The Use of Bacteriophages in the Poultry Industry
Abstract
Simple Summary
Abstract
1. Introduction
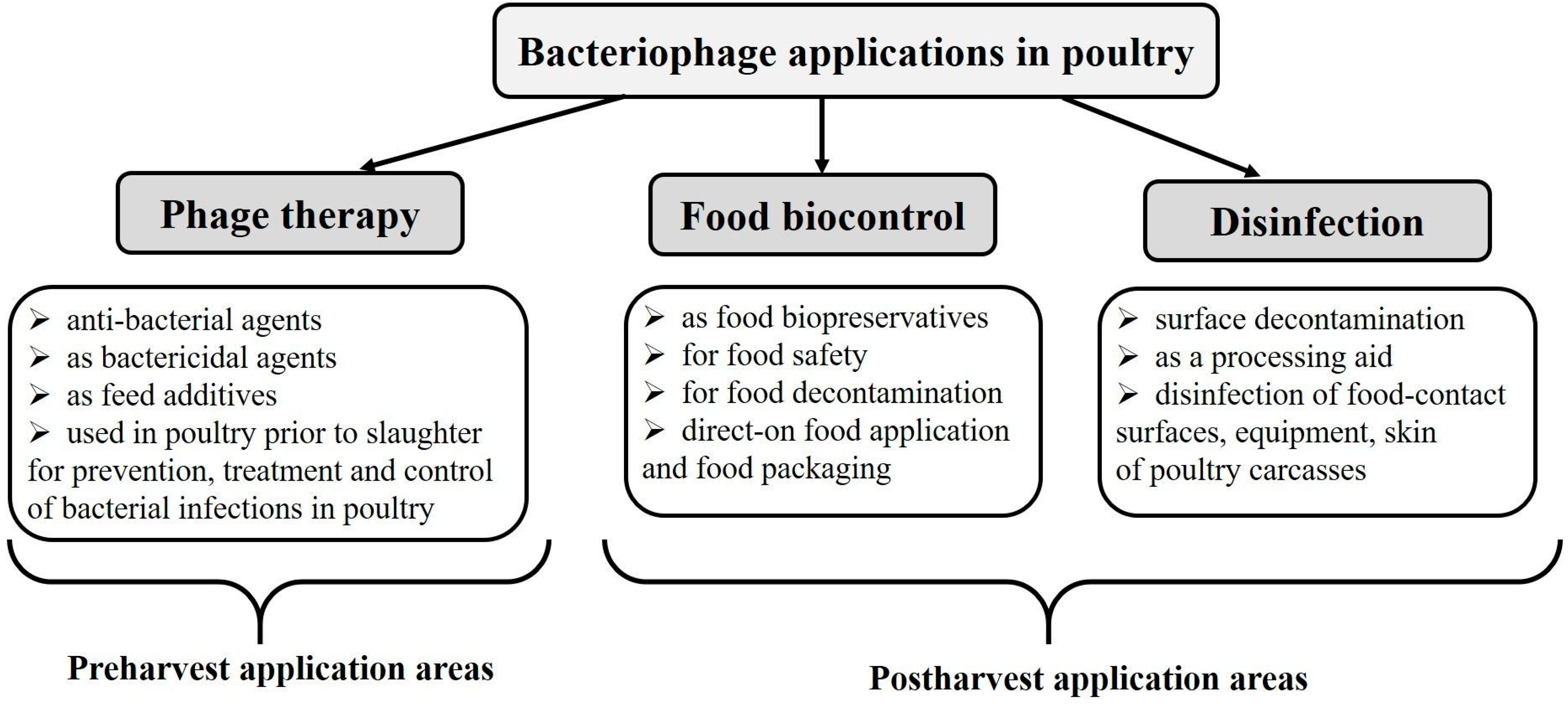
2. Phage Therapy of Bacterial Infections in Poultry
2.1. Campylobacter
2.2. Salmonella
2.3. Escherichia coli
2.4. Staphylococcus Aureus
2.5. Clostridium
3. Reduction of Food Contaminations (Biocontrol)
4. Use of Bacteriophages as Disinfectants
5. Threats Arising from the Use of Bacteriophages in Poultry
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Twort, F.W. An investigation on the nature of ultramicroscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- d’Hérelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. Crit. Rev. Acad. Sci. Paris 1917, 165, 373. [Google Scholar]
- Duckworth, D.H. Who Discovered Bacteriophage? Bacteriol. Rev. 1976, 40, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, C.; Blanco-Picazo, P.; Brown-Jaque, M.; Quirós, P.; Rodríguez-Rubio, L.; Cerdà-Cuellar, M.; Muniesa, M. Infectious phage particles packaging antibiotic resistance genes found in meat products and chicken feces. Sci. Rep. 2019, 9, 13281. [Google Scholar] [CrossRef] [PubMed]
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W. Bacteriophage observations and evolution. Res. Microbiol. 2003, 154, 245–251. [Google Scholar] [CrossRef]
- Wommack, K.E.; Hill, R.T.; Kessel, M.; Russek-Cohen, E.; Colwell, R.R. Distribution of viruses in the Chesapeake Bay. Appl. Environ. Microbiol. 1992, 58, 2965–2970. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Rath, N.C.; Donoghue, A.M. Critical Evaluation of Bacteriophage to Prevent and Treat Colibacillosis in Poultry. JAAS 2009, 63, 93–98. [Google Scholar]
- Fortier, L.C.; Sekulovic, O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 2013, 4, 354–365. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Ross, R.P.; Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 2009, 33, 801–819. [Google Scholar] [CrossRef]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogen. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Proença, D.; Cantante, C.; Silva, F.A.; Leandro, C.; Lourenço, S.; Milheiriço, C.; de Lencastre, H.; Cavaco-Silva, P.; Pimentel, M.; et al. Novel chimerical endolysins with broad antimicrobial activity against methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2012, 18, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kirbis, A.; Krizmana, M. Spread of antibiotic resistant bacteria from food of animal origin to humans and vice versa. Procedia Food Sci. 2015, 5, 148–151. