Same Space, Different Species: The Influence of Exhibit Design on the Expression of Zoo-Housed Apes’ Species-Typical Retiring Behaviors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
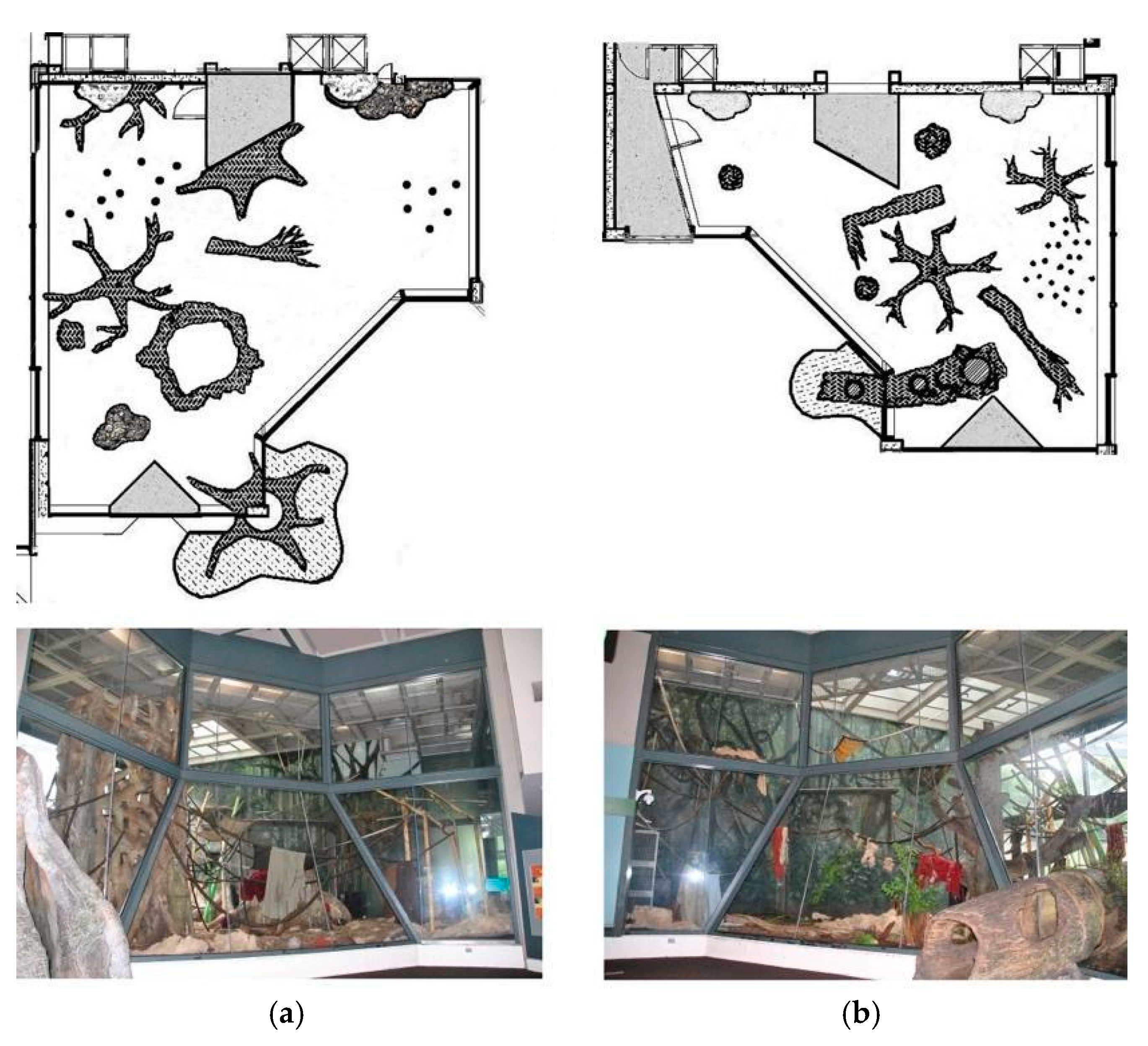
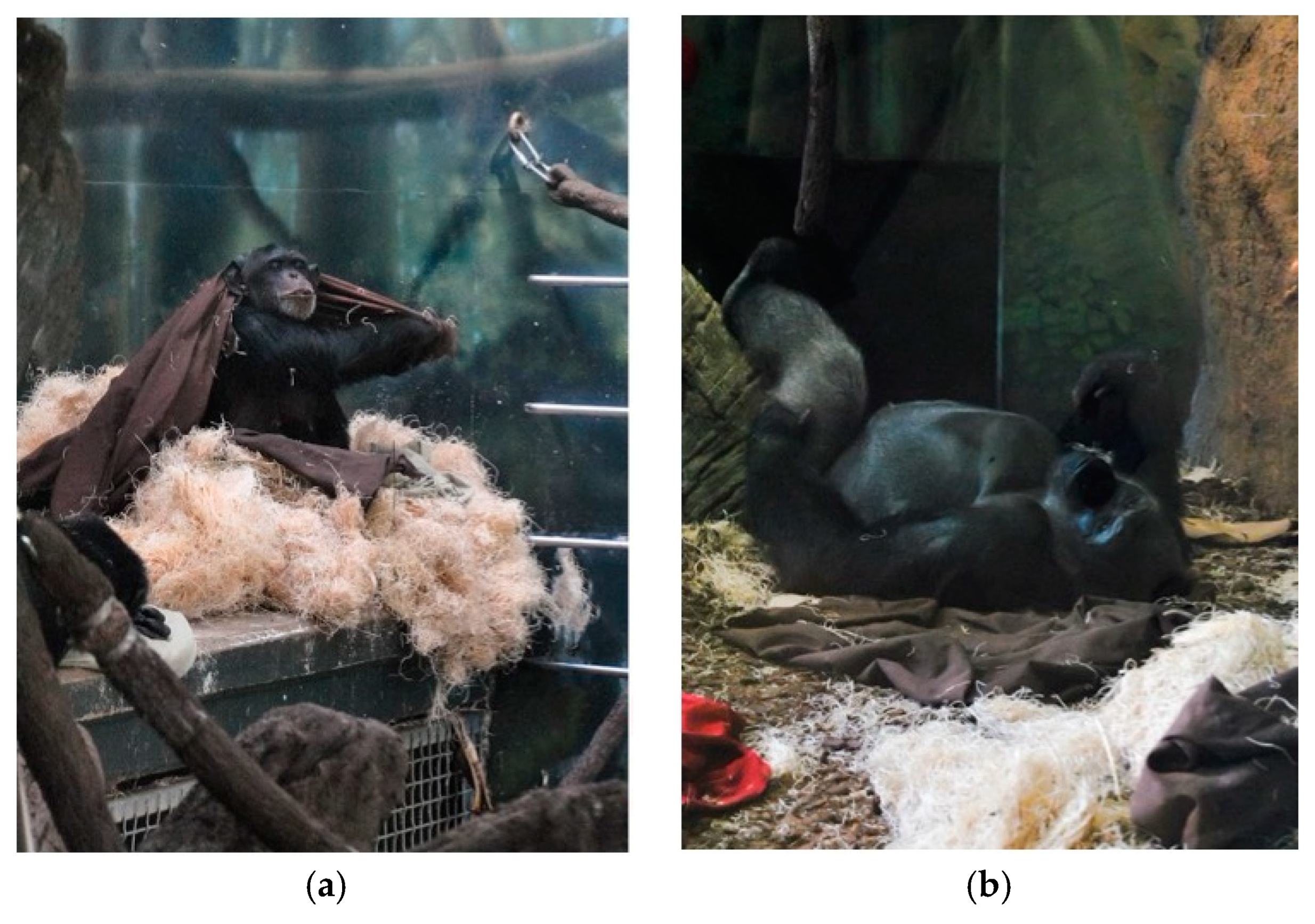
2.1. Subjects and Housing
2.2. Protocol
2.3. Data Collection
2.4. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doran, D. Influence of Seasonality on Activity Patterns, Feeding Behavior, Ranging, and Grouping Patterns in Tai Chimpanzees. Int. J. Primatol. 1997, 18, 183–206. [Google Scholar] [CrossRef]
- Masi, S.; Cipolletta, C.; Robbins, M.M. Western Lowland Gorillas (Gorilla gorilla gorilla) Change Their Activity Patterns in Response to Frugivory. Am. J. Primatol. 2009, 71, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Preutz, J.D.E.; McGrew, W.C. What Does a Chimpanzee Need? Using Natural Behavior to Guide the Care and Management of Captive Populations. In Special Topics in Primatology, Volume 2: The Care and Management of Captive Chimpanzees; Brent, L., Ed.; American Society of Primatologists: San Antonio, TX, USA, 2001; pp. 17–37. [Google Scholar]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 approach to promoting optimal welfare for captive wild animals. Behav. Process. 2018, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.J.; Sabater Pí, J.; McGrew, W.C.; Tutin, C.E.G. Comparisons of Nests Made by Different Populations of Chimpanzees (Pan troglodytes). Primates 1981, 22, 474–486. [Google Scholar] [CrossRef]
