Effect of Feed Restriction on the Behaviour and Welfare of Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing
2.2. Animals and Experimental Groups
2.3. Behavioural Observations
2.4. Sampling and Analysis for Corticosterone in Plasma and Faeces
2.5. Statistical Analysis
3. Results
3.1. Behavioural Observations During the Trial
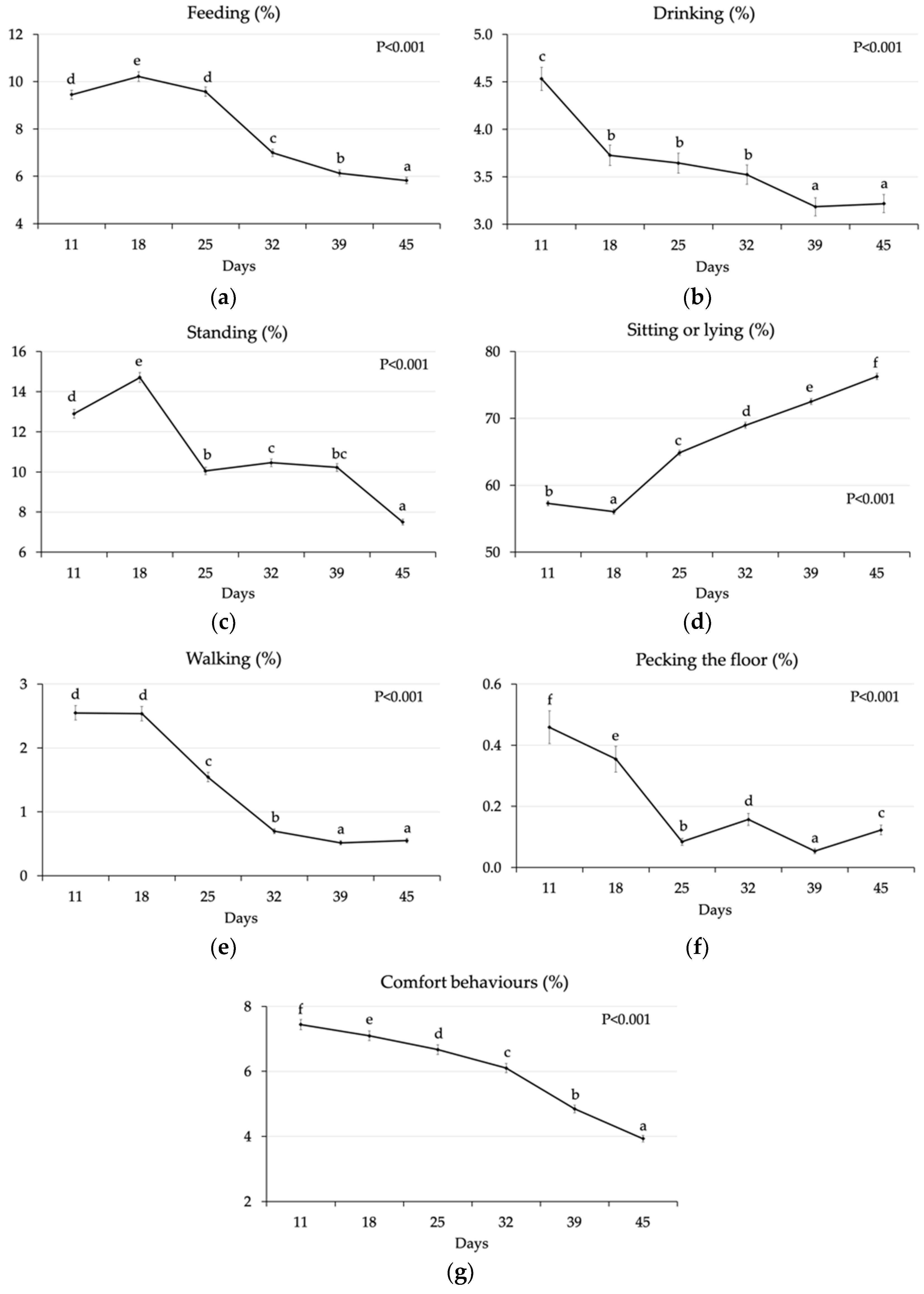
3.1.1. Effect of Age
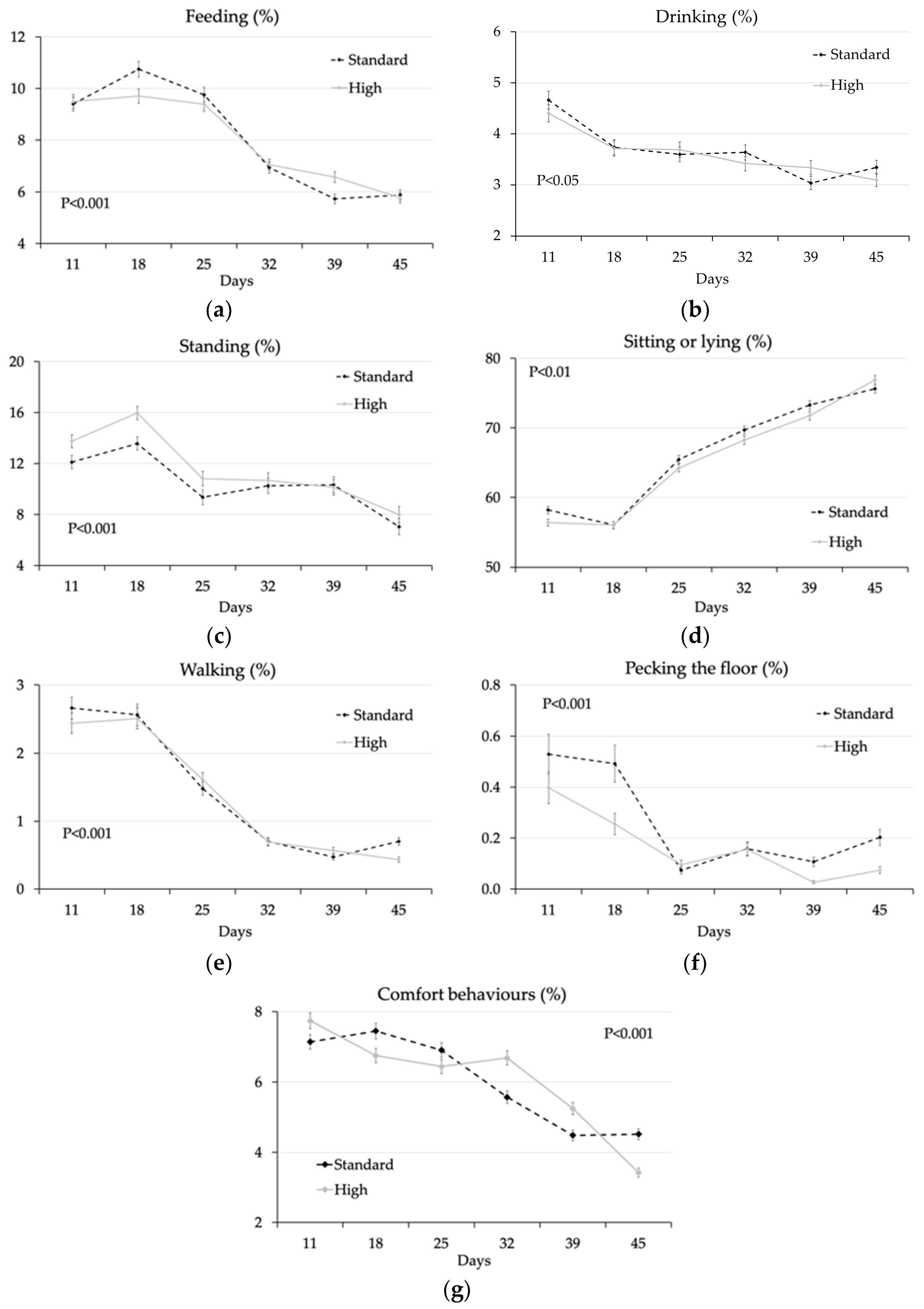
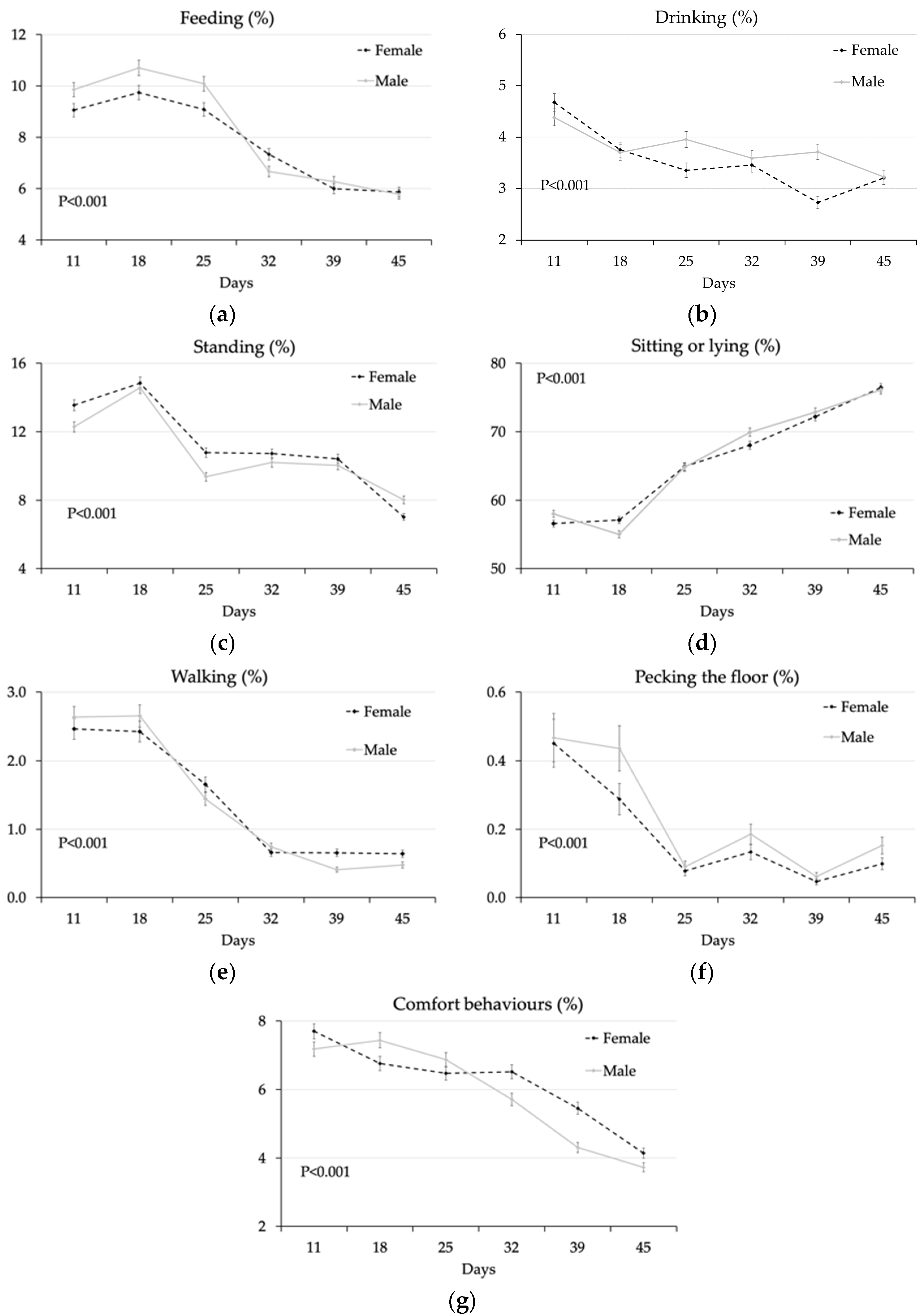
3.1.2. Effect of Feeding System, Genotype, and Sex
3.2. Behavioural Observations during Feed Administration in the Restriction Period
3.3. Corticosterone Changes in Plasma and Faeces
4. Discussion
4.1. Effect of the Feeding System
4.2. Effect of Sex
4.3. Effect of Genotype
4.4. Effect of Age
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petracci, M.; Mudalal, S.; Babini, E.; Claudio, C. Effect of white striping on chemical composition and nutritional value of chicken breast meat. Ital. J. Anim. Sci. 2014, 13, 3138. [Google Scholar] [CrossRef]
- De Jong, I.C.; van Voorst, A.S.; Blockhuis, H.J. Parameters for quantification of hunger in broiler breeders. Physiol. Behav. 2003, 78, 773–783. [Google Scholar] [CrossRef]
