Transcriptome Functional Analysis of Mammary Gland of Cows in Heat Stress and Thermoneutral Condition
Simple Summary
Abstract
1. Introduction
2. Materials and methods
2.1. Animals, Management and Experimental Treatments
2.2. Sample Collection
2.3. RNA Isolation and Library Preparation
2.4. Quality Control and Mapping
2.5. RNA-Seq Data Analysis
2.6. Quantitative Real-Time PCR Analysis (qRT-PCR).
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
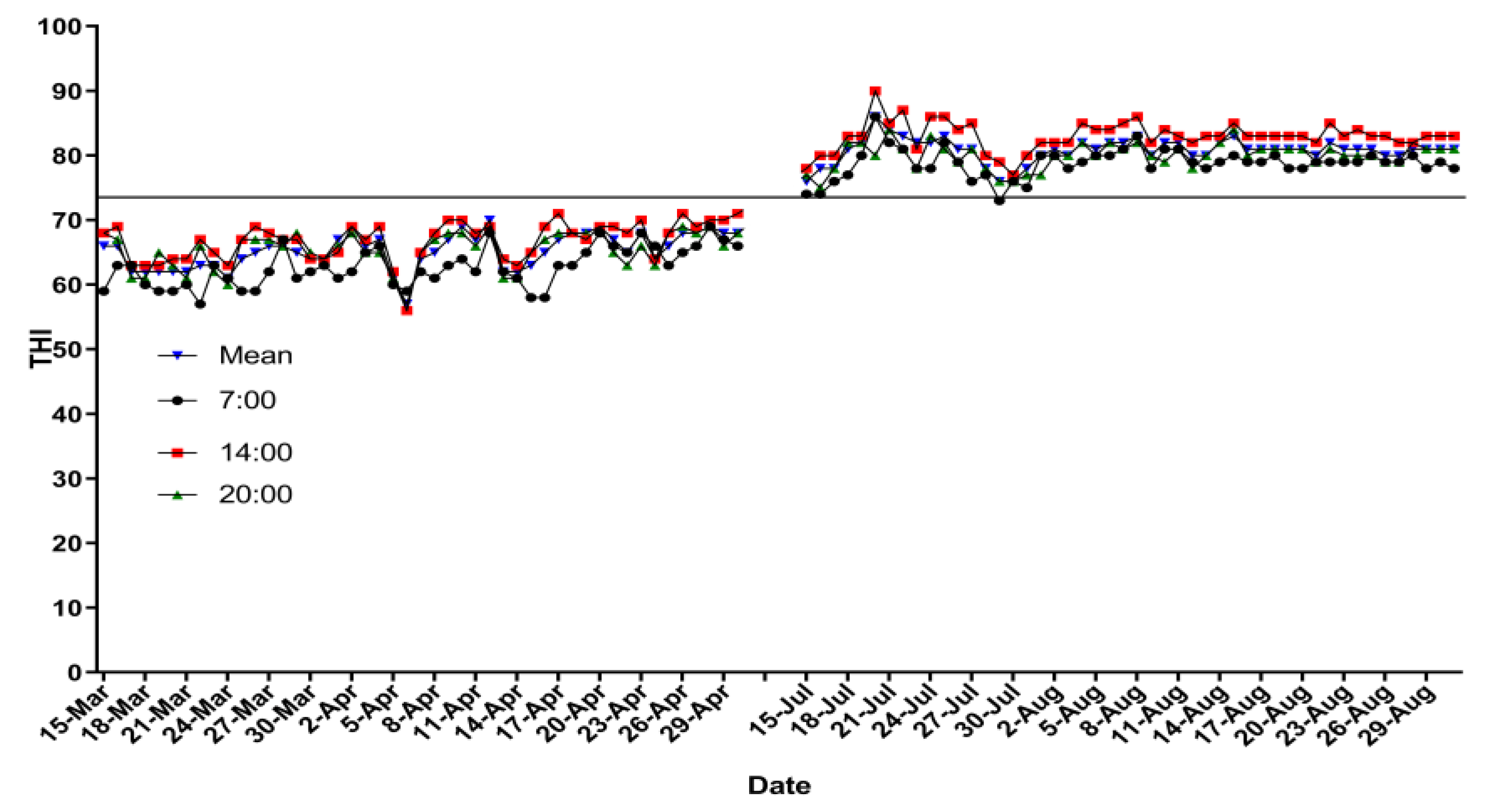
3.1. Temperature–Humidity Index and Physiological Index
3.2. Effect of Exposure of Heat Stress on Milking Performance and Blood Biochemical Indexes
3.3. HS Group Changed the Transcriptome in Mammary Gland Tissues
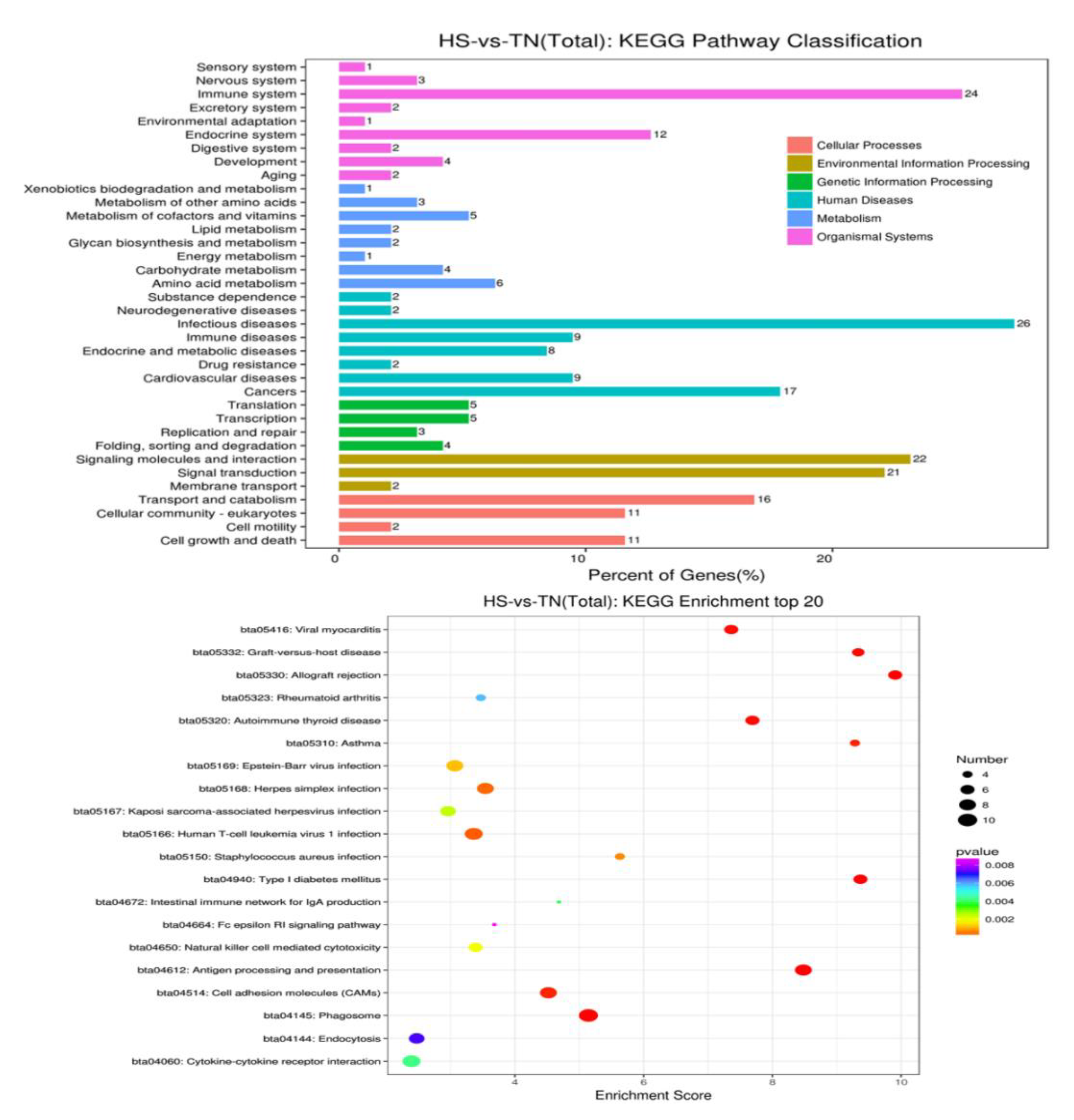
3.4. Gene Ontology Enrichment Analysis
3.5. Biological Pathway Affected by HS
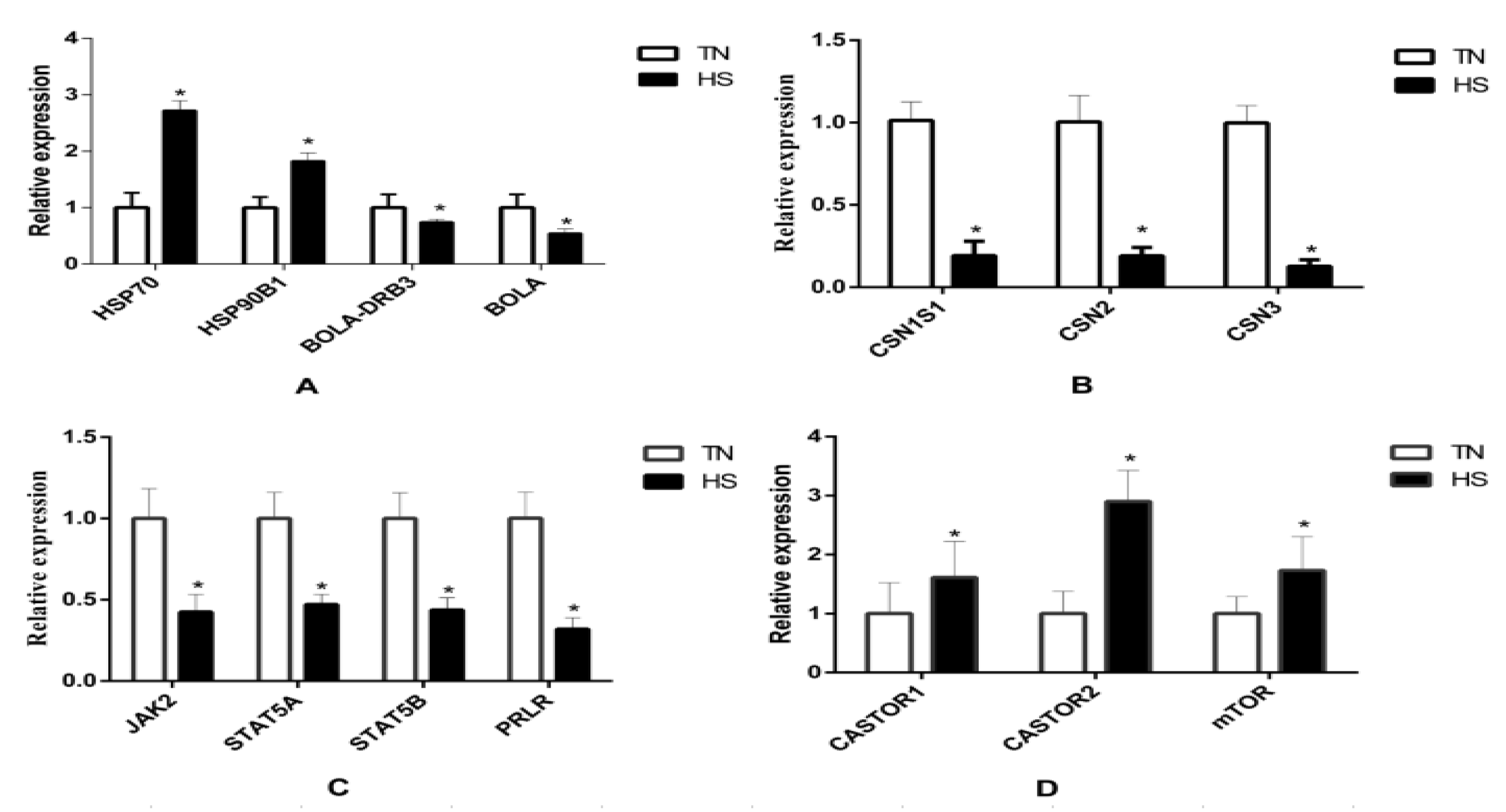
3.6. Identification of Differentially Expressed Genes in Response to HS Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Kim, J.W.; Collier, R.J.; Crooker, B.A.; Boisclair, Y.R.; Baumgard, L.H.; Rhoads, R.P. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 2010, 93, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of heat stress on body temperature, milkproduction, and reproduction in dairy cows: A novel idea for monitoring and evaluation of heat stress—A review. Asian Australas. J. Anim. Sci. 2019, 32, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Wheeock, J. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Fuquay, J.W. Heat stress as it affects animal production. J. Anim. Sci. 1981, 52, 164. [Google Scholar] [CrossRef] [PubMed]
- West, J. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Collier, R.J.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H. Invited review: Genes involved in the bovine heat stress response. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Stiening, C.M.; Pollard, B.C.; Vanbaale, M.J.; Coussens, P.M. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J. Anim. Sci. 2006, 84 (Suppl. S13), E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Mu, T.; Yang, Z. Methionine protects against hyperthermia-induced cell injury in cultured bovine mammary epithelial cells. Cell Stress Chaperon. 