- Goodall, J.M. Nest building behavior in the free ranging chimpanzee. Ann. N. Y. Acad. Sci. 1962, 102, 455–467. [Google Scholar] [CrossRef]
- Hernandez-Aguilar, R.A.; Moore, J.I.M.; Stanford, C.B. Chimpanzee Nesting Patterns in Savanna Habitat: Environmental Influences and Preferences. Am. J. Primatol. 2013, 75, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Sanz, C.; Morgan, D.; Strindberg, S.; Onononga, J.R. Distinguishing between the nests of sympatric chimpanzees and gorillas. J. Appl. Ecol. 2007, 44, 263–272. [Google Scholar] [CrossRef]
- Koops, K.; Humle, T.; Sterck, E.H.M.; Matsuzawa, T. Ground-Nesting by the Chimpanzees of the Nimba Mountains, Guinea: Environmentally or Socially Determined? Am. J. Primatol. 2007, 69, 407–419. [Google Scholar] [CrossRef]
- Stewart, F.A.; Pruetz, J.D. Do Chimpanzee Nests Serve an Anti-Predatory Function? Am. J. Primatol. 2013, 75, 593–604. [Google Scholar] [CrossRef]
- Boesch, C. Innovation in Wild Chimpanzee (Pan troglodytes). Int. J. Primatol. 1995, 16, 1–16. [Google Scholar] [CrossRef]
- Remis, M.J. Nesting Behavior of Lowland Gorillas in the Dzanga-Sangha Reserve, Central African Republic: Implications for Population Estimates and Understandings of Group Dynamics. Tropics 1993, 2, 245–255. [Google Scholar] [CrossRef]
- Tutin, C.E.G.; Parnell, R.J.; White, L.J.T.; Fernandez, M. Nest Building by Lowland Gorillas in the Lopé Reserve, Gabon: Environmental Influences and Implications for Censusing. Int. J. Primatol. 1995, 16, 53–76. [Google Scholar] [CrossRef]