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Monk, A.B.; Rees, C.D.; Barrow, P.; Hagens, S.; Harper, D.R. Bacteriophage applications: Where are we now? Lett. Appl. Microbiol. 2010, 51, 363–369. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control): The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 1–276. [CrossRef]
- Wójcik, E.A.; Wojtasik, A.; Górecka, E.; Stańczyk, M.; Dastych, J. Application of bacteriophage preparation BAFASAL® in broiler chickens experimentally exposed to Salmonella spp. SSRCI Vet. Med. Prod. Feed Add. 2015, 16, 241–251. [Google Scholar]
- Proteon-Pharmaceuticals. Bafasal®. Available online: https://www.proteonpharma.com/products/bafasal-poultry/ (accessed on 4 April 2020).
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.J. Don’t Shut the Stable Door after the Phage Has Bolted-The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef]
- Phagelux Inc. SalmoPro®. Available online: https://www.fda.gov/media/95017/download (accessed on 4 April 2020).
- Micreos Food Safety BV. Salmonelex™. Available online: https://www.fda.gov/media/98485/download (accessed on 4 April 2020).
- Micreos Food Safety BV. PhageGuard S. Available online: https://www.micreos.com/content/contact.aspx (accessed on 4 April 2020).
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, M.A.; Clavijo, V.; Vaglio, C.; González-Barrios, A.F.; Vives-Flórez, M.J.; Rito-Palomares, M. Economic evaluation of the development of a phage therapy product for the control of Salmonella in poultry. Biotechnol. Prog. 2019, 35, e2852. [Google Scholar] [CrossRef] [PubMed]
- Intralytix Inc. Bacteriophage Products-Food Safety Products. Available online: http://www.intralytix.com/index.php?page=prod (accessed on 4 April 2020).
- Perera, M.N.; Abuladze, T.; Li, M.; Woolston, J.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food. Microbiol. 2015, 52, 42–48. [Google Scholar] [CrossRef]
- Micreos Food Safety BV. Listex™ P100. Available online: https://phageguard.com/wp-content/uploads/2020/02/2020-02-11-PhageGuard-Application-Data-Sheet-RTE-.pdf (accessed on 4 April 2020).