2015, 20, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, J.; Gao, H.; Li, S.; Zhang, Y.; Zheng, N. Heat-induced apoptosis and gene expression in bovine mammary epithelial cells. Anim. Prod. Sci. 2016, 56, 918. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Wu, J.; Li, X.; Luo, M.; Wang, G. The global effect of heat on gene expression in cultured bovine mammary epithelial cells. Cell Stress Chaperon. 2015, 20, 381–389. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Farr, V.C.; Stelwagen, K.; Cate, L.R.; Molenaar, A.J.; Mcfadden, T.B.; Davis, S.R. An improved method for the routine biopsy of bovine mammary tissue. J. Dairy Sci. 1996, 79, 543–549. [Google Scholar] [CrossRef]
- Simon, A.; Theodor, P.P.; Wolfgang, H. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 2014, 31, 166–169. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. Hisat: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Proto. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D. Heat stress interaction with shade and cooling. J. Dairy Sci. 2002, 77, 2044–2050. [Google Scholar] [CrossRef]
- Bouraoui, R.; Lahmar, M.; Majdoub, A.; Belyea, R. The relationship of temperature-humidity index with milk production of dairy cows in a Mediterranean climate. Anim. Res. 2002, 51, 479–491. [Google Scholar] [CrossRef]
- Dikmen, S.; Alava, E.; Pontes, E.; Fear, J.M.; Dikmen, B.Y.; Olson, T.A.; Hansen, P.J. Differences in thermoregulatory ability between slick-haired and wild-type lactating holstein cows in response to acute heat stress. J. Dairy Sci. 2008, 91, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Hansen, P. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zimbelman, R.; Rhoads, R.; Rhoads, M.; Duff, G.; Baumgard, L.; Collier, R. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the 24h Southwest Nutrition and management Conference, Savoy, IL, USA, 26–27 February 2009; pp. 158–169. [Google Scholar]
