Effects of Dietary Iron Level on Growth Performance, Immune Organ Indices and Meat Quality in Chinese Yellow Broilers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Chickens and Husbandry
2.3. Diets
2.4. Measurements
2.4.1. Growth Performance
2.4.2. Sampling
2.4.3. Meat Quality
2.4.4. Biochemical Indices in Plasma, Liver and Kidney
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Indices of Immune Organs
3.3. Biochemical Analyses
3.4. Meat Quality
3.5. Regression Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Von, D.A.; Adamson, J.W. Iron metabolism in man. JPEN-Parenter. Enter. 2013, 37, 599–606. [Google Scholar]
- Taschetto, D.; Vieira, S.L.; Angel, C.R.; Stefanello, C.; Kindlein, L.; Ebbing, M.A.; Simoes, C.T. Iron requirements of broiler breeder hens. Poult. Sci. 2017, 96, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrition Requirements of Poultry, 9th ed.; National Academy Press: Cambridge, MA, USA, 1994.
- Southern, L.L.; Baker, D.H. Iron status of the chick as affected by Eimeria acervulina infection and by variable iron ingestion. J. Nutr. 1982, 112, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y. Study on Relative Bioavailability of Iron Proteinate and an Optimal Dietary Iron Level for Broilers. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]
- Davis, P.N.; Norris, L.C.; Kratzer, F.H. Iron Utilization and Metabolism in the Chick. J. Nutr. 1968, 94, 407–416. [Google Scholar] [CrossRef]
- Bao, Y.M.; Choct, M.; Iji, P.A.; Bruerton, K. Effect of organically complexed copper, iron, manganese, and zinc on broiler performance, mineral excretion, and accumulation in tissues. J. Appl. Poult. Res. 2007, 16, 448–455. [Google Scholar] [CrossRef]
- Waddell, D.G.; Sell, J.L. Effects of dietary calcium and phosphorus on the utilization of dietary iron by the chick. Poult. Sci. 1964, 43, 1249–1257. [Google Scholar] [CrossRef]
- Conrad, M.E.; Barton, J.C. Factors affecting iron balance. Am. J. Hematol. 1981, 10, 199–225. [Google Scholar] [CrossRef]
- Crichton, R.R.; Charloteaux-Wauters, M. Iron storage and transport. Eur. J. Biochem. 1987, 164, 485–506. [Google Scholar] [CrossRef]
- Strube, Y.N.J.; Beard, J.L.; Ross, A.C. Iron deficiency and marginal vitamin A deficiency affect growth, hematological indices and the regulation of iron metabolism genes in rats. J. Nutr. 2002, 132, 3607–3615. [Google Scholar] [CrossRef]
- Rincker, M.J.; Hill, G.M.; Link, J.E.; Rowntree, J.E. Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J. Anim. Sci. 2004, 82, 3189–3197. [Google Scholar] [CrossRef]
- Anderson, G.J.; Vulpe, C.D. Mammalian iron transport. Cell. Mol. Life. Sci. 2009, 66, 3241–3261. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.C.; Broxterman, R.M.; Wilcox, S.L.; Chen, C.; Barstow, T.J. Effect of adipose tissue thickness, muscle site, and sex on near-infrared spectroscopy derived total-[hemoglobin+ myoglobin]. J. Appl. Physiol. 2017, 123, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Apple, J.K.; Wallis-Phelps, W.A.; Maxwell, C.V.; Rakes, L.K.; Sawyer, J.T.; Hutchison, S.; Fakler, T.M. Effect of supplemental iron on finishing swine performance, carcass characteristics, and pork quality during retail display. J. Anim. Sci. 2007, 85, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.K.; Gomez, J.V.; Smith, V.L.; Kanner, J.; Miller, D.D. Dietary iron in swine rations affects nonheme iron and TBARS in pork skeletal muscles. J. Food. Sci. 1994, 59, 747–750. [Google Scholar] [CrossRef]
- Farrow, G.; Glassman, A.S.; Vohra, P.; Kratzer, F.H. Effect of high fat and iron levels on the growth and mortality of chickens. Poult. Sci. 1983, 62, 85–90. [Google Scholar] [CrossRef]
