Understanding Temporal Social Dynamics in Zoo Animal Management: An Elephant Case Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Subjects and Study Sites
2.3. Data Collection
2.4. Social Interactions
2.5. Data Analysis
2.6. Frequency of Social Interactions Given by Individual Elephants
2.7. Fluidity in Herd Relationships Over Time and Dyadic Reciprocity
2.8. Identification of Key Individuals in Social Networks
3. Results
3.1. Change Over Time
3.2. Reciprocity in Dyads
3.3. Herd Social Matrices and Identification of Key Individuals
4. Discussion
4.1. Dyadic Relationships and Herd Hierarchies
4.2. Temporal Changes in Social Interactions
4.3. Recommendations and Areas for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Massen, J.J.M.; Sterck, E.H.M.; de Vos, H. Close social associations in animals and humans: Functions and mechanisms of friendship. Behaviour 2010, 147, 1379–1412. [Google Scholar] [CrossRef]
- Silk, J.B.; Cheney, D.; Seyfarth, R. A practical guide to the study of social relationships. Evol. Anthr. 2013, 22, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte-Saller, M.; Mench, J.A. 2018. Assessing the dyadic social relationships of female African (Loxodonta africana) and Asian (Elephas maximus) zoo elephants using proximity, tactile contact, and keeper surveys. Appl. Anim. Behav. Sci. 2018, 199, 45–51. [Google Scholar] [CrossRef]
- Yasui, S.; Idani, G. Social significance of trunk use in captive Asian elephants. Ethol. Ecol. Evol. 2017, 29, 330–350. [Google Scholar] [CrossRef]
- Cooper, M.A.; Bernstein, I.S. Social grooming in assamese macaques (Macaca assamensis). Am. J. Primatol. 2000, 50, 77–85. [Google Scholar] [CrossRef]
- Dudzinski, K.M.; Ribic, C.A. Pectoral fin contact as a mechanism for social bonding among dolphins. Anim. Cogn. 2017, 4, 30–48. [Google Scholar] [CrossRef]
- Williams, E.; Bremner-Harrison, S.; Ward, S.J. Can we meet the needs of social species in zoos? An overview of the impact of group housing on welfare in socially housed mammals. In Zoo Animals: Husbandry, Welfare and Public Interactions; Berger, M., Corbett, S., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2018. [Google Scholar]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 approach to promoting optimal welfare for captive wild animals. Behav. Process. 2018, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.C.; Glasper, E.R.; Detillion, C.E. Social modulation of stress responses. Physiol. Behav. 2003, 79, 399–407. [Google Scholar] [CrossRef]
- Beisner, B.A.; Jin, J.; Fushing, H.; Mccowan, B. Detection of social group instability among captive rhesus macaques using joint network modelling. Curr. Zool. 2015, 61, 70–84. [Google Scholar] [CrossRef]
- Christensen, J.W.; Sondergaard, E.; Thodberg, K.; Halekoh, U. Effects of repeated regrouping on horse behaviour and injuries. Appl. Anim. Behav. Sci. 2011, 133, 199–206. [Google Scholar] [CrossRef]
- Guttman, A.K.; Spinka, M.; Winckler, C. Long-term familiarity creates preferred social partners in dairy cattle. Appl. Anim. Behav. Sci. 2015, 169, 1–8. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Mattiello, S. The importance of social behaviour for goat welfare in livestock farming. Small Eumin. Res. 2010, 90, 1–10. [Google Scholar] [CrossRef]