- Sahraei, M. Feed restriction in broiler chickens production: A review. Glob. Vet. 2012, 8, 449–458. [Google Scholar] [CrossRef]
- Reiter, K.; Bessei, W. Effect of locomotor activity on leg disorder in fattening chicken. Berl. Und Munch. Tierarztl. Wochenschr. 2009, 122, 264–270. [Google Scholar]
- Baxter, M.; Bailie, C.L.; O’Connell, N.E. Play behaviour, fear responses and activity levels in commercial broiler chickens provided with preferred environmental enrichments. Animal 2019, 13, 171–179. [Google Scholar] [CrossRef]
- Bokkers, E.; Koene, P. Sex and type of feed effects on motivation and ability to walk for a food reward in fast growing broilers. Appl. Anim. Behav. Sci. 2002, 79, 247–261. [Google Scholar] [CrossRef]
- Millman, S.T.; Duncan, I.J. Effect of male-to-male aggressiveness and feed-restriction during rearing on sexual behaviour and aggressiveness towards females by male domestic fowl. Appl. Anim. Behav. Sci. 2000, 70, 63–82. [Google Scholar] [CrossRef]
- Sahraei, M. Effects of feed restriction on metabolic disorders in broiler chickens: A review. Biotechnol. Anim. Husb. 2014, 30, 1–13. [Google Scholar] [CrossRef]