- Cao, J.; Luo, X.G.; Henry, P.R.; Ammerman, C.B.; Littell, R.C.; Miles, R.D. Effect of dietary iron concentration, age, and length of iron feeding on feed intake and tissue iron concentration of broiler chicks for use as a bioassay of supplemental iron sources. Poult. Sci. 1996, 75, 495–504. [Google Scholar] [CrossRef]
- Gou, Z.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Jiang, Z.Y.; Zheng, C.T.; Ding, F.Y.; Jiang, S.Q. Effects of oxidative stress induced by high dosage of dietary iron ingested on intestinal damage and caecal microbiota in Chinese Yellow broilers. J. Anim. Physiol. An. N. 2018, 102, 924–932. [Google Scholar] [CrossRef]
- Ma, C.Y. Study on an Optimal Dietary Iron Level for Broilers Fed a Corn-Soybean Meal Diet from 22 to 42 Days of Age. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Sun, X.X. Effects of Supplementing Copper, Iron, Zinc, and Manganese on Growth Performance, Meat Quality, and Inmmunity of Broilers. Ph.D. Thesis, Northwest A & F University, Yanglin, China, 2009. [Google Scholar]
- Sauvant, D.; Perez, J.M.; Tran, G. Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish; Wageningen Academic Publishers: Cambridge, MA, USA, 2004. [Google Scholar]
- Xiong, B.H.; Luo, Q.Y.; Zhou, Z.K. Specification of Chinese feed ingredients and nutritional value table. Chin. Feed 2017, 21, 31–41. [Google Scholar]
- Erf, G.F.; Bottje, W.G.; Bersi, T.K.; Headrick, M.D.; Fritts, C.A. Effects of dietary vitamin E on the immune system in broilers: Altered proportions of CD4 T cells in the thymus and spleen. Poult. Sci. 1998, 77, 529–537. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, W.; Li, Z.Z.; Zhang, C.; Huang, C.; Yang, J.; Kong, G.Y.; Li, Z.F. Repetitive restraint stress changes spleen immune cell subsets through glucocorticoid receptor or β-adrenergic receptor in a stage dependent manner. Biochem. Biophys. Res. Commun. 2018, 495, 1108–1114. [Google Scholar] [CrossRef]
- Kataranovski, M.; Mirkov, I.; Zolotarevski, L.; Popov, A.; Belij, S.; Stošić, J.; Kataranovski, D. Basic indices of spleen immune activity in natural populations of Norway rats (RattusnorvegicusBerkenhout, 1769) in Serbia. Arch. Biol. Sci. 2009, 61, 723–732. [Google Scholar] [CrossRef]
- Guang, S.; Xiao, M.F.; Xue, W.; Xiao, H.; Lei, T.; Jian, L. Effects of organic acid in Folium Isatidis on the immune organ index and activity of enzymes involved in free radicals of immunosuppressed mice. In Proceedings of the 2011 International Conference on Human Health and Biomedical Engineering, Jilin, China, 19–22 August 2011. [Google Scholar]
- Chen, N.H.; Wu, S.J. Analysis on the characteristics of histological location of bursin in immune organ of chicken and duck. Acta. Bio. Ex. Sinica 2003, 36, 155–162. [Google Scholar]
- Cherayil, B.J. Iron and immunity: Immunological consequences of iron deficiency and overload. Arch. Immunol. Ther. Ex. 2010, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C. Iron, infection and immune function. P. Nutr. Soc. 1993, 52, 165–174. [Google Scholar] [CrossRef]
- Feng, J.; Ma, W.Q.; Xu, Z.R.; He, J.X.; Wang, Y.Z.; Liu, J.X. The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim. Feed. Sci. Tech. 2009, 150, 106–113. [Google Scholar] [CrossRef]
- Rothenbacher, H.; Sherman, A.R. Target Organ Pathology in Iron-Deficient Suckling Rats. J. Nutr. 1980, 110, 1648–1654. [Google Scholar] [CrossRef]
- Wu, J.S.; Guo YMZhou, Y.P. Studies on effects of dietary iron on performance and immune function in broiler chick. Acta Zoonutrimenta Sin. 1999, 11, 19–24. [Google Scholar]