- Soni, K.A.; Nannapaneni, R.; Hagens, S. Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodborne Pathog. Dis. 2010, 7, 427–434. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards): Scientific opinion on the evaluation of the safety and efficacy of ListexTM P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, 1–94. [CrossRef]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Zhang, Q. Campylobacter in Poultry: Ecology and Potential Interventions. Avian Dis. 2015, 59, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Wysok, B.; Pastuszczak-Frąk, M.; Uradziński, J.; Gomółka-Pawlicka, M.; Dzisko, J.; Dziedziech, M.; Marko, A. Występowanie i antybiotykooporność szczepów Campylobacter spp. wyizolowanych od zwierząt rzeźnych i ludzi. Med. Weter. Vet. Med. Sci. Prac. 2015, 71, 801–806. [Google Scholar]
- Nowaczek, A.; Urban-Chmiel, R.; Dec, M.; Puchalski, A.; Stępień-Pyśniak, D.; Marek, A.; Pyzik, E. Campylobacter spp. and bacteriophages from broiler chickens: Characterization of antibiotic susceptibility profiles and lytic bacteriophages. MicrobiologyOpen 2019, 8, e784. [Google Scholar] [CrossRef] [PubMed]
- Marotta, F.; Garofolo, G.; Di Donato, G.; Aprea, G.; Platone, I.; Cianciavicchia, S.; Alessiani, A.; Di Giannatale, E. Population diversity of Campylobacter jejuni in poultry and its dynamic of contamination in chicken meat. Biomed Res. Int. 2015, 2015, 859845. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Connerton, P.L.; Dodd, C.E.; Rees, C.E.; Connerton, I.F. Isolation and characterization of Campylobacter bacteriophages from retail poultry. Appl. Environ. Microbiol. 2003, 69, 4511–4518. [Google Scholar] [CrossRef] [PubMed]
- Firlieyanti, A.S.; Connerton, P.L.; Connerton, I.F. Campylobacters and their bacteriophages from chicken liver: The prospect for phage biocontrol. Int. J. Food Microbiol. 2016, 237, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Connerton, P.L.; Connerton, I.F. Phage Biocontrol of Campylobacter jejuni in Chickens Does Not Produce Collateral Effects on the Gut Microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Jäckel, C.; Hammerl, J.A.; Hertwig, S. Campylobacter Phage Isolation and Characterization: What We Have Learned So Far. Methods Protoc. 2019, 2, 18. [Google Scholar] [CrossRef]
- Gast, R.K. Salmonella Infections. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 677–736. [Google Scholar]
- Berchieri, A.; Lovell, M.A.; Barrow, P.A. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 1991, 142, 541–549. [Google Scholar] [CrossRef]
- Bardina, C.; Spricigo, D.A.; Cortés, P.; Llagostera, M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012, 78, 6600–6607. [Google Scholar] [CrossRef]
- Hong, S.S.; Jeong, J.; Lee, J.; Kim, S.; Min, W.; Myung, H. Therapeutic effects of bacteriophages against Salmonella gallinarum infection in chickens. J. Microbiol. Biotechnol. 2013, 23, 1478–1483. [Google Scholar] [CrossRef]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, T.; Chae, S.J.; Kim, J.H.; Kang, Y.H.; Chung, G.T.; Kim, D.W.; Lee, D.Y. Complete Genome Sequence of Bacteriophage MA12, Which Infects both Campylobacter jejuni and Salmonella enterica Serovar Enteritidis. Genome Announc. 2016, 4, e00810–e00816. [Google Scholar] [CrossRef]
- Kim, J.-W.; Cho, Y.-W.; Im, H.-J.; Shin, E.-M.; Seo, H.-S.; Bae, G.-D.; Son, B.-K.; Yang, S.-Y. Bacteriophages: The Alternatives to Antibiotics for Animal Feeds. In International Symposium: Alternatives to Antibiotisc (ATA) Challenges and Solutions in Animal Production; OIE: Paris, France, 2012. Available online: https://www.ars.usda.gov/alternativestoantibiotics/PDF/IABS%20Abstracts%20Book.pdf (accessed on 7 April 2020).