- Hocking, P.M.; Hughes, B.O.; Keer-Keer, S. Comparison of food intake, rate of consumption, pecking activity and behaviour in layer and broiler breeder males. Br. Poult. Sci. 1997, 38, 237–240. [Google Scholar] [CrossRef]
- Savory, C.J.; Maros, K. Influence of degree of food restriction, age and time of day on behaviour of broiler breeder chickens. Behav. Process. 1993, 29, 179–189. [Google Scholar] [CrossRef]
- Savory, J.; Kostal, L. Temporal patterning of oral stereotypies in restricted-fed fowls: 1. Investigations with a single daily meal. Int. J. Comp. Psychol. Iscp; Spons. 2016, 9, 117–139. [Google Scholar]
- Mench, J.A. Broiler breeders: Feed restriction and welfare. World’s Poult. Sci. J. 2002, 58, 23–29. [Google Scholar] [CrossRef]
- Rettenbacher, S.; Möstl, E.; Hackl, R.; Ghareeb, K.; Palme, R. Measurement of corticosterone metabolites in chicken droppings. Br. Poult. Sci. 2004, 45, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 31 March 2020).
- Trocino, A.; Piccirillo, A.; Birolo, M.; Radaelli, G.; Bertotto, D.; Filiou, E.; Petracci, M.; Xiccato, G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015, 94, 2996–3004. [Google Scholar] [CrossRef]
- Radaelli, G.; Piccirillo, A.; Birolo, M.; Bertotto, D.; Gratta, F.; Ballarin, C.; Vascellari, M.; Xiccato, G.; Trocino, A. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poult. Sci. 2016, 96, 309–319. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Thodberg, K.; Malmkvist, J.; Steenfeldt, S. Proportion of insoluble fibre in the diet affect behaviour and hunger in broiler breeders growing at similar rates. Animal 2011, 5, 1246–1258. [Google Scholar] [CrossRef]
- Simontacchi, C.; Negrato, E.; Pazzaglia, M.; Bertotto, D.; Poltronieri, C.; Radaelli, G. Whole-body concentrations of cortisol and sex steroids in white sturgeon (Acipenser transmontanus, Richardson 1836) during early development and stress response. Aquac. Int. 2009, 17, 7–14. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System Institute, Inc.). SAS/STAT(R) 9.3 User’s Guide, 3rd ed.; SAS Institute: Cary, NC, USA, 2013; Available online: https://support.sas.com/documentation/cdl/en/statug/63962/HTML/default/viewer.htm#titlepage.htm (accessed on 14 February 2020).
