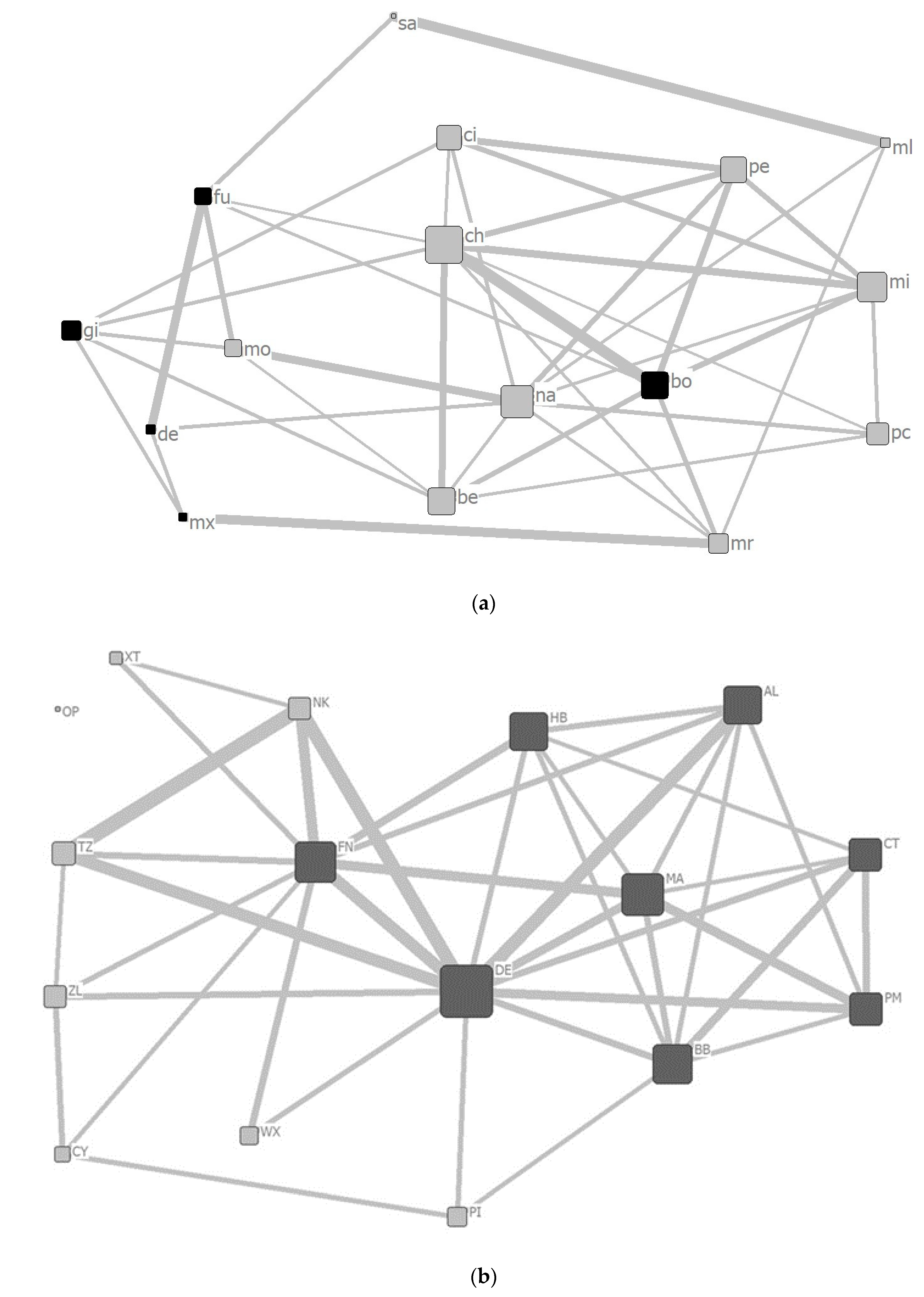
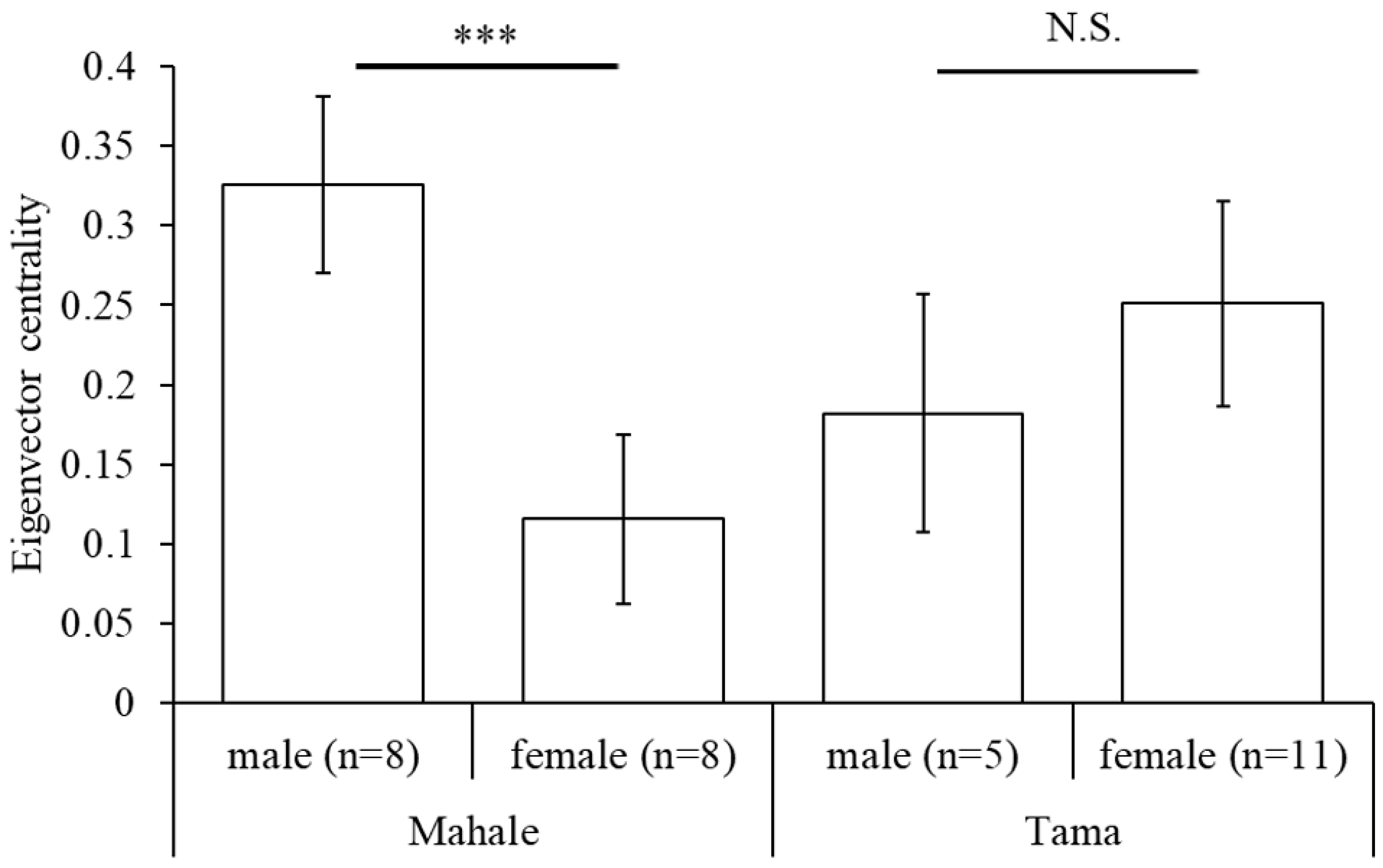
3.3. Social Networks and Network Indices
4. Discussion
4.1. Activity Budgets
4.2. Features of Grooming Interactions in Tama and Mahale
4.3. Social Characteristics of Captive Chimpanzees
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloomsmith, M.A.; Brent, L.Y.; Schapiro, S.J. Guidelines for developing and managing an environmental enrichment program for nonhuman primates. Lab. Anim. Sci. 1991, 41, 372–377. [Google Scholar]
- Barber, J.C. Programmatic approaches to assessing and improving animal welfare in zoos and aquariums. Zoo Biol. 2009, 28, 519–530. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M. Environmental enrichment for primates in laboratories. Adv. Sci. Res. 2010, 5, 41–56. [Google Scholar] [CrossRef]
- Keeling, M.E.; Alford, P.L.; Bloomsmith, M.A. Decision analysis for developing programs of psychological well-being: A bias-for-action approach. In Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates; Novak, M.A., Petto, A.J., Eds.; American Psychological Association: Washington, DC, USA, 1991; pp. 57–65. [Google Scholar] [CrossRef]
- Morimura, N. Note on effects of a daylong feeding enrichment program for Chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 2007, 106, 178–183. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Matsunaga, M.; Shimada, K.; Kado, R.; Tanaka, M. Introducing tool-based feeders to zoo-housed chimpanzees as a cognitive challenge: Spontaneous acquisition of new types of tool use and effects on behaviours and use of space. J. Zoo Aquar. Res. 2016, 4, 147–155. [Google Scholar] [CrossRef]
- Ochiai-Ohira, T.; Matsuzawa, T. Introduction of two wooden climbing frames as environmental enrichment for captive chimpanzees (Pan troglodytes) and its assessment. Jpn. J. Anim. Psychol. 2001, 51, 1–9. [Google Scholar] [CrossRef]
- Pruetz, J.D.; McGrew, W.C. What does a chimpanzee need? Using natural behavior to guide the care and management of captive populations. In The Care and Management of Captive Chimpanzees; Brent, L.E., Ed.; American Society of Primatologists: San Antonio, TX, USA, 2001; pp. 17–37. [Google Scholar]
- Gilloux, I.; Gurnell, J.; Shepherdson, D. An enrichment device for great apes. Anim. Welf. 1992, 1, 279–289. [Google Scholar]
- Shepherdson, J.D. Tracing the path of environmental enrichment in zoos. In Second Nature: Environmental Enrichment for Captive Animals Shepherdson; Mellen, J.D., Hutchins, M., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 1–12. [Google Scholar]
- Kaplan, H.; Hill, K.; Lancaster, J.; Hurtado, A.M. A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. 2000, 9, 156–185. [Google Scholar] [CrossRef]