- Sun, J.; Liu, D.; Shi, R. Supplemental dietary iron glycine modifies growth, immune function, and antioxidant enzyme activities in broiler chickens. Livest. Sci. 2015, 176, 129–134. [Google Scholar] [CrossRef]
- Amine, E.K.; Neff, R.; Hegsted, D.M. Biological estimation of available iron using chicks or rats. J. Agric. Food. Chem. 1972, 20, 246–251. [Google Scholar] [CrossRef]
- Chausow, D.G.; Czarnecki-Maulden, G.L. The Relative Bioavailability of Iron from Feedstuffs of Plant and Animal Origin to the Chick. Nutr. Res. 1988, 8, 175–185. [Google Scholar] [CrossRef]
- Biehl, R.R.; Emmert, J.L.; Baker, D.H. Iron Bioavailability in Soybean Meal as Affected by Supplemental Phytase and 1α-Hydroxycholecalciferol. Poult. Sci. 1997, 76, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Rutzke, M.A.; Glahn, R.P. Using the domestic chicken (Gallus gallus) as an in vivo model for iron bioavailability. Poult. Sci. 2010, 89, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Adbelrahim, A.I.; WensiDg, T.; Schonnan, A.J.H. Distribution of iron and copper in the liver and spleen of veal calves in relation to the concentration of iron in the diet. J. Vet. Med. Sci. 1986, 40, 209–211. [Google Scholar]
- Wensing, T.; Abdelrahim, A.I.; Schotman, A.J.H. Some aspects of extra iron supply in veal calf fattening. Vet. Res. Commun. 1986, 10, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Lee, H.K.; Lee, W.S.; Shin, K.S.; Paik, I.K. The effect of level and period of Fe-methionine chelate supplementation on the iron content of boiler meat. Asian. Austral. J. Anim. 2008, 21, 1501–1505. [Google Scholar] [CrossRef]
- Furugouri, K. Effect of elevated dietary levels of iron on iron store in liver, some blood constituents and phosphorus deficiency in young swine. J. Anim. Sci. 1972, 34, 573–577. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; Mao, Y.; Zhang, Y.; Niu, L.; Liang, R. Effect of Ultimate pH on Postmortem Myofibrillar Protein Degradation and Meat Quality Characteristics of Chinese Yellow Crossbreed Cattle. Sci. World J. 2014, 4, 1742–1753. [Google Scholar] [CrossRef]
- Nache, M.; Hinrichs, J.; Scheier, R.; Schmidt, H.; Hitzmann, B. Prediction of the pH as indicator of porcine meat quality using Raman spectroscopy and metaheuristics. Chemometr. Intell. Lab. 2016, 154, 45–51. [Google Scholar] [CrossRef]
- Xinru, W.; Ying, Z.; Minyi, H. Harmonized methodology for evaluating the water-holding capacity of chicken breast and its correlation with meat color, tenderness and pH24h. Food. Sci. 2014, 35, 50–56. [Google Scholar]
- Mackenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid Redox Sign. 2008, 10, 997–1030. [Google Scholar] [CrossRef]
- Tugiyanti, E.; Yuwanta, T.; Zuprizal, Z.; Rusman, R. Supplementation of vitamin E and C in feed on meat quality, thiobarbituric acid reactive substance (TBARs) and myoglobin level of muscovy duck meat. J. Indones. Trop. Anim. Agric. 2014, 39, 37–44. [Google Scholar] [CrossRef]
- Wensing, T.; Miltenburg, G.A.J.; Breukink, H.J. Iron Status of New-Born Calves and Effects of Supplementation with Different Amounts of Iron in Veal Calf Fattening. Available online: http://agris.fao.org/agris-search/search.do?recordID=NL9107233 (accessed on 28 December 2019).
| Phases | Starter | Grower | Finisher |
|---|---|---|---|
| Ingredients, % | 1 to 21 d | 22 to 42 d | 43 to 63 d |
| Corn | 61.50 | 63.30 | 67.05 |
| Soy protein concentrate (CP65%) | 9.4 | 9.4 | 9.4 |
| Feather meal | 8.0 | 5.5 | 2.0 |
| DDGS | 15.7 | 15.7 | 15.0 |
| Soybean oil | 0.40 | 1.20 | 1.71 |
| L-lysine HCL | 0.29 | 0.26 | 0.19 |
| L-threonine | 0.28 | 0.36 | 0.43 |
| DL-methioine | 0.15 | 0.10 | 0.06 |
| Calcium carbonate | 1.15 | 1.00 | 0.93 |