- Snijders, L.; Blumstein, D.T.; Stanley, C.R.; Franks, D.W. Animal Social Network Theory Can Help Wildlife Conservation. Trends Ecol. Evol. 2017, 32, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.J.; Poole, J.H. Relationships and social structure in African elephants. In Primate Social Relationships: An Integrated Approach; Hinde, R.A., Ed.; Blackwell Scientific Publications: Oxford, UK, 1983. [Google Scholar]
- Archie, E.A.; Moss, C.J.; Alberts, S.C. The ties that bind: Genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc. B Biol. Sci. 2006, 273, 513–522. [Google Scholar] [CrossRef]
- De Silva, S.; Wittemyer, G. A Comparison of Social Organization in Asian Elephants and African Savannah Elephants. Int. J. Primatol. 2012, 33, 1125–1141. [Google Scholar] [CrossRef]
- Fishlock, V.; Lee, P.C. Forest elephants: Fission-fusion and social arenas. Anim. Behav. 2013, 85, 357–363. [Google Scholar] [CrossRef]
- Meehan, C.L.; Hogan, J.N.; Bonaparte-Saller, M.; Mench, J.A. Housing and social environments of African (Loxodonta africana) and Asian (Elephas maximus) elephants in North American Zoos. PLoS ONE 2016, 11, e0146703. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G.M. A Review of the Welfare of Zoo Elephants in Europe; A Report Commissioned by the RSPCA; University of Oxford: Oxford, UK, 2002. [Google Scholar]
- Harris, M.; Sherwin, C.; Harris, S. The Welfare, Housing and Husbandry of Elephants in UK Zoos; Defra: Bristol, UK, 2008. [Google Scholar]
- Wittemyer, G.; Douglas-Hamilton, I.; Getz, W.M. The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim. Behav. 2005, 69, 1357–1371. [Google Scholar] [CrossRef]
- Vance, E.A.; Archie, E.A.; Moss, C.J. Social networks in African elephants. Comput. Math. Organ. Theory 2009, 15, 273–293. [Google Scholar] [CrossRef]
- Kaczensky, P.; Ganbaatar, O.; von Wehrden, H.; Walzer, C. Resource selection by sympatric wild equids in the Mongolian Gobi. J. Appl. Ecol. 2008, 45, 1762–1769. [Google Scholar] [CrossRef]
- Carter, K.D.; Seddon, J.M.; Frere, C.H.; Carter, J.K.; Goldizen, A.W. Fission-fusion dynamics in wild giraffes may be driven by kinship, spatial overlap and individual social preferences. Anim. Behav. 2013, 85, 385–394. [Google Scholar] [CrossRef]
- De Silva, S.; Ranjeewa, A.D.G.; Kryazhimskiy, S. The dynamics of social networks among female Asian elephants. BMC Ecol. 2011, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Defra. Secretary of State’s Standards of Modern Zoo Practice Appendix 8—Specialist Exhibits, Elephants; Defra: London, UK, 2017. [Google Scholar]
- Veasey, J. Concepts in the care and welfare of captive elephants. Int. Zoo Yearb. 2006, 40, 63–79. [Google Scholar] [CrossRef]
- Koontz, F.S.; Roush, R.S. Communication and social behaviour. In Wild Mammals in Captivity: Principles and Techniques; Kleiman, D.G., Allen, M.E., Thompson, K.V., Lumpkin, S., Eds.; The University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Sach, F.; Tatchley, C.; Needham, N.; Pullen, K. Guidelines for the Management of Elephants within BIAZA zoos: Incorporating BIAZA’s policy on the Management of Elephants; Biaza: London, UK, 2019. [Google Scholar]