- Nolan, L.K.; Barnes, H.J.; Vaillancourt, J.-P.; Abdul-Aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 751–805. [Google Scholar]
- Barrow, P.; Lovell, M.; Berchieri, A., Jr. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 1998, 5, 294–298. [Google Scholar] [CrossRef]
- Xie, H.; Zhuang, X.; Kong, J.; Ma, G.; Zhang, H. Bacteriophage Esc-A is an efficient therapy for Escherichia coli 3-1 caused diarrhea in chickens. J. Gen. Appl. Microbiol. 2005, 51, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, M.M.; Nabil, N.M.; Samy, A. Evaluation of bacteriophage efficacy in reducing the impact of single and mixed infections with Escherichia coli and infectious bronchitis in chickens. Infect. Ecol. Epidemiol. 2019, 9, 1686822. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.B. Staphylococcosis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 971–977. [Google Scholar]
- Feßler, A.T.; Kadlec, K.; Hassel, M.; Hauschild, T.; Eidam, C.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Food and Food Products of Poultry Origin in Germany. Appl. Environ. Microbiol. 2011, 77, 7151–7157. [Google Scholar] [CrossRef]
- Leskinen, K.; Tuomala, H.; Wicklund, A.; Horsma-Heikkinen, J.; Kuusela, P.; Skurnik, M.; Kiljunen, S. Characterization of vB_SauM-fRuSau02, a Twort-Like Bacteriophage Isolated from a Therapeutic Phage Cocktail. Viruses 2017, 9, 258. [Google Scholar] [CrossRef]
- Marek, A.; Pyzik, E.; Stępień-Pyśniak, D.; Urban-Chmiel, R.; Nowaczek, A. Characterization of bacteriophages and their carriage in Staphylococcus aureus isolated from broilers in Poland. Br. Poult. Sci. 2019, 60, 373–380. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef]
- Smith, H.W. The bacteriophages of Clostridium perfringens. J. Gen. Microbiol. 1959, 21, 622–630. [Google Scholar] [CrossRef]
- Seal, B.S. Characterization of bacteriophages virulent for Clostridium perfringens and identification of phage lytic enzymes as alternatives to antibiotics for potential control of the bacterium. Poult. Sci. 2013, 92, 526–533. [Google Scholar] [CrossRef]
- Zimmer, M.; Vukov, N.; Scherer, S.; Loessner, M.J. The murein hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl. Environ. Microbiol. 2002, 68, 5311–5317. [Google Scholar] [CrossRef]
- Nariya, H.; Miyata, S.; Tamai, E.; Sekiya, H.; Maki, J.; Okabe, A. Identification and characterization of a putative endolysin encoded by episomal phage phiSM101 of Clostridium perfringens. Appl. Microbiol. Biotechnol. 2011, 90, 1973–1979. [Google Scholar] [CrossRef]
- Gervasi, T.; Horn, N.; Wegmann, U.; Dugo, G.; Narbad, A.; Mayer, M.J. Expression and delivery of an endolysin to combat Clostridium perfringens. Appl. Microbiol. Biotechnol. 2014, 98, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W.; Skinner, J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage therapy for control of necrotic enteritis of broilerchickens experimentally infected with Clostridium perfringens. Avian Dis. 2010, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Kim, M.G.; Kwon, M.; Lee, H.S.; Kim, G.B. Inhibition of Clostridium perfringens using Bacteriophages and Bacteriocin Producing Strains. Korean J. Food Sci. Anim. Resour. 2018, 38, 88–98. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Bigot, B.; Lee, W.J.; McIntyre, L.; Wilson, T.; Hudson, J.A.; Billington, C.; Heinemann, J.A. Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiol. 2011, 28, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.J.; Jung, S.J.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Characterization of Salmonella spp.-specific bacteriophages and their biocontrol application in chicken breast meat. J. Food Sci. 2020, 85, 526–534. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult. Sci. 2016, 95, 668–675. [Google Scholar] [CrossRef]
- Garcia, K.C.O.D.; Corrêa, I.M.O.; Pereira, L.Q.; Silva, T.M.; Mioni, M.S.R.; Izidoro, A.C.M.; Bastos, I.H.V.; Gonçalves, G.A.M.; Okamoto, A.S.; Andreatti Filho, R.L. Bacteriophage use to control Salmonella biofilm on surfaces present in chicken slaughterhouses. Poult Sci. 2017, 96, 3392–3398. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Connerton, P.L.; Dodd, C.E.; Rees, C.E.; Connerton, I.F. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 2003, 69, 6302–6306. [Google Scholar] [CrossRef]
- Hungaro, H.M.; Mendonça, R.C.S.; Gouvêa, D.M.; Vanetti, M.C.D.; Pinto, C.L.D. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res. Int. 2013, 52, 75–81. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Huff, W.E.; Huff, G.R.; Rath, N.C.; Zhou, Z.Y.; Donoghue, A.M. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens. Poult Sci. 2014, 93, 2788–2792. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ): Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2009 update). EFSA J. 2009, 7, 1–92.