- Kadzere, C.; Murphy, M.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Trinklein, N.D.; Murray, J.I.; Hartman, S.J.; Botstein, D.; Myers, R.M. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 2004, 15, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.; Bonner, S.; Loxton, I.; Mader, T. Effects of chronic heat stress on plasma concentration of secreted heat shock protein 70 in growing feedlot cattle. J. Dairy Sci. 2013, 91, 120–129. [Google Scholar]
- Romero, R.D.; Pardo, A.M.; Montaldo, H.H.; Rodríguez, A.D.; Cerón, J.H. Differences in body temperature, cell viability, and HSP-70 concentrations between Pelibuey and Suffolk sheep under heat stress. Trop. Anim. Heal. Prod. 2013, 45, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Patir, H.; Upadhyay, R. Purification, characterization and expression kinetics of heat shock protein 70 from Bubalus bubalis. Res. Veter. Sci. 2010, 88, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Monteiro, A.P.A.; Guo, J.; Li, C.; Orellana, R.M.; Marins, T.N.; Bernard, K.; Tomlinson, D.J.; Defrain, J.M.; Wohgemuth, S.; et al. Effects of heat stress and dietary zinc source on performance and mammary epithelial integrity of lactating dairy cows. J. Dairy Sci. 2018, 101, 2617–2630. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guo, J.; Quan, S.; Nan, X.; Fernandez, M.S.; Baumgard, L.; Bu, D. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017, 100, 5040–5049. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Rhoads, M.; Rhoads, R.; VanBaale, M.; Collier, R.J.; Sanders, S.; Weber, W.J.; Crooker, B.A.; Baumgard, L. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Elvinger, F.; Hansen, P.J.; Natzke, R.P. Modulation of function of bovine polymorphonuclear leukocytes and lymphocytes by high temperature in vitro and in vivo. Am. J. Vet. Res. 1991, 52, 1692–1698. [Google Scholar] [PubMed]
- Nardone, A. Heat stress elicits different responses in peripheral blood mononuclear cells from brown swiss and holstein cows. J. Dairy Sci. 2006, 89, 4606–4612. [Google Scholar]
- Kumar, A.; Ashraf, S.; Goud, T.S.; Grewal, A.; Singh, S.V.; Yadav, B.R.; Upadhyay, R.C. Expression profiling of major heat shock protein genes during different seasons in cattle (Bos indicus) and buffalo (Bubalus bubalis) under tropical climatic condition. J. Therm. Biol. 2015, 51, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Sajjanar, B.; Singh, U.; Kumar, S.; Singh, R.; Sengar, G.; Sharma, A. Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus×Bos taurus) breed of cattle: A comparative study. Gene 2014, 536, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Nagayach, R.; Gupta, U.D.; Prakash, A. Expression profiling of hsp70 gene during different seasons in goats (Capra hircus) under sub-tropical humid climatic conditions. Small Rumin. Res. 2017, 147, 41–47. [Google Scholar] [CrossRef]
- Randow, F.; Seed, B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001, 3, 891–896. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Dai, J.; Srivastava, P.K.; Zammit, D.J.; Lefrançois, L.; Li, Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 2007, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.; Rammensee, H.G. gp96—the immune system’s swiss army knife. Nat. Immunol. 2000. [Google Scholar] [CrossRef] [PubMed]
- Galea-Lauri, J.; Latchman, D.S.; Katz, D.R. The role of the 90-kDa heat shock protein in cell cycle control and differentiation of the monoblastoid cell line U937. Exp. Cell Res. 1996, 226, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Usinger, W.R.; Curie-Cohen, M.; Benforado, K.; Pringnitz, D.; Stone, W.H. The bovine major histocompatibility complex (BoLA): Close linkage of the genes controlling serologically defined antigens and mixed lymphocyte reactivity. Immunogenetics 1981, 14, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Soloski, M.J.; Szperka, M.E.; Davies, A.; Wooden, S.L. Host immune response to intracellular bacteria: A role for MHC-linked class-Ib antigen-presenting molecules. Exp. Bio. Med. 2000, 224, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.G. The Role of the Bovine Mammary Gland in Immunological Responses to StreptococcusUberis. Ph.D. Thesis, University of Bristol, Bristol, UK, 1995. [Google Scholar]
- Fitzpatrick, J.; Cripps, P.; Hill, A.; Bland, P.; Stokes, C. MHC class II expression in the bovine mammary gland. Veter. Immunol. Immunopathol. 1992, 32, 13–23. [Google Scholar] [CrossRef]
