Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Instrumentation
2.3.1. Sample Preparation, ICP–MS
2.3.2. HPLC–ICP MS
2.3.3. HPLC–ESI–MS/MS
2.4. Experiment
2.4.1. Freeze-Drying of Tissues
2.4.2. Extraction Procedure
2.4.3. Total Se Determination
2.4.4. HPLC–ICP–MS Analysis
2.4.5. LC–ESI–MS/MS Analysis
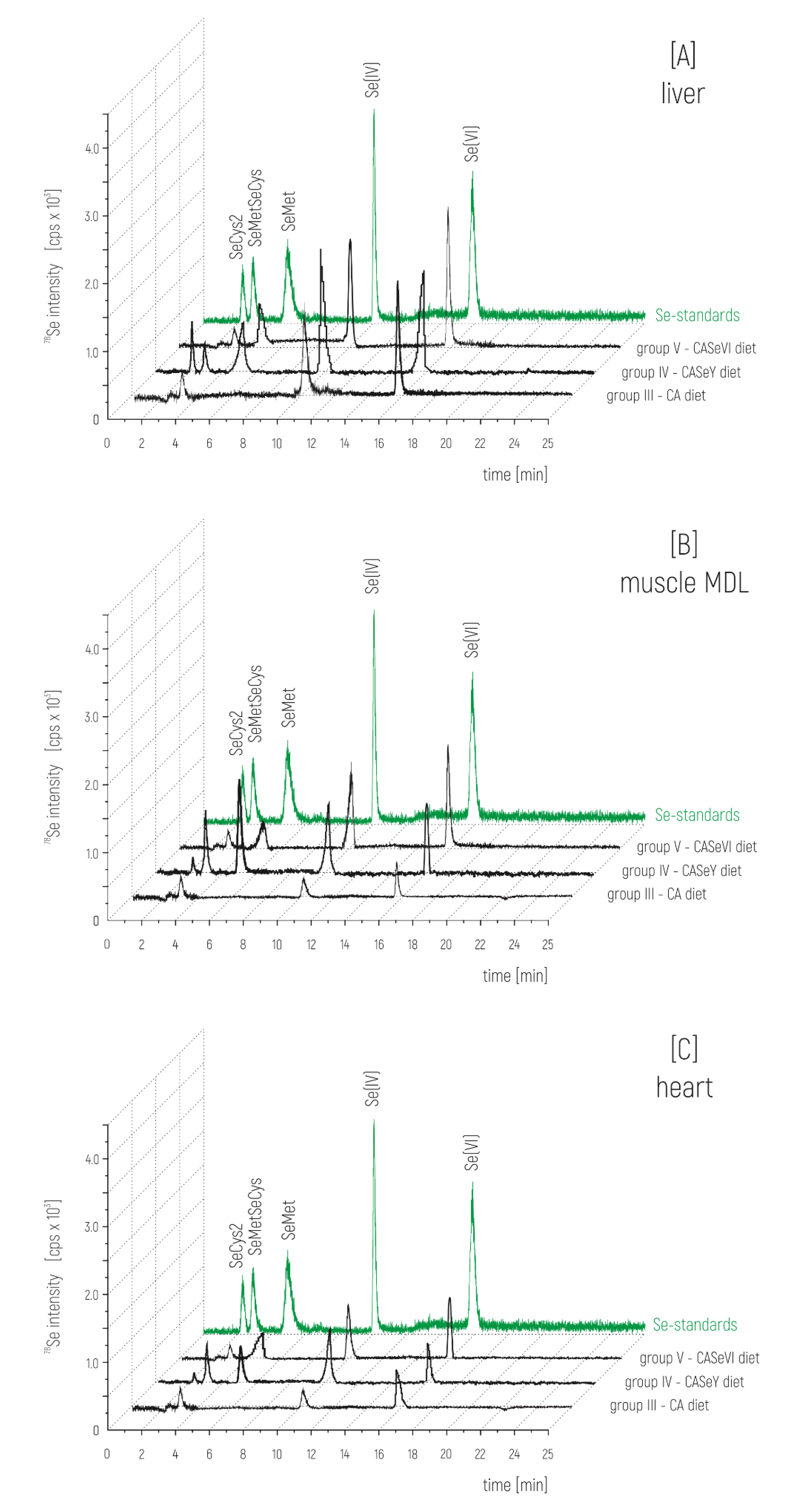
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Combs, G.F.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effectsof selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Willett, W.C.; Morris, S.J.; Stampfer, M.J.; Spiegelman, D.; Rimm, E.B.; Giovannucci, E. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J. Natl. Cancer Inst. 1998, 90, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effectof Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers. JAMA 2009, 301, 39. [Google Scholar] [CrossRef]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Seleniumand Its Supplementation in Cardiovascular Disease—What do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef]
- Stone, C.A.; Kawai, K.; Kupka, R.; Fawzi, W.W. Roleof selenium in HIV infection. Nutr. Rev. 2010, 68, 671–681. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenocompoundsin Plants and Animals and their Biological Significance. J. Am. Coll. Nutr. 2002, 21, 223–232. [Google Scholar] [CrossRef]
- Einbond, L.S.; Wu, H.; Kashiwazaki, R.; He, K.; Roller, M.; Su, T.; Wang, X.; Goldsberry, S. Carnosicacid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin. Fitoterapia 2012, 83, 1160–1168. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Effectof the phenological stage on the chemical composition, and antimicrobial and antioxidant properties of Rosmarinus officinalis L essential oil and its polyphenolic extract. Ind. Crops Prod. 2013, 48, 144–152. [Google Scholar] [CrossRef]
- Morán, L.; Giráldez, F.J.; Panseri, S.; Aldai, N.; Jordán, M.J.; Chiesa, L.M.; Andrés, S. Effectof dietary carnosic acid on the fatty acid profile and flavour stability of meat from fattening lambs. Food Chem. 2013, 138, 2407–2414. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipidmetabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Foster, L.; Sumar, S. Hydridegeneration atomic absorption spectrometric (HGAAS) determination of selenium in term and preterm infant formulae available in the United Kingdom. Food Chem. 1996, 55, 293–298. [Google Scholar] [CrossRef]
- Walcerz, M.; Bulska, E.; Hulanicki, A. Studyof some interfering processes in the arsenic, antimony and selenium determination by hydride generation atomic absorption spectrometry. Fresenius J. Anal. Chem. 1993, 346, 622–626. [Google Scholar] [CrossRef]