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage. 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Donoghue, A.M. Immune interference of bacteriophage efficacy when treating colibacillosis in poultry. Poult. Sci. 2010, 89, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Zaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef]
- Cisek, A.A.; Dąbrowska, I.; Gregorczyk, K.P.; Wyżewski, Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years After the Discovery of Bacteriophages. Curr. Microbiol. 2017, 74, 277–283. [Google Scholar] [CrossRef]
- Majewska, J.; Kaźmierczak, Z.; Lahutta, K.; Lecion, D.; Szymczak, A.; Miernikiewicz, P.; Drapała, J.; Harhala, M.; Marek-Bukowiec, K.; Jędruchniewicz, N.; et al. Induction of Phage-Specific Antibodies by Two Therapeutic Staphylococcal Bacteriophages Administered per os. Front. Immunol. 2019, 10, 2607. [Google Scholar] [CrossRef]
- Bruttin, A.; Brüssow, H. Human volunteers receiving Escherichia coli phage T4 orally: A safety test of phage therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef]
- Żaczek, M.; Łusiak-Szelachowska, M.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Owczarek, B.; Kopciuch, A.; Fortuna, W.; Rogóż, P.; Górski, A. Antibody Production in Response to Staphylococcal MS-1 Phage Cocktail in Patients Undergoing Phage Therapy. Front. Microbiol. 2016, 7, 1681. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Karimi Torshizi, M.A.; Rahimi, S.; Engberg, R.M.; Sørensen Dalgaard, T. Effect of serum anti-phage activity on colibacillosis control by repeated phage therapy in broilers. Vet. Microbiol. 2019, 234, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Choińska-Pulita, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
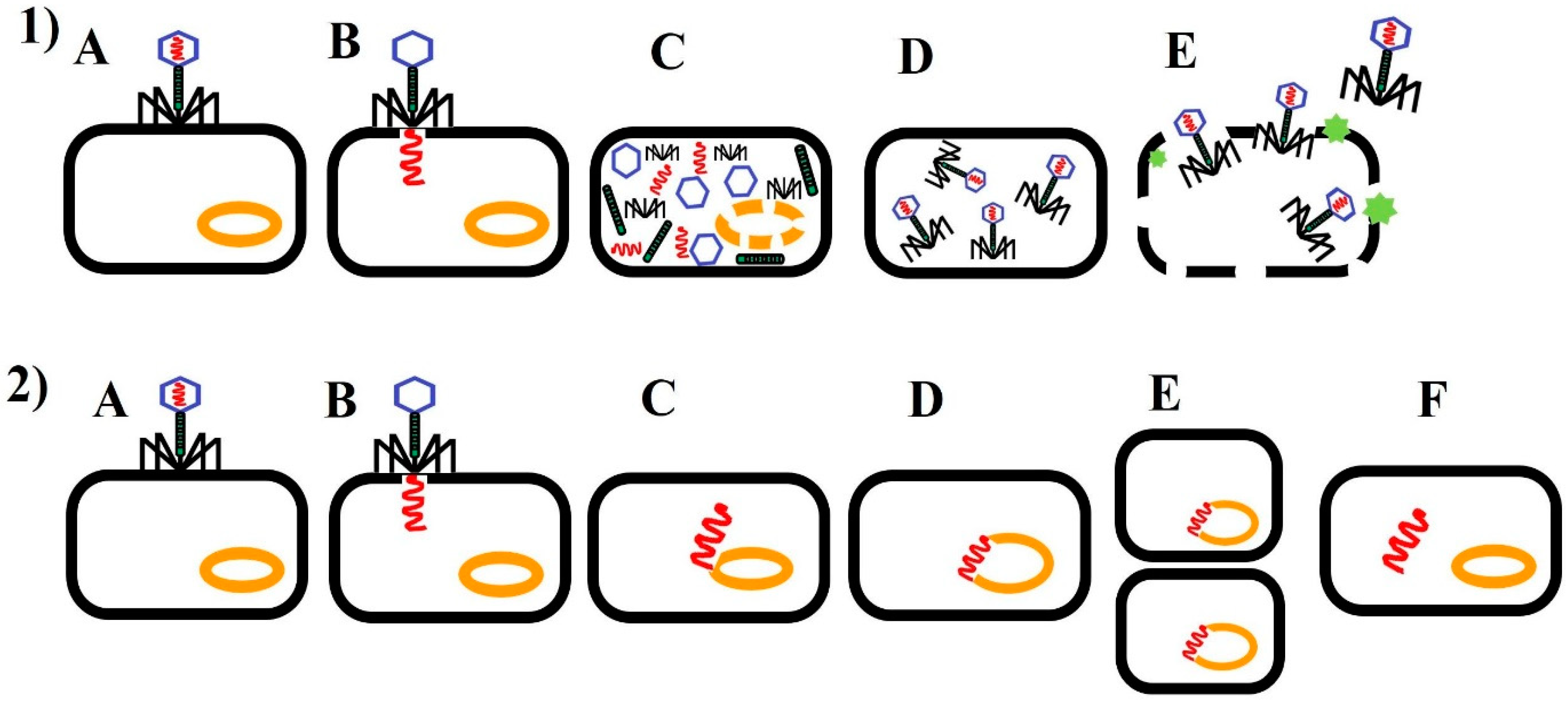
| Target Bacteria | Product Name | Manufacturer | Bacteriophages | Notes | Ref. |
|---|---|---|---|---|---|
| Salmonella | Bafasal® | Proteon Pharmaceuticals (Łódź, Poland) | 3 phages: 3ent1, 8sent65 and 8sent1748, mixed in equal concentration |