- Matsuzawa, T. Field experiments on use of stone tools by chimpanzees in the wild. In Chimpanzee Cultures; Wrangham, R.W., McGrew, W.C., de Waal, F.B.M., Heltne, P.G., Eds.; Harvard University Press: Cambridge, MA, USA, 1994; pp. 351–370. [Google Scholar]
- Nishida, T.; Hiraiwa, M. Natural history of a tool-using behavior by wild chimpanzees in feeding upon wood-boring ants. J. Hum. Evol. 1982, 11, 73–99. [Google Scholar] [CrossRef]
- Nishida, T.; Hasegawa, T.; Hayaki, H.; Takahata, Y.; Uehara, S. Meat-sharing as a coalition strategy by an alpha male chimpanzee. Top. Primatol. 1992, 1, 159–174. [Google Scholar]
- Newton-Fisher, N.E. The Hunting Behavior and Carnivory of Wild Chimpanzees; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1295–1320. [Google Scholar]
- Tama Zoological Park. Chimpanzees Teach You What’s Important as a Human; Tokuma Shoten Publishing Co.: Tokyo, Japan, 2011. (In Japanese) [Google Scholar]
- Nishida, T. The ant-gathering behaviour by the use of tools among wild chimpanzees of the Mahali Mountains. J. Hum. Evol. 1973, 2, 357–370. [Google Scholar] [CrossRef]
- Nishie, H. Natural history of Camponotus ant-fishing by the M group chimpanzees at the Mahale Mountains National Park, Tanzania. Primates 2011, 52, 329–342. [Google Scholar] [CrossRef]
- Kakinuma, M.; Hamano, S.; Hatakeyama, H. Chimpanzee mother-child contact at infancy and later exploratory behavior. Bull. Nippon Vet. Zootech. Col. 2003, 52, 11–17. (In Japanese) [Google Scholar]
- Kakinuma, M.; Hatakeyama, H.; Tsuchida, A.; Nose, I. Diversities in the development of social, physical and cognitive abilities of captive chimpanzees (Pan troglodytes) compared. Bull. Nippon Vet. Life Sci. Univ. 2015, 64, 13–19. [Google Scholar]
- Nakamura, M. Grooming hand-clasp in Mahale M group chimpanzees: Implications for culture in social behaviors. In Behavioural Diversity in Chimpanzees and Bonobos; Boesch, C., Hohmann, G., Marchant, L.F., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 71–83. [Google Scholar]
- Nakamura, M. ‘Gatherings’ of social grooming among wild chimpanzees: Implications for evolution of sociality. J. Hum. Evol. 2003, 44, 59–71. [Google Scholar] [CrossRef]
- Seyfarth, R.M. A model of social grooming among adult female monkeys. J. Theor. Biol. 1977, 65, 671–698. [Google Scholar] [CrossRef]
- Newton-Fisher, N.E.; Lee, P.C. Grooming reciprocity in wild male chimpanzees. Anim. Behav. 2011, 81, 439–446. [Google Scholar] [CrossRef]
- Nishida, T. The social group of wild chimpanzees in the Mahali Mountains. Primates 1968, 9, 167–224. [Google Scholar] [CrossRef]