- Williams, E.; Carter, A.; Hall, C.; Bremner-Harrison, S. Social interactions in zoo-housed elephants: Factors affecting social relationships. Animals 2019, 9, e747. [Google Scholar] [CrossRef]
- Garai, M.E. Special Relationships between Female Asian Elephants (Elephas maximus) in Zoological Gardens. Ethology 1992, 90, 187–205. [Google Scholar] [CrossRef]
- Asher, L.; Williams, E.; Yon, L. Developing Behavioural Indicators, as Part of a Wider set of Indicators, to Assess the Welfare of Elephants in UK Zoos; Defra Project WC1081; Defra: London, UK, 2015. [Google Scholar]
- Borgatti, S.P.; Everett, M.G.; Freeman, L.C. Ucinet 6 for Windows: Software for Social Network Analysis; Analytic Technologies: Harvard, MA, USA, 2002. [Google Scholar]
- Borgatti, S.P. NetDraw Software for Network Visualisation; Analytic Technologies: Lexington, KY, USA, 2000. [Google Scholar]
- Wey, T.W.; Blumstein, D.T.; Shen, W.; Jordan, F. Social network analysis of animal behaviour: A promising tool for the study of sociality. Anim. Behav. 2008, 75, 333–344. [Google Scholar] [CrossRef]
- Lutz, C.; Well, A.; Novak, M. Stereotypic and self-injurious behaviour in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am. J. Primatol. 2003, 60, 1–15. [Google Scholar] [CrossRef]
- Rommeck, I.; Anderson, K.; Heagerty, A.; Cameron, A.N.; McCowan, B. Risk factors and remediation of self-injurous and self-abuse behaviour in rhesus macaques. J. Appl. Anim. Welf. Sci. 2009, 12, 61–72. [Google Scholar] [CrossRef]
- Inglett, B.J.; French, J.A.; Simmons, L.G.; Vires, K.W. Dynamics of intrafamily aggression and social reintegration in lion tamarins. Zoo Biol. 1989, 8, 67–78. [Google Scholar] [CrossRef]
- Waples, K.A.; Gales, N.J. Evaluating and minimising social stress in the care of captive bottlenose dolphins (Tursiops aduncus). Zoo Biol. 2002, 21, 5–26. [Google Scholar] [CrossRef]
- Schel, A.M.; Rawlings, B.; Claidiere, N.; Wilke, C.; Wathan, J.; Richardson, J.; Pearson, S.; Herrelko, E.S.; Whiten, A.; Slocombe, K. Network analysis of social changes in a captive chimpanzee community following the successful integration of two adult groups. Am. J. Primatol 2013, 75, 254–266. [Google Scholar] [CrossRef]
- Martin-Wintle, M.S.; Shepherdson, D.; Zhang, G.; Zhang, H.; Li, D.; Zhou, X.; Li, R.; Swaisgood, R.R. Free mate choice enhances conservation breeding in the endangered giant panda. Nat. Commun. 2017, 6, 10125. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, C.L.; Williams, E.; Asher, L.; Yon, L. Incorporating stakeholder perspectives into the assessment and provision of captive elephant welfare. Anim. Welf. 2017, 26, 461–472. [Google Scholar] [CrossRef]
- Harvey, N.; Daly, C.; Clark, N.; Ransford, E.; Wallace, S.; Yon, L. Social Interactions in Two Groups of Zoo-Housed Adult Female Asian Elephants (Elephas maximus) that Differ in Relatedness. Animals 2018, 8, e132. [Google Scholar] [CrossRef] [PubMed]