- Larsen, E.H.; Ekelund, J. Determinationof total selenium in nutritional supplements and selenised yeast by Zeeman-effect graphite furnace atomic absorption spectrometry. Analyst 1989, 114, 915. [Google Scholar] [CrossRef]
- Pinto, F.G.; Andrada, D.; Magalhães, C.G.; Nunes, B.R.; de Amorim, F.R.; Franco, M.B.; Saint’Pierre, T.D.; da Silva, J.B.B.; Curtius, A.J. Directdetermination of selenium in urine samples by electrothermal atomic absorption spectrometry using a Zr plus Rh-treated graphite tube and co-injection of Rh as chemical modifier. Anal. Bioanal. Chem. 2005, 383, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Watson, R.P.; Lindstrom, R.M. Accurateand Precise Measurement of Selenium by Instrumental Neutron Activation Analysis. Anal. Chem. 2011, 83, 3493–3498. [Google Scholar] [CrossRef]
- Czauderna, M.; Białek, M.; Krajewska, K.; Ruszczyńska, A.; Bulska, E. Seleniumsupplementation into diets containing carnosic acid, fish and rapeseed oils affects the chemical profile of whole blood in lambs. J. Anim. Feed Sci. 2017, 26, 192–203. [Google Scholar] [CrossRef]
- Jagielska, A.; Ruszczyńska, A.; Wagner, B.; Bulska, E.; Skrajnowska, D.; Bobrowska-Korczak, B. ICP-MS analysis of diet supplementation influence on the elemental content of rat prostate gland. Monatshefte Chem. Chem. Mon. 2019, 150, 1681–1690. [Google Scholar] [CrossRef]
- Donner, M.W.; Siddique, T. A rapid and sensitive IC-ICP-MS method for determining selenium speciation in natural waters. Can. J. Chem. 2018, 96, 795–802. [Google Scholar] [CrossRef]
- Kurek, E.; Ruszczyńska, A.; Wojciechowski, M.; Czauderna, M.; Bulska, E. Studyon speciation of selenium in animal tissues using high performance liquid chromatography with On-line detection by inductively coupled plasma mass spectrometry. Chem. Anal. 2009, 54, 43–57. [Google Scholar]
- Ruszczyńska, A.; Konopka, A.; Kurek, E.; Elguera, J.C.T.; Bulska, E. Investigationof biotransformation of selenium in plants using spectrometric methods. Spectrochim. Acta Part B At. Spectrosc. 2017, 130, 7–16. [Google Scholar] [CrossRef]
- Wróbel, K.; Wróbel, K.; Kannamkumarath, S.S.; Caruso, J.A.; Wysocka, I.A.; Bulska, E.; Świa̧tek, J.; Wierzbicka, M. HPLC–ICP-MS speciation of selenium in enriched onion leaves–a potential dietary source of Se-methylselenocysteine. Food Chem. 2004, 86, 617–623. [Google Scholar] [CrossRef]
- Bodnar, M.; Konieczka, P. Evaluationof candidate reference material obtained from selenium-enriched sprouts for the purpose of selenium speciation analysis. LWT 2016, 70, 286–295. [Google Scholar] [CrossRef]
- Sele, V.; Ørnsrud, R.; Sloth, J.J.; Berntssen, M.H.G.; Amlund, H. Selenium and selenium species in feeds and muscle tissue of Atlantic salmon. J. Trace Elem. Med. Biol. 2018, 47, 124–133. [Google Scholar] [CrossRef]