- Whiten, A.; Arnold, K. Grooming interactions among the chimpanzees of the Budongo Forest, Uganda: Tests of five explanatory models. Behaviour 2003, 140, 519–552. [Google Scholar] [CrossRef]
- de Waal, F.B.M. Chimpanzee Politics: Power and Sex Among Apes; John Hopkins University Press: Baltimore, MD, USA, 1989. [Google Scholar]
- Wrangham, R.W.; Smuts, B.B. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fert. 1980, 28, 13–31. [Google Scholar]
- Nishida, T. Alpha status and agonistic alliance in wild chimpanzees (Pan troglodytes schweinfurthii). Primates 1983, 24, 318–336. [Google Scholar] [CrossRef]
- Nishida, T.; Kawanaka, K. Inter-unit-group relationships among wild chimpanzees of the Mahali Mountains. Kyoto Univ. Afr. Stud. 1972, 7, 131–169. [Google Scholar]
- Langergraber, K.; Mitani, J.; Vigilant, L. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 2009, 71, 840–851. [Google Scholar] [CrossRef]
- Baker, K.C.; Smuts, B.B. Social relationships of female chimpanzees. In Chimpanzee Cultures; Wrangham, R.W., McGrew, W.C., de Waal, F.B.M., Heltne, P.G., Eds.; Harvard University Press: Cambridge, MA, USA, 1994; pp. 227–241. [Google Scholar]
- de Waal, F.B.M. The chimpanzee’s adaptive potential: A comparison of social life under captive and wild conditions. In Chimpanzee Cultures; Wrangham, R.W., McGrew, W.C., de Waal, F.B.M., Heltne, P., Eds.; Harvard University Press: Cambridge, MA, USA, 1994; pp. 243–260. [Google Scholar]
- Koski, S.E.; de Vries, H.; van de Kraats, A.; Sterck, E.H. Stability and change of social relationship quality in captive chimpanzees (Pan troglodytes). Int. J. Primatol. 2012, 33, 905–921. [Google Scholar] [CrossRef]
- Croft, D.P.; James, R.; Krause, J. Exploring Animal Social Networks; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Nakamura, M.; Hosaka, K.; Itoh, N.; Zamma, K. (Eds.) Mahale Chimpanzees: 50 years of Research; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Nishida, T.; Zamma, K.; Matsusaka, T.; Inaba, A.; McGrew, W.C. Chimpanzee Behavior in the Wild: An Audio-Visual Encyclopedia; Springer: Tokyo, Japan, 2010. [Google Scholar]
- Birkett, L.P.; Newton-Fisher, N.E. How abnormal is the behaviour of captive, zoo-living chimpanzees? PLoS ONE 2011, 6, e20101. [Google Scholar] [CrossRef]
- Jacobson, S.L.; Ross, S.R.; Bloomsmith, M.A. Characterizing abnormal behavior in a large population of zoo-housed chimpanzees: Prevalence and potential influencing factors. PeerJ 2016, 4, e2225. [Google Scholar] [CrossRef]
- Nakamura, M. Grooming at a gathering. In Chimpanzees in Mahale: 37 years of Panthropology; Nishida, T., Uehara, S., Kawanaka, K., Eds.; Kyoto University Press: Kyoto, Japan, 2002; pp. 345–367. (In Japanese) [Google Scholar]