| [19,20] |
| Salmonella Gallinarum, Salmonella Pullorum | Biotector® S | CJ CheilJedang Research Institute of Biotechnology (Seoul, South Korea) | nd |
| [21] |
| Salmonella enterica | SalmoFresh™ | Intralytix Inc. (Columbia, MD, USA) | 6 lytic phages |
| [15,21] |
| Salmonella enterica | SalmoPro® | Phagelux (Montreal, QC, Canada) | 2 phages: BP-63, BP-12 |
| [15,21,22] |
| Salmonella | Salmonelex™ (PhageGuard) | Micreos Food Safety BV (The Netherlands) | 2 phages |
| [23] |
| Salmonella | PhageGuard STM | Micreos Food Safety BV (Wageningen, The Netherlands) | 2 phages: Fo1a and S16 |
| [15,24] |
| Salmonella | BacWashTM | OmniLytics Inc. (Sandy, UT, USA) | nd |
| [25] |
| Salmonella | SalmoFREE® | Sciphage (Bogotá, Colombia) | 6 lytic phages |
| [26,27] |
| Escherichia coli O157:H7 | EcoShieldTM | Intralytix Inc. (Columbia, MD, USA) | 3 lytic phages: ECML-4, ECML-117, ECML-134 in the Myoviridae family isolated from the environment 1010 PFU/mL in PBS, pH 7.4 |
| [15,28] |
| Escherichia coli O157:H7 | Ecolicide PX™ | Intralytix Inc. (Columbia, MD, USA) | nd |
| [21] |
| Listeria monocytogenes | ListShieldTM | Intralytix Inc. (Columbia, MD, USA) | 6 phages: LIST-36, LMSP-25, LMTA-34, LMTA-57, LMTA-94, LMTA-148 |
| [15,28,29] |
| Listeria monocytogenes | Listex™ P100 (PhageGuard) | Micreos Food Safety BV. (Wageningen The Netherlands) | Phage P100 |
| [15,25,30,31,32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. https://doi.org/10.3390/ani10050872
Żbikowska K, Michalczuk M, Dolka B. The Use of Bacteriophages in the Poultry Industry. Animals. 2020; 10(5):872. https://doi.org/10.3390/ani10050872
Chicago/Turabian StyleŻbikowska, Katarzyna, Monika Michalczuk, and Beata Dolka. 2020. "The Use of Bacteriophages in the Poultry Industry" Animals 10, no. 5: 872. https://doi.org/10.3390/ani10050872
APA StyleŻbikowska, K., Michalczuk, M., & Dolka, B. (2020). The Use of Bacteriophages in the Poultry Industry. Animals, 10(5), 872. https://doi.org/10.3390/ani10050872