- Anderson, J.R.; Ang, M.Y.L.; Lock, L.C.; Weiche, I. Nesting, sleeping, and nighttime behaviors in wild and captive great apes. Primates 2019, 60, 321–332. [Google Scholar] [CrossRef]
- Lukas, K.E.; Stoinski, T.S.; Burks, K.; Snyder, R.; Bexell, S.; Maple, T.L. Nest Building in Captive Gorilla gorilla gorilla. Int. J. Primatol. 2003, 24, 103–124. [Google Scholar] [CrossRef]
- Riss, D.; Goodall, J. Sleeping Behavior and Associations in a Group of Captive Chimpanzees. Folia Primatol. 1976, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Videan, E.N. Sleep in captive chimpanzee (Pan troglodytes): The effects of individual and environmental factors on sleep duration and quality. Behav. Brain Res. 2006, 169, 187–192. [Google Scholar] [CrossRef]
- Ross, S.R.; Wagner, K.E.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Transfer and acclimatization effects on the behavior of two species of African great ape (Pan troglodytes and Gorilla gorilla gorilla) moved to a novel and naturalistic zoo environment. Int. J. Primatol. 2011, 32, 99–117. [Google Scholar] [CrossRef]
- Fowler, K.J.; Hopper, L.M.; Ross, S.R.; Leahy, M.; Santymire, R.M. Monitoring the behavior and stress physiology of male gorillas (Gorilla gorilla gorilla) during a bachelor group formation. Zoo Biol. (in review).
- Ross, M.R.; Niemann, T.; Wark, J.D.; Heintz, M.R.; Horrigan, A.; Cronin, K.A.; Shender, M.A.; Gillespie, K. ZooMonitor (Version 1) [Mobile Application Software]. 2016. Available online: https://zoomonitor.org (accessed on 1 January 2020).