- McGrew, W.C.; Tutin, C.E. Evidence for a social custom in wild chimpanzees? Man 1978, 13, 234–251. [Google Scholar] [CrossRef]
- McGrew, W.C. Chimpanzee Material Culture: Implications for Human Evolution; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Nakamura, M.; Nishida, T. Ontogeny of a social custom in wild chimpanzees: Age changes in grooming hand-clasp at Mahale. Am. J. Primatol. 2013, 75, 186–196. [Google Scholar] [CrossRef]
- Shimada, M.; Sueur, C. The importance of social play network for infant or juvenile wild chimpanzees at Mahale Mountains National Park, Tanzania. Am. J. Primatol. 2014, 76, 1025–1036. [Google Scholar] [CrossRef]
- Farine, D.R.; Whitehead, H. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 2015, 84, 1144–1163. [Google Scholar] [CrossRef]
- Shimizu, H. An introduction to the statistical free software HAD: Suggestions to improve teaching, learning and practice data analysis. J. Media Inf. Commun. 2016, 1, 59–73. [Google Scholar]
- Borgatti, S.P.; Everett, M.G.; Freeman, L.C. UCINET 6 for Windows: Software for Social Network Analysis; Analytic Technologies: Harvard, MA, USA, 2002. [Google Scholar]
- Morimura, N.; Ueno, Y. Influences on the feeding behavior of three mammals in the Maruyama Zoo: Bears, elephants, and chimpanzees. J. Appl. Anim. Welf. Sci. 1999, 2, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Fulk, R.; Garland, C. (Eds.) The Care and Management of Chimpanzees (Pan troglodytes) in Captive Environments. A husbandry Manual Developed for the Chimpanzee Species Survival Plan; North Carolina Zoological Society: Asheboro, NC, USA, 1992. [Google Scholar]
- Boesch, C.; Boesch-Achermann, H. The chimpanzees of the Taï Forest: Behavioural Ecology and Evolution; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kosheleff, V.P.; Anderson, C.N. Temperature’s influence on the activity budget, terrestriality, and sun exposure of chimpanzees in the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 2009, 139, 172–181. [Google Scholar] [CrossRef]
- Potts, K.B.; Watts, D.P.; Wrangham, R.W. Comparative feeding ecology of two communities of chimpanzees (Pan troglodytes) in Kibale National Park, Uganda. Int. J. Primatol. 2011, 32, 669–690. [Google Scholar] [CrossRef]
- Green, S.J.; Boruff, B.J.; Niyigaba, P.; Ndikubwimana, I.; Grueter, C.C. Chimpanzee ranging responses to fruit availability in a high-elevation environment. Am. J. Primatol. 2020, e23119. [Google Scholar] [CrossRef] [PubMed]
- AZA Ape TAG. Chimpanzee (Pan troglodytes) Care Manual; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2010. [Google Scholar]