- Deitrich, C.L.; Cuello-Nuñez, S.; Kmiotek, D.; Torma, F.A.; Busto, M.E.d.; Fisicaro, P.; Goenaga-Infante, H. AccurateQuantification of Selenoprotein P (SEPP 1 ) in Plasma Using Isotopically Enriched Seleno-peptides and Species-Specific Isotope Dilution with HPLC Coupled to ICP-MS/MS. Anal. Chem. 2016, 88, 6357–6365. [Google Scholar] [CrossRef]
- Kurek, E.; Ruszczyńska, A.; Wojciechowski, M.; Łuciuk, A.; Michalska-Kacymirow, M.; Motyl, I.; Bulska, E. Bio-transformation of selenium in Se-enriched bacterial strains of Lactobacillus casei. Rocz. Panstw. Zakl. Hig. 2016, 67, 253–262. [Google Scholar] [PubMed]
- Acosta, M.; Torres, S.; Mariño-Repizo, L.; Martinez, L.D.; Gil, R.A. Novelmethod for metalloproteins determination in human breast milk by size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. J. Pharm. Biomed. Anal. 2018, 158, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Rumpler, A.; Francesconi, K.A.; Pergantis, S.A. Quantitativeselenium speciation in human urine by using liquid chromatography–electrospray tandem mass spectrometry. Anal. Chim. Acta. 2012, 731, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Frascarolo, P.; Polati, S.; Medana, C.; Gianotti, V.; Palma, P.; Aigotti, R.; Baiocchi, C.; Gennaro, M.C. Speciationof selenium in diet supplements by HPLC–MS/MS methods. Food Chem. 2007, 105, 1738–1747. [Google Scholar] [CrossRef]
- Konopka, A.; Winter, D.; Konopka, W.; Busto, M.E.d.; Nunez, S.; Goenaga-Infante, H.; Fisicaro, P.; Lehmann, W.D. [Sec-to-Cys]selenoprotein–a novel type of recombinant, full-length selenoprotein standard for quantitative proteomics. J. Anal. At. Spectrom. 2016, 31, 1929–1938. [Google Scholar] [CrossRef]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelinesfor terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Michalke, B.; Witte, H.; Schramel, P. Effectof different extraction procedures on the yield and pattern of Se-species in bacterial samples. Anal. Bioanal. Chem. 2002, 372, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Kacymirow, M.; Kurek, E.; Smolis, A.; Wierzbicka, M.; Bulska, E. Biologicaland chemical investigation of Allium cepa L. response to selenium inorganic compounds. Anal. Bioanal. Chem. 2014, 406, 3717–3722. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.G.; Mataveli, L.R.V.; Arruda, M.A.Z. Speciationanalysis of selenium in plankton, Brazil nut and human urine samples by HPLC–ICP-MS. Talanta 2013, 110, 53–57. [Google Scholar] [CrossRef]
- Montes-Bayón, M.; Yanes, E.G.; de León, C.P.; Jayasimhulu, K.; Stalcup, A.; Shann, J.; Caruso, J.A. InitialStudies of Selenium Speciation in Brassica juncea by LC with ICPMS and ES-MS Detection: An Approach for Phytoremediation Studies. Anal. Chem. 2002, 74, 107–113. [Google Scholar] [CrossRef]