- Archie, E.A.; Morrison, T.A.; Foley, C.A.H.; Moss, C.J.; Alberts, S.C. Dominance rank relationships among wild African elephants, Loxodonta africana. Anim. Behav. 2006, 71, 117–127. [Google Scholar] [CrossRef]
- de Silva, S.; Schmid, V.; Wittemyer, G. Fission-fusion processes weaken dominance networks of female Asian elephants in a productive habitat. Behav. Ecol. 2017, 28, 243–252. [Google Scholar] [CrossRef]
- Farine, D.R.; Whitehead, H. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 2015, 84, 1144–1163. [Google Scholar] [CrossRef]
- Moreno, K.; Acevedo-Gutierrez, A. The social structure of Golfo Dulce bottlenose dolphins (Tursiops truncatus) and the influence of behavioural state. R. Soc. Open Sci. 2016, 3, 160010. [Google Scholar] [CrossRef]
- Sundaresan, S.R.; Fischhoff, I.R.; Dushoff, J.; Rubenstein, D.I. Network metrics reveal differences in social organization between two fission-fusion species, Grevy’s zebra and onager. Oecologia 2007, 151, 140–149. [Google Scholar] [CrossRef]
- Pinter-Wollman, N.; Isbell, L.A.; Hart, L.A. The relationship between social behaviour and habitat familiarity in African elephants (Loxodonta africana). Proc. R. Soc. B Biol. Sci. 2009, 276, 1009–1014. [Google Scholar] [CrossRef]
- Nandini, S.; Keerthipriya, P.; Vidya, T.N.C. Seasonal variation in female Asian elephant social structre in Nagarahole-Bandipur, southern India. Anim. Behav. 2017, 134, 135–145. [Google Scholar] [CrossRef]
- Murph, D.; Mumby, H.S.; Henley, M.D. Age differences in the temporal stability of a male African elephant (Loxodonta africana) social network. Behav. Ecol. 2020, 31, 21–31. [Google Scholar] [CrossRef]
- Elzanowski, A.; Sergiel, A. Stereotypic behaviour of a female Asiatic elephant (Elephas maximus) in a zoo. J. Appl Anim. Welf. Sci. 2006, 9, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Krebs, B.L.; Marrin, D.; Phelps, A.; Krol, L.; Watters, J. Managed aged animals in zoos to promote positive welfare: A review and future directions. Animals 2018, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Williams, E. An Investigation into Social Relationships and Social Structure in UK and Irish Zoo Elephant Herds. Ph.D. Thesis, Nottingham Trent University, Nottingham, UK, 2019. [Google Scholar]
- Yon, L.; Williams, E.; Harvey, N.D.; Asher, L. Development of a behavioural welfare assessment tool for routine use with captive elephants. PLoS ONE 2019, 14, e0210783. [Google Scholar] [CrossRef]
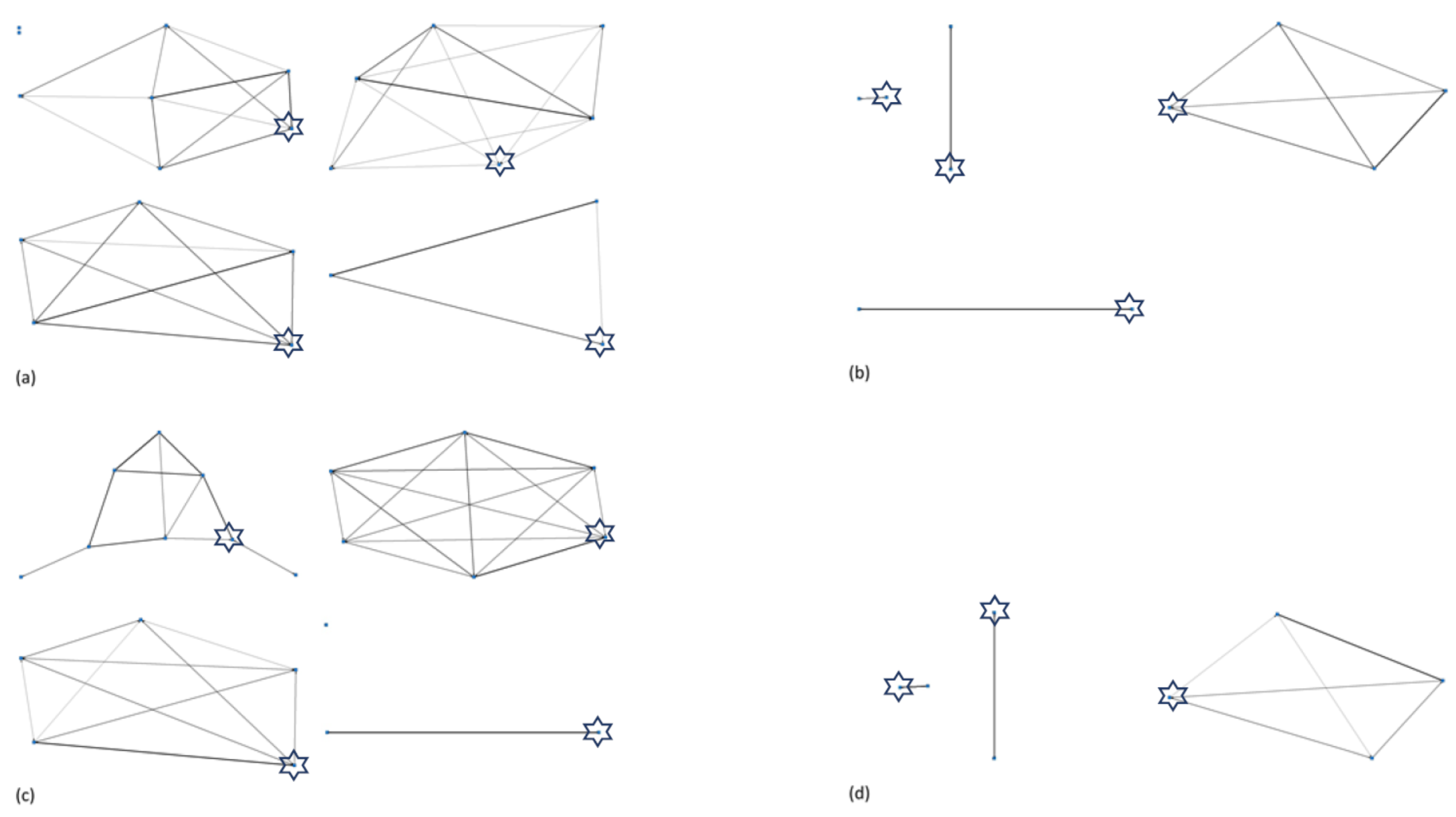
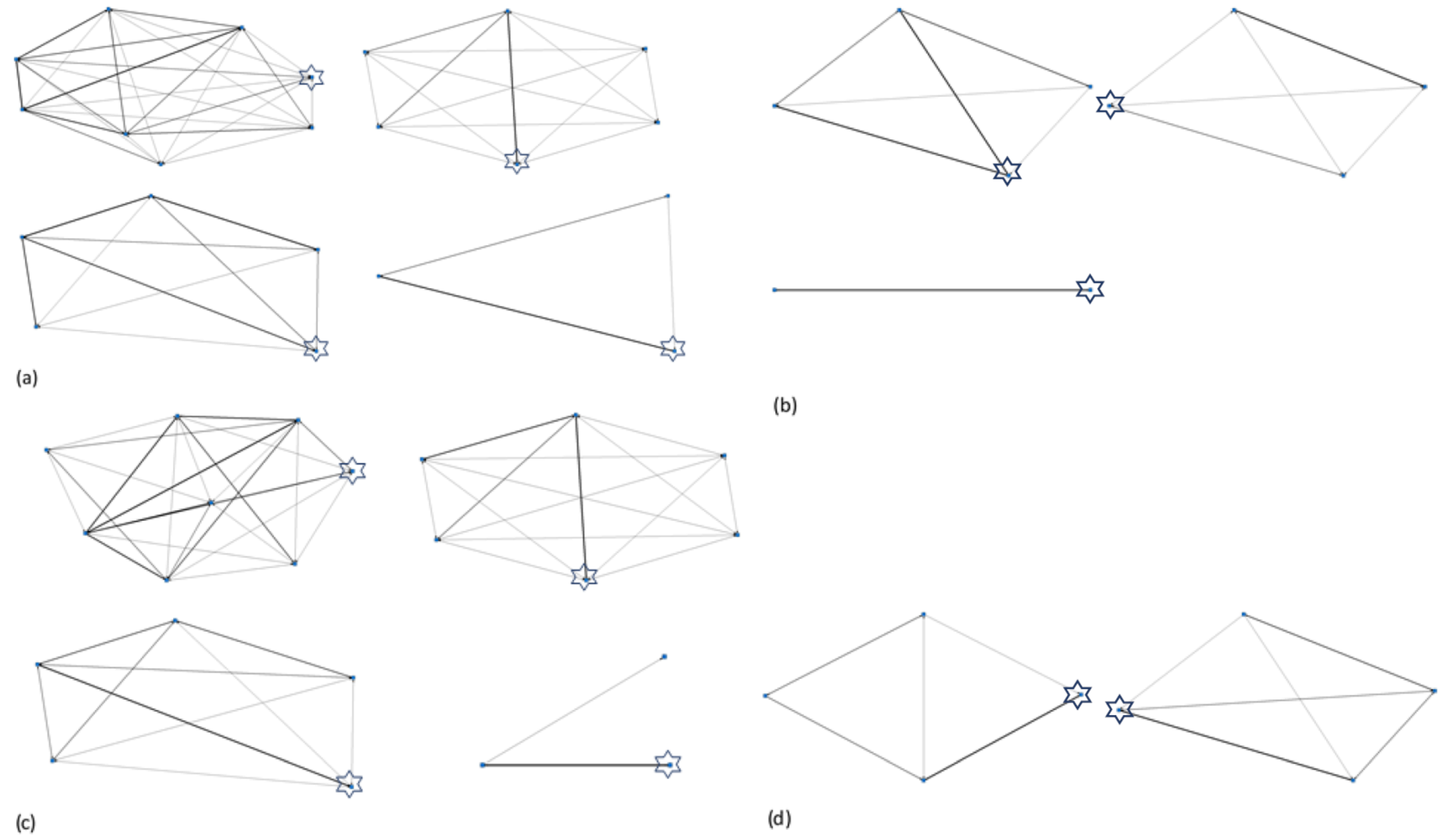
| Zoo | Elephant | Species | Sex | Age | No. Relatives in Herd | Wild or Captive Born | If Zoo Born, at Natal Zoo? | Observation Period (mins) | Proportion Observations in Sight |
|---|---|---|---|---|---|---|---|---|---|
| A | E1 | African | F | 45 | 0 | Wild | N/A | 5817 | 0.66 |
| E2 | African | F | 47 | 0 | Wild | N/A | 5817 | 0.98 | |
| B | E3 | Asian | F | 54 | 0 | Wild | N/A | 5842 | 0.89 |
| E4 | Asian | F | 44 | 0 | Wild | N/A | 5842 | 0.89 | |
| E5 | Asian | F | 40 | 0 | Wild | N/A | 5842 | 0.85 | |
| C | E6 | Asian | F | 49 | 0 | Captive | N | 5838 | 0.75 |
| E7 | Asian | M | 15 | 1 | Captive | N | 5838 | 0.16 | |