- Sakamaki, T. Social grooming among wild bonobos (Pan paniscus) at Wamba in the Luo Scientific Reserve, DR Congo, with special reference to the formation of grooming gatherings. Primates 2013, 54, 349–359. [Google Scholar] [CrossRef]
- Hare, B.; Yamamoto, S. (Eds.) Bonobos: Unique in Mind, Brain, and Behavior; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Furuichi, T. Factors underlying party size differences between chimpanzees and bonobos: A review and hypotheses for future study. Primates 2009, 50, 197–209. [Google Scholar] [CrossRef]
- Gomes, C.M.; Mundry, R.; Boesch, C. Long-term reciprocation of grooming in wild West African chimpanzees. Proc. R. Soc. B. 2008, 276, 699–706. [Google Scholar] [CrossRef]
- Fedurek, P.; Dunbar, R.I. What does mutual grooming tell us about why chimpanzees groom? Ethology 2009, 115, 566–575. [Google Scholar] [CrossRef]
- Bonnie, K.E.; de Waal, F.B. Affiliation promotes the transmission of a social custom: Handclasp grooming among captive chimpanzees. Primates 2006, 47, 27–34. [Google Scholar] [CrossRef]
- Whiten, A.; Goodall, J.; McGrew, W.C.; Nishida, T.; Reynolds, V.; Sugiyama, Y.; Tutin, C.E.G.; Wrangham, R.W.; Boesch, C. Cultures in chimpanzees. Nature 1999, 399, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Whiten, A.; Goodall, J.; McGrew, W.C.; Nishida, T.; Reynolds, V.; Sugiyama, Y.; Tutin, C.E.G.; Wrangham, R.W.; Boesch, C. Charting cultural variation in chimpanzees. Behaviour 2001, 138, 1481–1516. [Google Scholar] [CrossRef]
- Mitani, J.C. Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 2009, 77, 633–640. [Google Scholar] [CrossRef]
- Bloomsmith, M.A.; Baker, K.C.; Ross, S.R.; Lambeth, S.P. Early rearing conditions and captive chimpanzee behavior: Some surprising findings. In Nursery Rearing of Nonhuman Primates in the 21st Century; Sackett, G.P., Ruppentahal, G.C., Elias, K., Eds.; Springer: Boston, MA, USA, 2006; pp. 289–312. [Google Scholar]
- Watts, D.P.; Mitani, J.C. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour 2001, 138, 299–327. [Google Scholar] [CrossRef]
- Reinhardt, V.; Roberts, A. Effective feeding enrichment for non-human primates: A brief review. Anim. Welf. 1997, 6, 265–272. [Google Scholar]
- Morimura, N. A note on enrichment for spontaneous tool use by chimpanzees (Pan troglodytes). Appl. Anim. Behav. Sci. 2003, 82, 241–247. [Google Scholar] [CrossRef]
- Bloomsmith, M.A.; Alford, P.L.; Maple, T.L. Successful feeding enrichment for captive chimpanzees. Am. J. Primatol. 1988, 16, 155–164. [Google Scholar] [CrossRef]