- Kápolna, E.; Fodor, P. Speciationanalysis of selenium enriched green onions (Allium fistulosum) by HPLC-ICP-MS. Microchem. J. 2006, 84, 56–62. [Google Scholar] [CrossRef]
- Kannamkumarath, S.S.; Wrobel, K.; Wrobel, K.; Vonderheide, A.; Caruso, J.A. HPLC–ICP–MS determination of selenium distribution and speciation in different types of nut. Anal. Bioanal. Chem. 2002, 373, 454–460. [Google Scholar] [CrossRef]
- Shiobara, Y.; Ogra, Y.; Suzuki, K.T. Speciationof metabolites of selenate in rats by HPLC-ICP-MS. Analyst 1999, 124, 1237–1241. [Google Scholar] [CrossRef]
- Bierla, K.; Dernovics, M.; Vacchina, V.; Szpunar, J.; Bertin, G.; Lobinski, R. Determinationof selenocysteine and selenomethionine in edible animal tissues by 2D size-exclusion reversed-phase HPLC-ICP MS following carbamidomethylation and proteolytic extraction. Anal. Bioanal. Chem. 2008, 390, 1789–1798. [Google Scholar] [CrossRef]
- Czauderna, M.; Ruszczyńska, A.; Bulska, E.; Krajewska, K.A. Seleno-compounds and Carnosic Acid Added to Diets with Rapeseed and Fish Oils Affect Concentrations of Selected Elements and Chemical Composition in the Liver, Heart and Muscles of Lambs. Biol. Trace Elem. Res. 2018, 184, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Daun, C.; Lundh, T.; Önning, G.; Åkesson, B. Separationof soluble selenium compounds in muscle from seven animal species using size exclusion chromatography and inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2004, 19, 129–134. [Google Scholar] [CrossRef]
- Jaworska, D.; Czauderna, M.; Przybylski, W.; Rozbicka-Wieczorek, A.J. Sensoryquality and chemical composition of meat from lambs fed diets enriched with fish and rapeseed oils, carnosic acid and seleno-compounds. Meat Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamashita, M. Identificationof a Novel Selenium-containing Compound, Selenoneine, as the Predominant Chemical Form of Organic Selenium in the Blood of Bluefin Tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, P.; Teng, T.; Shi, B.; Shan, A.; Lei, X.G. Effectsof Dietary Selenium Deficiency or Excess on Selenoprotein Gene Expression in the Spleen Tissue of Pigs. Animals 2019, 9, 1122. [Google Scholar] [CrossRef]
- Bryszewska, M.A.; Måge, A. Determinationof selenium and its compounds in marine organisms. J. Trace Elem. Med. Biol. 2015, 29, 91–98. [Google Scholar] [CrossRef]
- Lorentzen, M.; Maage, A.; Julshamn, K. Effectsof dietary selenite or selenomethionine on tissue selenium levels of Atlantic salmon (Salmo salar). Aquaculture 1994, 121, 359–367. [Google Scholar] [CrossRef]