- Lundén, A.; Sigurdardóttir, S.; Edfors-Lilja, I.; Danell, B.; Rendel, J.; Andersson, L. The effect of bovine MHC class. II polymorphism on bull breeding values for clinical mastitis and somatic cell counts in milk. In Proceedings of the 4th World Congress on Genetics applied to Livestock Production, Edinburgh, UK, 23–27 July 1990. [Google Scholar]
- Cook, N.B.; Bennett, T.B.; Emery, K.M. Monitoring nonlactating cow intramammary infection dynamics using DHI somatic cell count data. J. Dairy Sci. 2002, 85, 1119–1126. [Google Scholar] [CrossRef]
- Bertocchi, L.; Vitali, A.; Lacetera, N.; Nardone, A.; Varisco, G.; Bernabucci, U. Seasonal variations in the composition of Holstein cow's milk and temperature-humidity index relationship. Animal 2014, 8, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.W.; Frisan, T.; Vandewalle, A.; Normark, S.; Richterdahlfors, A. Toll-like receptor 4 resides in the golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 2002, 195, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.D.; Paape, M.J.; Hare, W.R.; Sohn, E.J. Increased levels of LPS-binding protein in bovine blood and milk following bacterial lipopolysaccharide challenge. J. Dairy Sci. 2003, 86, 3128–3137. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.S.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Yulee, L.; Luo, G.; Book, M.L.; Morris, S.M. Lactogenic hormone signal transduction. Biol. Reprod. 1998, 58, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.J.; Li, N.; Cheung, J.; Lowe, E.T.; Lambert, E.; Marlow, R.; Wang, P.; Schatzmann, F.; Wintermantel, T.; Schüetz, G.; et al. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 2005, 171, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Groner, B. Transcription factor regulation in mammary epithelial cells. Domest. Anim. Endocrinol. 2002, 23, 25–32. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, G.W.; Wagner, K.U.; Garrett, L.; Wynshaw-Boris, A.; Hennighausen, L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.H.; Auchtungmontgomery, T.L.; Dahl, G.E.; Mcfadden, T.B. Short communication: Short-day photoperiod during the dry period decreases expression of suppressors of cytokine signaling in mammary gland of dairy cows. J. Dairy Sci. 2005, 88, 3145–3148. [Google Scholar] [CrossRef]