| E8 | Asian | F | 1 | 4 | Captive | Y | 5838 | 0.90 | |
| E9 | Asian | F | 36 | 3 | Wild | N/A | 5838 | 0.78 | |
| E10 | Asian | F | 19 | 3 | Captive | Y | 5838 | 0.87 | |
| E11 | Asian | F | 13 | 3 | Captive | Y | 5838 | 0.87 | |
| D | E12 | African | M | 34 | 0 | Wild | N/A | 7666 | 0.20 |
| E13 | African | F | 35 | 0 | Wild | N/A | 7666 | 0.27 | |
| E14 | African | F | 35 | 0 | Wild | N/A | 7666 | 0.67 | |
| E15 | African | F | 31 | 0 | Wild | N/A | 7666 | 0.69 | |
| E | E16 | Asian | F | 32 | 8 | Captive | N | 3267 | 0.65 |
| E17 | Asian | F | 26 | 8 | Captive | N | 3267 | 0.66 | |
| E18 | Asian | F | 13 | 8 | Captive | N | 3267 | 0.71 | |
| E19 | Asian | F | 10 | 8 | Captive | Y | 3267 | 0.75 | |
| E20 | Asian | M | 2 | 9 | Captive | Y | 3267 | 0.61 | |
| E21 | Asian | F | 2 | 9 | Captive | Y | 3267 | 0.65 | |
| E22 | Asian | M | 2 | 9 | Captive | Y | 3267 | 0.60 | |
| E23 | Asian | F | <1 | 9 | Captive | Y | 1569 | 0.51 | |
| - | Asian | M | 22 | 9 | Captive | N | |||
| F | E24 | African | F | 14 | 1 | Captive | Y | 5031 | 0.79 |
| E25 | African | F | 30 | 0 | Wild | N/A | 5031 | 0.76 | |
| E26 | African | F | 14 | 2 | Captive | Y | 5031 | 0.81 | |
| E27 | African | F | 30 | 1 | Wild | N/A | 5031 | 0.80 | |
| G | E28 | Asian | F | 33 | 0 | Wild | N/A | 5016 | 0.69 |
| E29 | Asian | F | 22 | 1 | Captive | N | 5016 | 0.70 | |
| E30 | Asian | F | 3 | 1 | Captive | Y | 5016 | 0.63 | |
| E31 | Asian | F | 19 | 1 | Captive | Y | 5016 | 0.68 | |
| E32 | Asian | F | 34 | 1 | Wild | N/A | 5016 | 0.67 |
| Zoo | Data Collection Period (Study Months, Days) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| A | January and February 2016 (10 days) | April and May 2016 (10 days) | July and August 2016 (10 days) | October and November 2016 (10 days) |
| B | May 2016 (5 days) | August 2016 (5 days) | December 2016 (5 days) | February 2017(5 days) |
| C | January and February 2016 (10 days) | April and May 2016 (10 days) | July and August 2016 (10 days) | October and November 2016 (10 days) |
| D | January and February 2016 (10 days) | April and May 2016 (10 days) | July and August 2016 (10 days) | October and November 2016 (10 days) |
| E | February 2016 | April and May 2016 | September 2016 | October and November 2016 |
| F | January and February 2016 (10 days) | April and May 2016 (10 days) | July and August 2016 (10 days) | October and November 2016 (10 days) |
| G | January and February 2016 (10 days) | April and May 2016 (10 days) | July and August 2016 (10 days) | October and November 2016 (10 days) |
| Behaviour | Description | ||
|---|---|---|---|
| Positive | Positive physical | Conspecific play | Engaging in active play with another elephant, including head-to-head sparring, trunk wrestling, mounting, chasing and rolling on one another. Does not include behaviours observed following an agonistic encounter or courtship. |
| Touching (trunk to) | Touching another elephant with the trunk in a non-aggressive manner. | ||
| Touching (body to) | Touching/rubbing another elephant with the body. | ||
| Positive non-physical | Protecting | Standing over another elephant. | |