- Mattioli, S.; Rosignoli, P.; D’Amato, R.; Fontanella, M.C.; Regni, L.; Castellini, C.; Proietti, P.; Elia, A.C.; Fabiani, R.; Beone, G.M.; et al. Effectof Feed Supplemented with Selenium-Enriched Olive Leaves on Plasma Oxidative Status, Mineral Profile, and Leukocyte DNA Damage in Growing Rabbits. Animals 2020, 10, 274. [Google Scholar] [CrossRef]
- Rozbicka-Wieczorek, A.; Czauderna, M.; Więsyk, E.; Radzik-Rant, A. Seleniumspecies in diet containing carnosic acid, fish and rapeseed oils affect fatty acid profiles in lamb muscles. J. Anim. Feed Sci. 2016, 25, 216–225. [Google Scholar] [CrossRef]
- Beilstein, M.A.; Whanger, P.D. ChemicalForms of Selenium in Rat Tissues after Administration of Selenite or Selenomethionine. J. Nutr. 1986, 116, 1711–1719. [Google Scholar] [CrossRef]
- Strzetelski, J.; Brzoska, F.; Kowalski, Z.M.; Osięgowski, S. Feeding Recommendation for Ruminants and Feed Tables; National Research Institute of Animal Production: Cracow, Poland, 2014. [Google Scholar]
- Czauderna, M.; Rochalska, M. Studieson the differences in the effects of SeO2 and organic Se-compounds on the distribution of Hg, Co, Fe, Zn and Rb in mice by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. Artic. 1986, 99, 265–277. [Google Scholar] [CrossRef]
- Czauderna, M.; Konecki, J.; Glowacka, M. Studies on the interaction between Se, Te, Cd, As and Zn and the distribution of Fe, Co, Rb and Hg in mice by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. Artic. 1987, 109, 79–88. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulationof Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenomethionine: A Review of Its Nutritional Significance, Metabolism and Toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef]
| Group | Supplements Added to the Basal Diet (BD) | FCE for 35 Days of the Experimental Period (kg BWG/kg Diet Intake) | The Body Weight of Lambs | BWG 3 (kg) | |
|---|---|---|---|---|---|
| BWinitial 1 (kg) | BW35days 2 (kg) | ||||
| Group I 4 (control group) | 3% RO (the RO diet 6) | 0.150 ± 0.001 a,α | 30.7 ± 2.5 a | 36.3 ± 2.8 a | 5.6 ± 1.0 a,α (0.16 ± 0.03) |
| Group II 5 | 2% RO and 1% FO (the FO diet 6) | 0.189 ± 0.001 a,b,β | 30.6 ± 2.1 a | 37.7 ± 1.8 a,b | 7.1 ± 1.0 a,b,β (0.20 ± 0.03) |
| Group III 5 | 2% RO, 1% FO and 0.1% CA (the CA diet 6) | 0.174 ± 0.001 a | 30.6 ± 1.9 a | 37.2 ± 1.4 a | 6.6 ± 0.8 a (0.19 ± 0.02) |
| Group IV 5 | 2% RO, 1% FO, 0.1% CA and 0.35 mg Se as SeY in 1 kg BD (the CASeY diet 6) | 0.174 ± 0.001 a | 30.3 ± 1.3 a | 36.8 ± 1.4 a | 6.5 ± 0.7 a (0.19 ± 0.02) |
| Group V 5 | 2% RO, 1% FO, 0.1% CA and 0.35 mg Se as Se (VI) in 1 kg BD (the CASeVI diet 6) | 0.215 ± 0.001 b | 30.3 ± 2.1 a | 38.5 ± 2.7 b | 8.2 ± 0.7 b (0.23 ± 0.02) |