- Nielsen, B.L.; Litherland, M.; Nøddegaard, F. Effects of qualitative and quantitative feed restriction on the activity of broiler chickens. Appl. Anim. Behav. Sci. 2003, 83, 309–323. [Google Scholar] [CrossRef]
- Sandilands, V.; Tolkamp, B.J.; Kuriazakis, I. Behaviour of food restricted broilers during rearing and lay-effects of an alternative feeding method. Physiol. Behav. 2005, 85, 115–123. [Google Scholar] [CrossRef]
- Sandilands, V.; Tolkamp, B.J.; Savory, C.J.; Kyriazakis, I. Behaviour and welfare of broiler breeders fed qualitatively restricted diets during rearing: Are there viable alternatives to quantitative restriction. Appl. Anim. Behav. Sci. 2006, 96, 53–67. [Google Scholar] [CrossRef]
- Hocking, P.M.; Maxwell, M.H.; Robertson, G.W.; Mitchell, M.A. Welfare assessment of modified rearing programmes for broiler breeders. Br. Poult. Sci. 2001, 42, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.L. Adrenergic and noradrenergic regulation of poultry behavior and production. Domest. Anim. Endocrinol. 2016, 56, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.; Su, G.; Kestin, S.C. Effects of age and stocking density on leg weakness in broiler chickens. Poult. Sci. 2000, 79, 864–870. [Google Scholar] [CrossRef]
- Van Krimpen, M.M.; De Jong, I.C. Impact of nutrition on welfare aspects of broiler breeder flocks. World’s Poult. Sci. J. 2014, 70, 139–150. [Google Scholar] [CrossRef]
- Richards, M.P.; Proszkowiec-Weglarz, M. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poult. Sci. 2007, 86, 1478–1490. [Google Scholar] [CrossRef]
- Newberry, R.C.; Hunt, J.R.; Gardiner, E.E. Influence of light intensity on behaviour and performance of broiler chickens. Poult. Sci. 1988, 67, 1020–1025. [Google Scholar] [CrossRef]
- Reiter, K.; Bessie, W. Effect of locomotor activity on bone development and leg disorders in broilers. Arch. Fur Geflugelkd. 1998, 62, 247–253. [Google Scholar]
- Newberry, R.C. Exploratory behaviour of young domestic fowl. Appl. Anim. Behav. Sci. 1999, 63, 311–321. [Google Scholar] [CrossRef]
- Barbato, G.F.; Siegel, P.B.; Cherry, J.A.; Nir, I. Selection for body weight at eight weeks of age. 17. Overfeeding. Poult. Sci. 1984, 63, 11–18. [Google Scholar] [CrossRef]



| Behaviour | Description |
|---|---|
| Feeding | Chickens pecking in feeders |
| Drinking | Chickens at drinkers |
| Standing | Stand up |
| Sitting/lying | Sitting or lie down |
| Walking | Walking or running with no other discernible activity |
| Pecking floor | Pecking the floor (including feeding fodder) |
| Pecking other bird | Pecking any body part of other birds |
| Comfort | Other comfort behaviours, such as preening, scratching or wing stretching |
| Feeding System (F) | Genotype (T) | Sex (S) | p-Value 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behaviours | Ad libitum | Restricted | Standard | High | Females | Males | F | T | S | Age |