- Sasaki, A.T.; Yasukawa, H.; Suzuki, A.; Kamizono, S.; Syoda, T.; Kinjyo, I.; Sasaki, M.; Johnston, J.A.; Yoshimura, A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 1999, 4, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Trottier, N.L. Linking our understanding of mammary gland metabolism to amino acid nutrition. Amino Acids 2014, 46, 2447–2462. [Google Scholar]
- Stransky, L.A.; Forgac, M. Amino acid availability modulates vacuolar H+-ATPase assembly. J. Biol. Chem. 2015, 290, 27360–27369. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.N.; Hu, H.; Zheng, N.; Wang, J.Q. Leucine and histidine independently regulate milk protein synthesis in bovine mammary epithelial cells via mTOR signaling pathway. J. Zhejiang Univ. Sci. B 2015, 16, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.D.; Kassube, K.R.; Almeida, R.A.; Ríus, A.G. Short communication: High incubation temperature in bovine mammary epithelial cells reduced the activity of the mTOR signaling pathway. J. Dairy. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.K.; Duque, M.; Wang, L.; Shahzad, K.; Olivera, M.; Loor, J. Enhanced supply of methionine or arginine alter MTOR signaling proteins, mRNA, and microRNA abundance in heat-stressed bovine mammary epithelial cells in vitro. J. Dairy. Sci. 2019, 102, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Li, D.; Wang, Y.; Li, M.; Chen, C.; Fang, X.; Chen, H.; Zhang, C. Milk protein synthesis is regulated by T1R1/T1R3, a G protein-coupled taste receptor, through the mTOR pathway in the mouse mammary gland. Mol. Nutr. Food Res. 2017, 61, 1601017. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, X.; Zhang, Y.; Liu, H.; Liao, J.; Shao, K.; Chu, Y.; Liu, G. Modulation of TSC-mTOR signaling on immune cells in immunity and autoimmunity. J. Cell Physiol. 2013, 229, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Pruitt, M.; Tran, D.; Du Bois, W.; Zhang, K.; Patel, R.; Hoover, S.; Simpson, R.M.; Simmons, J.; Gary, J.; et al. B cell-specific deficiencies in mTOR limit humoral immune responses. J. Immunol. 2013, 191, 1692–1703. [Google Scholar] [CrossRef] [PubMed]



| Parameter | TN (thermal neutral) | HS (heat stress) | p-Value |