| Huddling | Formation of a tight circle with calves at the nucleus. Calves hidden in the middle, adults surrounding them. | ||
| Approach | Walking towards another elephant in a non-threatening manner. Recipient stays in position during and after the approach. | ||
| Approach with trunk | Trunk outstretched towards another elephant. Not close enough to make physical contact. | ||
| Walking with | Walking side by side with another elephant. | ||
| Following | Walking closely behind another elephant (within one elephant body length). | ||
| Negative | Negative physical | Pushing | One elephant forces or pushes against the body (usually the rump) of another elephant, resulting in the elephant that is being pushed moving at least two steps. |
| Pulling | Using the trunk to pull at another elephant in a non-playful manner. May pull at the trunk or an accessible body part such as tusks/tushes or the tail. | ||
| Sparring | An escalation of a push/pull incident into more physical aggression. | ||
| Hitting/kicking | Aggressive physical contact with the trunk or leg, e.g., trunk strike or kicking out. | ||
| Negative non-physical | Displace | Movement of one elephant results in another elephant leaving its location (within 10 s)—usually occurs when a more dominant elephant approaches a more subordinate individual. | |
| Approach | Walking towards another elephant in an aggressive or hostile manner (head held high, ears wide or flapping). Receiving elephant may either respond to this by standing as tall as possible, head raised, ears flapping or turning away from/walking away from the approaching elephant. | ||
| Walking/turning away from | Avoiding or shying away from elephants or people; the individual either walks forwards away from or backwards away from a particular elephant or person. | ||
| Frozen | Standing still and alert as another elephant approaches. | ||
| Charge/mock charge | Move towards another elephant with the head held high, pace usually quickens as the individual gets closer to the target elephant. In the case of a mock charge, the individual charging stops further away from the target elephant. | ||
| Blocking | Blocking from food source or other resource (e.g., door) | ||
| Interaction Type | Time Period | Median | IQR | Range (%) |
|---|---|---|---|---|
| Positive physical | 1 | 0.96 | 0.09–4.28 | 0–8.55 |
| 2 | 0.19 | 0.19–5.3 | 0–18.27 | |
| 3 | 0.86 | 0.86–4.77 | 0–14.03 | |
| 4 | 1.16 | 1.16–6.56 | 0–13.73 | |
| Negative physical | 1 | 0 | 0–0.05 | 0–0.3 |
| 2 | 0.02 | 0–0.07 | 0–0.19 | |
| 3 | 0.02 | 0–0.04 | 0–0.16 | |
| 4 | 0 | 0–0.06 | 0–0.48 | |
| Positive non-physical | 1 *, 2,3,4 | 3.35 | 3.35–8.19 | 0.13–50.65 |
| 2 *, 1,3,4 | 1.57 | 1.57–6 | 0.03–16.84 | |
| 3 *, 1,2 | 1.04 | 1.04–1.96 | 0–11.34 | |
| 4 *, 1,2 | 1.29 | 1.29–2.24 | 0.15–6.52 | |
| Negative non-physical | 1 | 0.19 | 0.06–0.34 | 0–1.1 |
| 2 | 0.09 | 0.04–0.23 | 0–0.85 | |
| 3 | 0.09 | 0.03–0.24 | 0–3.28 | |
| 4 | 0.06 | 0.03–0.18 | 0–0.52 |
| Interaction Type | Time Period | Median | IQR | Range (%) |
|---|---|---|---|---|
| Positive physical | P1 *,4 | 0 | 0–0.15 | 0–7.52 |