- De Waal, F. Mama’s Last Hug: Animal Emotions and what They Tell Us about Ourselves; WW Norton & Company: New York, NY, USA, 2019. [Google Scholar]
| Group | Name | Abbreviation | Sex | Age | Age Category | Place of Birth * |
|---|---|---|---|---|---|---|
| Tama | Bonbon | bo | Male | 14 | Young | Tama |
| Deckie | de | app. 41 | Adult | Wild | ||
| Gin | gi | 11 | Young | Tama | ||
| Max | mx | 12 | Young | Tama | ||
| Fubuki | fu | 5 | Juvenile | Tama | ||
| Peach | pc | Female | 29 | Adult | Izu | |
| Nana | na | 36 | Adult | Tama | ||
| Peco | pe | app. 58 | Adult | Wild | ||
| Chico | ci | 25 | Adult | Tama | ||
| Cherry | ch | 29 | Adult | Tama | ||
| Berry | be | 19 | Adult | Tama | ||
| Momoko | mo | 26 | Adult | KS | ||
| Mikan | mi | 13 | Adult | Tama | ||
| Marina | mr | 29 | Adult | KS | ||
| Mil | ml | 16 | Adult | Tama | ||
| Sakura | sa | 10 | Young | Noichi | ||
| Mahale | Alofu | AL | Male | 20 | Adult | M |
| Bonobo | BB | 21 | Adult | M | ||
| Carter | CT | 17 | Adult | M | ||
| Kalunde | DE | 39 | Adult | M | ||
| Fanana | FN | 24 | Adult | M | ||
| Hanby | HB | 22 | Adult | M | ||
| Masudi | MA | 25 | Adult | K | ||
| Pimu | PM | 14 | Young | M | ||
| Cynthia | CY | Female | 20 | Adult | O | |
| Nkombo | NK | 32 | Adult | K | ||
| Opal | OP | 31 | Adult | O | ||
| Pinky | PI | 30 | Adult | O | ||
| Totzy | TZ | 20 | Adult | M | ||
| Wakusi | WX | 41 | Adult | O | ||
| Christina | XT | 27 | Adult | O | ||
| Zola | ZL | 15 | Adult | O |
| Code of Clique Types | Configuration of Participating Individuals | Number of Participating Individuals |
|---|---|---|
| 001 | ○→● | 2 |
| 010 | ●→○ | 2 |
| 111 | ●↔○ | 2 |
| 011 | ○→●→○ | 3 |
| 002 | ○○→● | 3 |
| 112 | ○→●↔○ | 3 |
| 003 | ○○○→● | 4 |
| 012 | ○○→●→○ | 4 |
| 114 | ○○○→●↔○ | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, N.; Shimada, M. Comparisons of Activity Budgets, Interactions, and Social Structures in Captive and Wild Chimpanzees (Pan troglodytes). Animals 2020, 10, 1063. https://doi.org/10.3390/ani10061063
Inoue N, Shimada M. Comparisons of Activity Budgets, Interactions, and Social Structures in Captive and Wild Chimpanzees (Pan troglodytes). Animals. 2020; 10(6):1063. https://doi.org/10.3390/ani10061063
Chicago/Turabian StyleInoue, Nodoka, and Masaki Shimada. 2020. "Comparisons of Activity Budgets, Interactions, and Social Structures in Captive and Wild Chimpanzees (Pan troglodytes)" Animals 10, no. 6: 1063. https://doi.org/10.3390/ani10061063
APA StyleInoue, N., & Shimada, M. (2020). Comparisons of Activity Budgets, Interactions, and Social Structures in Captive and Wild Chimpanzees (Pan troglodytes). Animals, 10(6), 1063. https://doi.org/10.3390/ani10061063