|---|---|---|---|
| Number of cows | 10 | 10 | |
| Parity | 2.1 ± 1.0 | 2.2 ± 1.3 | 0.89 |
| Lactation days | 130.5 ± 15 | 123.4 ± 20 | 0.92 |
| 305-day milk yield (kg) | 8993.7 ± 767.5 | 8933.2 ± 757.1 | 0.95 |
| Average bodyweight (kg) | 605.8 ± 58.1 | 603.5 ± 45.3 | 0.97 |
| Ingredients (% of DM) | Content |
|---|---|
| Corn | 14.94 |
| Soybean meal | 4.98 |
| Cottonseed meal | 1.30 |
| Rapeseed meal | 0.42 |
| Extruded full-fat soybean | 2.21 |
| Corn gluten meal | 0.83 |
| Dried distillers grains | 1.11 |
| Oat hay | 5.03 |
| Alfalfa hay | 10.75 |
| Whole cottonseed | 3.42 |
| Whole corn silage | 38.87 |
| Beet pulp | 2.52 |
| Molasses | 3.42 |
| Fatty power | 0.57 |
| Brewer’s grains | 6.86 |
| Limestone | 0.77 |
| Dicalcium phosphate | 0.42 |
| Vitamin–mineral premix 1 | 0.48 |
| Sodium bicarbonate | 0.70 |
| MgO | 0.14 |
| NaCL | 0.28 |
| Chemical composition (%) | |
| NEL (Mcal/Kg) 2 | 1.70 |
| Crude protein | 16.6 |
| Ether extract | 5.50 |
| NDF 3 | 30.89 |
| ADF 4 | 19.27 |
| Ca | 0.78 |
| P | 0.43 |
| Ash | 7.50 |
| Table | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| TN | HS | |||
| Respiration rate (breath/min) | ||||
| 0700 | 35.6 | 72.4 | 2.51 | 0.03 |
| 1400 | 39.3 | 89.6 | 3.31 | <0.01 |
| 2000 | 41.3 | 84.3 | 4.32 | <0.01 |
| Rectal temperature (°C) | ||||
| 0700 | 38.2 | 39.2 | 0.08 | <0.01 |
| 1400 | 38.5 | 39.5 | 0.09 | <0.01 |
| 2000 | 38.4 | 39.3 | 0.07 | <0.01 |
| Items | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| TN | HS | |||
| Milk yield (kg/d) | 42.5 | 35.6 | 2.03 | <0.01 |
| DMI (kg/d) | 23.5 | 21.4 | 0.10 | <0.01 |
| Milk Protein % | 3.2 | 2.8 | 0.07 | 0.03 |
| Milk protien yield (kg/d) | 1.3 | 1.0 | 0.06 | 0.12 |
| Milk Fat % | 4.3 | 3.8 | 0.14 | 0.04 |
| Milk Lactose % | 4.6 | 4.7 | 0.03 | <0.01 |
| UN of milk (mg/dL) | 13.9 | 14.6 | 0.86 | 0.51 |
| LPS (EU/L) | 691.4 | 948.1 | 72.81 | 0.01 |
| HSP 70 (ng/mL) | 7.8 | 14.0 | 1.31 | <0.01 |
| Glucose (mm/L) | 2.8 | 2.2 | 0.22 | 0.03 |
| NEFA (μm/L) | 167.0 | 238.2 | 18.51 | 0.02 |
| SCC 1000/ML | 266.0 | 307.1 | 132.46 | 0.76 |
| Term | p-Value | Genes | Gene Expression |
|---|---|---|---|