| Feeding | 7.51 ± 0.18 | 8.17 ± 0.19 | 7.83 ± 0.19 | 7.84 ± 0.19 | 7.69 ± 0.18 | 7.98 ± 0.19 | 0.011 | 0.978 | 0.277 | <0.001 |
| Drinking | 3.51 ± 0.11 | 3.72 ± 0.11 | 3.64 ± 0.11 | 3.59 ± 0.11 | 3.48 ± 0.11 | 3.75 ± 0.11 | 0.181 | 0.750 | 0.089 | <0.001 |
| Standing | 9.84 ± 0.20 | 11.71 ± 0.23 | 10.22 ± 0.20 | 11.27 ± 0.22 | 10.92 ± 0.22 | 10.54 ± 0.21 | <0.001 | <0.001 | <0.001 | <0.001 |
| Sitting/lying | 67.03 ± 0.38 | 64.14 ± 0.37 | 65.99 ± 0.38 | 65.15 ± 0.37 | 65.47 ± 0.38 | 65.67 ± 0.38 | <0.001 | 0.118 | 0.700 | <0.001 |
| Walking | 1.10 ± 0.06 | 1.14 ± 0.06 | 1.15 ± 0.06 | 1.09 ± 0.06 | 1.18 ± 0.07 | 1.06 ± 0.06 | 0.682 | 0.491 | 0.182 | <0.001 |
| Pecking floor | 0.15 ± 0.02 | 0.16 ± 0.02 | 0.20 ± 0.03 | 0.12 ± 0.02 | 0.14 ± 0.02 | 0.18 ± 0.03 | 0.807 | 0.008 | 0.155 | <0.001 |
| Pecking bird | 0.10 ± 0.82 | 0.06 ± 0.63 | 0.10 ± 0.76 | 0.07 ± 0.71 | 0.05 ± 0.57 | 0.11 ± 0.86 | <0.001 | 0.990 | <0.001 | <0.001 |
| Comfort | 5.94 ± 0.14 | 5.80 ± 0.14 | 5.88 ± 0.14 | 5.86 ± 0.14 | 6.06 ± 0.14 | 5.68 ± 0.13 | 0.499 | 0.891 | 0.049 | <0.001 |
| Behaviours 1 | Time with Respect to Feed Administration (F) | p-value 2 | ||||
|---|---|---|---|---|---|---|
| −10 min | 0 | +5 min | +10 min | +15 min | ||
| Feeding | 0.14 a ± 0.41 | 33.08 c ± 2.28 | 32.26 c ± 2.29 | 21.20 b ± 1.84 | 11.14 a ± 1.08 | ≤0.001 |
| Drinking | 0.84 a ± 0.27 | 0.00 a ± 0.35 | 3.58 b ± 0.72 | 4.35 bc ± 0.86 | 6.97 c ± 1.07 | ≤0.001 |
| Standing | 10.44 b ± 0.92 | 27.28 c ± 1.71 | 11.33 b ± 0.92 | 5.08 a ± 0.58 | 11.48 b ± 1.01 | ≤0.001 |
| Sitting/lying | 74.40 d ± 3.41 | 8.87 a ± 0.81 | 7.33 a ± 1.38 | 27.81 b ± 1.85 | 43.45 c ± 2.33 | ≤0.001 |
| Walking | 0.83 a ± 0.46 | 6.72 b ± 1.95 | 3.66 ab ± 1.11 | 1.64 a ± 0.56 | 0.00 a ± 0.32 | ≤0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trocino, A.; White, P.; Bordignon, F.; Ferrante, V.; Bertotto, D.; Birolo, M.; Pillan, G.; Xiccato, G. Effect of Feed Restriction on the Behaviour and Welfare of Broiler Chickens. Animals 2020, 10, 830. https://doi.org/10.3390/ani10050830
Trocino A, White P, Bordignon F, Ferrante V, Bertotto D, Birolo M, Pillan G, Xiccato G. Effect of Feed Restriction on the Behaviour and Welfare of Broiler Chickens. Animals. 2020; 10(5):830. https://doi.org/10.3390/ani10050830
Chicago/Turabian StyleTrocino, Angela, Peter White, Francesco Bordignon, Valentina Ferrante, Daniela Bertotto, Marco Birolo, Giulio Pillan, and Gerolamo Xiccato. 2020. "Effect of Feed Restriction on the Behaviour and Welfare of Broiler Chickens" Animals 10, no. 5: 830. https://doi.org/10.3390/ani10050830
APA StyleTrocino, A., White, P., Bordignon, F., Ferrante, V., Bertotto, D., Birolo, M., Pillan, G., & Xiccato, G. (2020). Effect of Feed Restriction on the Behaviour and Welfare of Broiler Chickens. Animals, 10(5), 830. https://doi.org/10.3390/ani10050830