| Analytes | Precursor Ion (m/z) | Product Ion (m/z) | Dwell Time (ms) | Q1 Voltage Potential (V) | CE Collision Energy (V) | Q3 Voltage Potential (V) |
|---|---|---|---|---|---|---|
| SeMetSeCys 1 | 183.90 | 167.00 | 100.0 | −20.0 | −10.0 | −20.0 |
| 183.90 | 94.95 | 100.0 | −20.0 | −25.0 | −20.0 | |
| SeCys2 2 | 336.80 | 247.90 | 100.0 | −20.0 | −15.0 | −20.0 |
| 336.80 | 88.15 | 100.0 | −20.0 | −25.0 | −20.0 | |
| SeMet3 | 198.00 | 181.15 | 100.0 | −25.0 | −12.0 | −30.0 |
| 198.00 | 109.10 | 100.0 | −24.0 | −23.0 | −18.0 |
| Experimental Diet | Total Se Content | Extracted SeCys2 | Extracted SeMetSeCys | Extracted SeMet | |
|---|---|---|---|---|---|
| μg/kg dm | μg/kg dm | μg/kg dm | μg/kg dm | ||
| Liver | group I—RO diet | 853.5 ± 192.2 | 51.2 ± 15.1 | 69.7 ± 16.6 | 49.9 ± 2.7 |
| group II—FO diet | 700.1 ± 76.3 | 45.7 ± 16.3 | 73.3 ± 19.7 | 43.7 ± 5.4 | |
| group III—CA diet | 740.4 ± 113.4 | 55.2 ± 11.1 | 68.7 ± 11.1 | 49.7 ± 9.2 | |
| group IV—CASeY diet | 1381.4 ± 139.6 | 132.8 ± 12.3 | 166.2 ± 19.5 | 176.6 ± 14.8 | |
| group V—CASeVI diet | 1448.2 ± 179.2 | 44.7 ± 16.2 | 137.6 ± 12.6 | 103.1 ± 7.7 | |
| Muscle MLD | group I—RO diet | 243.7 ± 31.3 | 52.0 ± 4.6 | 58.3 ± 10.1 | 64.5 ±7.9 |
| group II—FO diet | 252.2 ± 25.0 | 60.7 ± 6.3 | 65.0 ± 15.2 | 60.1 ± 8.3 | |
| group III—CA diet | 257.1 ± 35.6 | 60.4 ± 2.3 | 65.5 ± 3.2 | 42.3 ± 20.9 | |
| group IV—CASeY diet | 352.5 ± 127.2 | 79.0 ± 4.1 | 156.1 ± 23.0 | 254.8 ± 11.3 | |
| group V—CASeVI diet | 317.7 ± 26.5 | 55.4 ± 3.6 | 50.9 ± 8.8 | 121.9 ± 16.9 | |
| Heart | group I—RO diet | 767.1 ± 99.6 | 68.6 ± 4.6 | 62.2 ± 9.0 | 55.7 ± 4.9 |
| group II—FO diet | 799.4 ± 121.7 | 68.9 ± 12.4 | 50.2 ± 10.6 | 43.2 ± 3.3 | |
| group III—CA diet | 871.2 ± 59.5 | 61.1 ± 15.2 | 47.9 ± 8.6 | 59.5 ± 3.0 | |
| group IV—CASeY diet | 1134.5 ± 142.4 | 92.1 ± 6.8 | 128.4 ± 6.5 | 89.0 ± 11.3 | |
| group V—CASeVI diet | 1138.5 ± 103.5 | 62.6 ± 3.5 | 60.3 ± 8.6 | 99.3 ± 12.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawor, A.; Ruszczynska, A.; Czauderna, M.; Bulska, E. Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals 2020, 10, 808. https://doi.org/10.3390/ani10050808
Gawor A, Ruszczynska A, Czauderna M, Bulska E. Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals. 2020; 10(5):808. https://doi.org/10.3390/ani10050808
Chicago/Turabian StyleGawor, Andrzej, Anna Ruszczynska, Marian Czauderna, and Ewa Bulska. 2020. "Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods" Animals 10, no. 5: 808. https://doi.org/10.3390/ani10050808
APA StyleGawor, A., Ruszczynska, A., Czauderna, M., & Bulska, E. (2020). Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals, 10(5), 808. https://doi.org/10.3390/ani10050808