| P2 | 0 | 0–0.07 | 0–12.01 | |
| P3 | 0 | 0–0.16 | 0–8.89 | |
| P4 *,1 | 0.03 | 0–0.71 | 0–11.65 | |
| Negative physical | P1 | 0 | 0–0 | 0–0.23 |
| P2 | 0 | 0–0 | 0–0.15 | |
| P3 | 0 | 0–0 | 0–0.09 | |
| P4 | 0 | 0–0 | 0–0.48 | |
| Positive non-physical | P1 *,2,3,4 | 0.27 | 0–0.97 | 0–17.16 |
| P2 *,1,3 | 0.13 | 0–0.53 | 0–10.81 | |
| P3 *,1,2,4 | 0.07 | 0–0.27 | 0–10.81 | |
| P4 *,1,3 | 0.13 | 0.12–0.31 | 0–6.14 | |
| Negative non-physical | P1 *, 2,3,4 | 0 | 0–0.06 | 0–0.76 |
| P2 *,1 | 0 | 0–0.04 | 0–0.85 | |
| P3 *,1 | 0 | 0–0.03 | 0–3.28 | |
| P4 *,1 | 0 | 0–0.03 | 0–0.44 |
| Zoo | Physical | Non-Physical | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| A | N/A | N/A | N/A | N/A |
| B | NS | NS | NS | NS |
| C | 0.8455 * | NS | 0.8965 ** | 0.8551 * |
| D | NS | NS | NS | NS |
| E | 0.5341 ** | NS | NS | 0.6821 ** |
| F | 0.9761 * | NS | NS | NS |
| G | 0.9348 * | NS | NS | NS |
| Interaction Type | Comparison Points | Zoo | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| Positive physical | 1 | N/A | NS | 0.9834 ** | NS | NS | NS | 0.9204 *** |
| 2 | N/A | NS | NS | NS | NS | 0.8279 * | NS | |
| 3 | N/A | NS | NS | NS | NS | NS | NS | |
| 4 | N/A | NS | 1 ** | NS | NS | NS | NS | |
| 5 | N/A | NS | NS | NS | 0.7266 ** | NS | NS | |
| 6 | N/A | NS | 0.9897 * | NS | NS | NS | NS | |
| Positive non-physical | 1 | N/A | NS | NS | NS | NS | NS | 0.9206 * |
| 2 | N/A | NS | NS | NS | NS | NS | 0.8353 * | |
| 3 | N/A | NS | 0.7289 * | NS | NS | 0.9113 * | 0.9444 * | |
| 4 | N/A | NS | 0.8054 ** | NS | 0.713 ** | NS | 0.8622 ** | |
| 5 | N/A | NS | NS | NS | 0.6924 * | NS | 0.8118 ** | |
| 6 | N/A | NS | 0.9704 ** | NS | NS | 0.9113 * | 0.7876 * | |
| Negative physical | 1 | N/A | N/A | 0.6784 * | N/A | NS | N/A | NS |
| 2 | N/A | NS | 0.8688 ** | N/A | N/A | N/A | NS | |
| 3 | N/A | NS | 0.93 ** | N/A | N/A | NS | NS | |
| 4 | N/A | N/A | 0.7917 ** | N/A | N/A | NS | NS | |
| 5 | N/A | NS | NS | N/A | NS | NS | NS | |
| 6 | N/A | NS | 0.9376 ** | N/A | NS | NS | NS | |
| Negative non-physical | 1 | N/A | NS | 0.6346 * | NS | NS | NS | NS |
| 2 | N/A | NS | 0.6478 * | NS | NS | NS | NS | |
| 3 | N/A | NS | 0.5476 * | NS | NS | NS | NS | |
| 4 | N/A | NS | NS | NS | 0.591 * | NS | NS | |
| 5 | N/A | NS | NS | NS | NS | NS | NS | |
| 6 | N/A | NS | 0.6828 * | NS | NS | NS | NS | |
| Zoo | Elephant | Species | Sex | Age | Related to Others in Herd | Betweenness Score | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive Physical | Positive Non-Physical | Negative Physical | Negative Non-Physical | ||||||
| A | E1 M | African | F | 45 | N | N/A | 0 | N/A | 0 |
| E2 | African | F | 47 | N | N/A | 0 | N/A | 0 | |
| B | E3 M | Asian | F | 54 | N | 1 | 0 | 0 | 0 |
| E4 | Asian | F | 44 | N | 0 | 0 | 0 | 0 | |
| E5 | Asian | F | 40 | N | 0 | 0 | 0 | 0 | |
| C | E6 | Asian | F | 49 | N | 0 | 0 | 0 | 0.25 |
| E7 | Asian | M | 15 | Y | 0 | 0 | 0 | 0 | |
| E8 M | Asian | F | 36 | Y | 0 | 0 | 0 | 0.25 | |
| E9 | Asian | F | 1 | Y | 0 | 0 | 0 | 0 | |
| E10 | Asian | F | 19 | Y | 0 | 0 | 0 | 0.25 | |
| E11 | Asian | F | 13 | Y | 0 | 0 | 0 | 0.25 | |