| bta05416, Viral myocarditis | <0.01 | BoLA; BOLA; BOLA-DRB3; BOLA-DYA; LOC512672; LOC524810 | Down |
| bta05169, Epstein-Barr virus infection | <0.01 | BoLA; BOLA; BOLA-DRB3; BOLA-DYA; LOC512672; LOC524810 | Down |
| bta05168, Herpes simplex infection | <0.01 | BoLA; BOLA; BOLA-DRB3; BOLA-DYA; LOC512672; SP100 | Down |
| bta05150, Staphylococcus aureus infection | <0.01 | BOLA-DRB3; BOLA-DYA; LOC524810 | Down |
| bta5140, Leishmaniasis | BOLA-DRB3; BOLA-DYA; LOC524810 | Down | |
| bta04612, Antigen processing and presentation | 0.001 | BoLA; BOLA; BOLA-DRB3; BOLA-DYA; LOC512672 | Down |
| bta05323, Rheumatoid arthritis | 0.003 | BOLA-DRB3; BOLA-DYA; LOC524810 | Down |
| bta05320, Autoimmune thyroid disease | <0.01 | BoLA; BOLA; BOLA-DRB3; BOLA-DYA; LOC512672; LOC524810 | Down |
| bta05310, Asthma | <0.01 | BOLA-DRB3; BOLA-DYA; LOC524810; MS4A2 | Down |
| bta04940, Type I diabetes mellitus | <0.01 | BoLA; BOLA; BOLA-DRB3; BOLA-DYA; CPE; LOC512672 | Down |
| bta04612, Antigen processing and presentation | 0.001 | HSPA1A; HSPA8; LOC618733, HSP90B1 | Up |
| bta04141, Protein processing in endoplasmic reticulum | 0.002 | DNAJA1; HSPA1A; HSPA8; HSPH1, HSP90B1 | Up |
| bta03040, Spliceosome | <0.01 | HSPA1A; HSPA8; PHF5A; SNRPG | Up |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, S.; Wang, Z.; Wang, L.; Peng, Q.; Xue, B. Transcriptome Functional Analysis of Mammary Gland of Cows in Heat Stress and Thermoneutral Condition. Animals 2020, 10, 1015. https://doi.org/10.3390/ani10061015
Yue S, Wang Z, Wang L, Peng Q, Xue B. Transcriptome Functional Analysis of Mammary Gland of Cows in Heat Stress and Thermoneutral Condition. Animals. 2020; 10(6):1015. https://doi.org/10.3390/ani10061015
Chicago/Turabian StyleYue, Shuangming, Zhisheng Wang, Lizhi Wang, Quanhui Peng, and Bai Xue. 2020. "Transcriptome Functional Analysis of Mammary Gland of Cows in Heat Stress and Thermoneutral Condition" Animals 10, no. 6: 1015. https://doi.org/10.3390/ani10061015
APA StyleYue, S., Wang, Z., Wang, L., Peng, Q., & Xue, B. (2020). Transcriptome Functional Analysis of Mammary Gland of Cows in Heat Stress and Thermoneutral Condition. Animals, 10(6), 1015. https://doi.org/10.3390/ani10061015