- Ross, S.R.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Space use as an indicator of enclosure appropriateness: A novel measure of captive animal welfare. Appl. Anim. Behav. Sci. 2009, 121, 42–50. [Google Scholar] [CrossRef]
- Nichols, J.; Kwiatt, A.C.; Ross, S.R.; Hopper, L.M. False Dusk But Not Visitor Numbers Influence Evening Retirement Behaviors In Zoo-Housed Chimpanzees (Pan troglodytes). In Proceedings of the 40th Annual Meeting of the American Society of Primatologistsm, WILEY, Washington, DC, USA, 27 August 2017. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Remis, M.J. Effects of Body Size and Social Context on the Arboreal Activities of Lowland Gorillas in the Central African Republic. Am. J. Phys. Anthropol. 1995, 97, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Remis, M.J. Tree Structure and Sex Differences in Arboreality among Western Lowland Gorillas (Gorilla gorilla gorilla) at Bai Hokou, Central African Republic. Primates 1999, 40, 383–396. [Google Scholar] [CrossRef]
- Pruetz, J.D.; Fulton, S.J.; Marchant, L.F.; McGrew, W.C.; Schiel, M.; Waller, M. Arboreal nesting as anti-predator adaptation by savanna chimpanzees (Pan troglodytes verus) in southeastern Senegal. Am. J. Primatol. 2008, 70, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Aguilar, R.A. Chimpanzee nest distribution and site reuse in a dry habitat: Implications for early hominin ranging. J. Hum. Evol. 2009, 57, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Koops, K.; McGrew, W.C.; de Vries, H.; Matsuzawa, T. Nest-Building by Chimpanzees (Pan troglodytes verus) at Seringbara, Nimba Mountains: Antipredation, Thermoregulation, and Antivector Hypotheses. Int. J. Primatol. 2012, 33, 356–380. [Google Scholar] [CrossRef]
- Caine, N.G.; Potter, M.P.; Mayer, E. Sleeping Site Selection by Captive Tamarins (Saguinus. labiatus). Ethology 1992, 90, 63–71. [Google Scholar] [CrossRef]

| Behavior | Definition |
|---|---|
| Inactive | Focal animal is not moving and not active in any other behavior listed but is not asleep. Behavior includes instances during which a subject holds or carries an object (including food or water), without actively manipulating it (including chewing or swallowing). If the state of wakefulness cannot be determined, the behavior is categorized as “inactive”. |
| Sleeping | Focal animal is not moving, is not alert, is in a prone position, has eyes closed, and appears to be asleep. |
| Nest Building | Focal animal manipulates materials in the act of constructing or modifying a nest site. Behavior is limited to modification or immediate construction of the nest itself and does not include gathering and carrying materials with which to nest. |
| Factors Included in Model | AIC | Model Rank |
|---|---|---|
| Exhibit | 201.8 | 1 |
| Exhibit × Species | 203.0 | 2 |
| Species | 224.3 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Earl, S.C.; Hopper, L.M.; Ross, S.R. Same Space, Different Species: The Influence of Exhibit Design on the Expression of Zoo-Housed Apes’ Species-Typical Retiring Behaviors. Animals 2020, 10, 836. https://doi.org/10.3390/ani10050836
Earl SC, Hopper LM, Ross SR. Same Space, Different Species: The Influence of Exhibit Design on the Expression of Zoo-Housed Apes’ Species-Typical Retiring Behaviors. Animals. 2020; 10(5):836. https://doi.org/10.3390/ani10050836
Chicago/Turabian StyleEarl, Samantha C., Lydia M. Hopper, and Stephen R. Ross. 2020. "Same Space, Different Species: The Influence of Exhibit Design on the Expression of Zoo-Housed Apes’ Species-Typical Retiring Behaviors" Animals 10, no. 5: 836. https://doi.org/10.3390/ani10050836
APA StyleEarl, S. C., Hopper, L. M., & Ross, S. R. (2020). Same Space, Different Species: The Influence of Exhibit Design on the Expression of Zoo-Housed Apes’ Species-Typical Retiring Behaviors. Animals, 10(5), 836. https://doi.org/10.3390/ani10050836