| D | E12 M | African | M | 34 | N | 0 | 0 | 0.5 | 0 |
| E13 | African | F | 35 | N | 0 | 0 | 0 | 0 | |
| E14 M | African | F | 35 | N | 0 | 0 | 0.5 | 0 | |
| E15 | African | F | 31 | N | 0 | 0 | 0 | 0 | |
| E | E16 M | Asian | F | 32 | Y | 0 | 0 | 6 | 0.25 |
| E17 | Asian | F | 26 | Y | 0.4 | 0 | 0 | 0.67 | |
| E18 | Asian | F | 13 | Y | 0.4 | 0 | 6.33 | 0.92 | |
| E19 | Asian | F | 10 | Y | 0 | 0 | 2 | 0.25 | |
| E20 | Asian | M | 2 | Y | 0.4 | 0 | 0.33 | 0.67 | |
| E21 | Asian | F | 2 | Y | 0.4 | 0 | 7 | 0.25 | |
| E22 | Asian | M | 2 | Y | 0.4 | 0 | 3.33 | 0 | |
| E23 | Asian | F | <1 | Y | 0 | 0 | 0 | 0 | |
| F | E24 | African | F | 14 | Y | 0 | 0 | 0 | 0 |
| E25 M | African | F | 30 | N | 0 | 0 | 0 | 0 | |
| E26 | African | F | 14 | Y | 0 | 0 | 0 | 0 | |
| E27 | African | F | 30 | Y | 0 | 0 | 0 | 0 | |
| G | E28 M | Asian | F | 33 | N | 0 | 0 | 0 | 0 |
| E29 | Asian | F | 22 | Y | 0 | 0 | 0 | 0 | |
| E30 | Asian | F | 3 | Y | 0 | 0 | 0 | 0 | |
| E31 | Asian | F | 19 | Y | 0 | 0 | 0 | 0 | |
| E32 | Asian | F | 34 | Y | 0 | 0 | 0 | 0 | |
| Zoo | Physical Positive | Physical Negative | Non-Physical Positive | Non-Physical Negative | ||||
|---|---|---|---|---|---|---|---|---|
| A | ||||||||
| B | ||||||||
| C | E6–E10 E7–E8 E8–E9 | Unrelated Unrelated Related | E6–E10 E6–E11 E7–E8 E7–E9 | Unrelated Unrelated Unrelated Related | E6–E8 E6–E9 E6–E10 E6–E11 E7–E8 E7–E10 E9–E10 E10–E11 | Unrelated Unrelated Unrelated Unrelated Related Unrelated Related Related | E6–E9 E6–E10 E10–E11 | Unrelated Unrelated Related |
| D | E14–E15 | Unrelated | E14–E15 | Unrelated | ||||
| E | E19–E21 E18–E22 E20–E21 | Related Related Related | E16–E17 | Related | E16–E17 E16–E18 E17–E19 E18–E19 E21–E23 E22–E23 | Related Related Related Related Related Related | E16–E21 E17–E19 E17–E20 | Related Related Related |
| F | E24–E25 E26–E27 | Unrelated Related | E24–E25 | Unrelated | E24–E25 E24–E26 E24–E27 E25–E27 | Unrelated Related Unrelated Unrelated | E24–E25 E26–E27 | Unrelated Related |
| G | E28–E30 E28–E31 E28–E32 E29–E30 E29–E31 E30–E31 | Unrelated Unrelated Unrelated Related Unrelated Unrelated | E28–E29 E28–E31 E29–E31 E30–E32 | Unrelated Unrelated Unrelated Unrelated | E29–E31 | Unrelated | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.; Bremner-Harrison, S.; Hall, C.; Carter, A. Understanding Temporal Social Dynamics in Zoo Animal Management: An Elephant Case Study. Animals 2020, 10, 882. https://doi.org/10.3390/ani10050882
Williams E, Bremner-Harrison S, Hall C, Carter A. Understanding Temporal Social Dynamics in Zoo Animal Management: An Elephant Case Study. Animals. 2020; 10(5):882. https://doi.org/10.3390/ani10050882
Chicago/Turabian StyleWilliams, Ellen, Samantha Bremner-Harrison, Carol Hall, and Anne Carter. 2020. "Understanding Temporal Social Dynamics in Zoo Animal Management: An Elephant Case Study" Animals 10, no. 5: 882. https://doi.org/10.3390/ani10050882
APA StyleWilliams, E., Bremner-Harrison, S., Hall, C., & Carter, A. (2020). Understanding Temporal Social Dynamics in Zoo Animal Management: An Elephant Case Study. Animals, 10(5), 882. https://doi.org/10.3390/ani10050882