| Dicalcium phosphate | 1.82 | 1.70 | 1.45 |
| Sodium chloride | 0.25 | 0.25 | 0.25 |
| Zeolite | 0.06 | 0.23 | 0.53 |
| Vitamin-mineral premix † | 1.00 1 | 1.00 2 | 1.00 3 |
| Nutritional level | |||
| ME (Mcal/kg) § | 2.90 | 3.00 | 3.10 |
| CP, % ‡ | 20.98 | 18.22 | 16.38 |
| Calcium, % ‡ | 1.06 | 0.87 | 0.83 |
| Total phosphorus, % ‡ | 0.68 | 0.65 | 0.61 |
| Lys, % § | 1.05 | 0.98 | 0.85 |
| Met, % § | 0.46 | 0.40 | 0.34 |
| Fe, mg/kg § | 50 | 50 | 50 |
| Fe, mg/kg ¶ | 48.3 | 49.1 | 48.7 |
| Variables ‡ | Dietary Fe level, mg/kg | SEM † | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 90 | 110 | 130 | 150 | |||
| ADG, g | ||||||||
| 1 to 21 d | 15.85 | 15.80 | 15.99 | 16.03 | 15.90 | 15.92 | 0.13 | 0.774 |
| 22 to 42 d | 40.58 | 37.86 | 39.22 | 37.57 | 37.65 | 38.33 | 2.46 | 0.957 |
| 43 to 63 d | 48.43 | 48.90 | 50.16 | 49.62 | 49.88 | 50.66 | 0.95 | 0.905 |
| ADFI, g | ||||||||
| 1 to 21 d | 29.06 | 29.44 | 31.12 | 31.17 | 31.75 | 30.95 | 0.78 | 0.956 |
| 22 to 42 d | 83.79 | 84.65 | 85.01 | 84.07 | 84.24 | 84.24 | 1.43 | 0.676 |
| 43 to 63 d | 120.35 | 120.24 | 122.21 | 119.28 | 120.98 | 117.29 | 2.33 | 0.832 |
| FCR | ||||||||
| 1 to 21 d | 1.83 | 1.86 | 1.95 | 1.94 | 2.00 | 1.94 | 0.04 | 0.261 |
| 22 to 42 d | 2.06 | 2.24 | 2.17 | 2.24 | 2.24 | 2.20 | 0.02 | 0.816 |
| 43 to 63 d | 2.49 | 2.46 | 2.44 | 2.40 | 2.43 | 2.32 | 0.08 | 0.933 |
| Variables ‡ | Dietary Fe level, mg/kg | SEM † | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 90 | 110 | 130 | 150 | |||
| Spleen, % of BW | ||||||||
| 1 to 21 d | 0.171 | 0.187 | 0.177 | 0.153 | 0.171 | 0.210 | 0.026 | 0.195 |
| 22 to 42 d | 0.168 | 0.171 | 0.182 | 0.178 | 0.166 | 0.163 | 0.008 | 0.805 |
| 43 to 63 d | 0.184 | 0.190 | 0.192 | 0.178 | 0.188 | 0.183 | 0.012 | 0.702 |
| Thymus, % of BW | ||||||||
| 1 to 21 d | 0.300 | 0.304 | 0.342 | 0.323 | 0.296 | 0.300 | 0.020 | 0.456 |
| 22 to 42 d | 0.300 | 0.304 | 0.342 | 0.323 | 0.296 | 0.300 | 0.020 | 0.456 |
| 43 to 63 d | 0.367 | 0.393 | 0.382 | 0.375 | 0.405 | 0.362 | 0.052 | 0.198 |
| Bursa of Fabricius, % of BW | ||||||||
| 1 to 21 d | 0.491 | 0.448 | 0.526 | 0.528 | 0.504 | 0.443 | 0.046 | 0.430 |
| 22 to 42 d | 0.353 | 0.376 | 0.390 | 0.366 | 0.378 | 0.429 | 0.034 | 0.603 |
| 43 to 63 d | 0.115 | 0.121 | 0.132 | 0.114 | 0.140 | 0.116 | 0.020 | 0.267 |
| Variables | Dietary Fe level, mg/kg | SEM † | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 90 | 110 | 130 | 150 | |||
| Hematocrit, % | ||||||||
| 1 to 21 d | 29.3 | 29.6 | 29.5 | 30.6 | 29.4 | 30.2 | 8.55 | 0.915 |
| 22 to 42 d | 30.8 | 31.5 | 32.2 | 32.4 | 32.4 | 31.5 | 6.23 | 0.552 |
| 43 to 63 d | 30.9 | 31.0 | 30.9 | 30.1 | 31.2 | 30.0 | 7.11 | 0.939 |
| Fe, (mol /g) | ||||||||
| Liver 43 to 63 d | 8.52 | 9.54 | 10.06 | 10.01 | 10.38 | 11.37 | 0.41 | 0.516 |
| Kidney 43 to 63 d | 5.65 | 6.04 | 6.04 | 6.73 | 6.48 | 7.52 | 0.24 | 0.262 |
| Variables | Dietary Fe level, mg/kg | SEM † | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 90 | 110 | 130 | 150 | |||||
| Breast muscle, % | 5.43 | 5.50 | 5.39 | 5.55 | 5.52 | 5.47 | 0.12 | 0.974 | ||
| Leg muscle, % | 6.43 | 7.00 | 6.63 | 7.06 | 6.66 | 6.51 | 0.15 | 0.139 | ||
| Meat color of breast muscle | 45 min | L* | 59.70 | 59.39 | 58.80 | 60.99 | 59.27 | 59.29 | 0.57 | 0.257 |
| a* | 15.17 | 15.85 | 15.08 | 14.65 | 15.42 | 15.47 | 0.45 | 0.714 | ||
| b* | 13.27 | 13.31 | 16.56 | 14.85 | 13.71 | 13.32 | 0.93 | 0.349 | ||
| 24 h | L* | 61.23 | 61.19 | 60.57 | 61.42 | 60.62 | 60.81 | 1.19 | 0.984 | |
| a* | 12.32 a | 12.71 a | 12.93 ab | 13.37 ab | 13.32 ab | 14.56 b | 0.15 | 0.041 | ||
| b* | 14.52 | 14.57 | 16.99 | 15.90 | 14.72 | 15.00 | 0.38 | 0.083 | ||
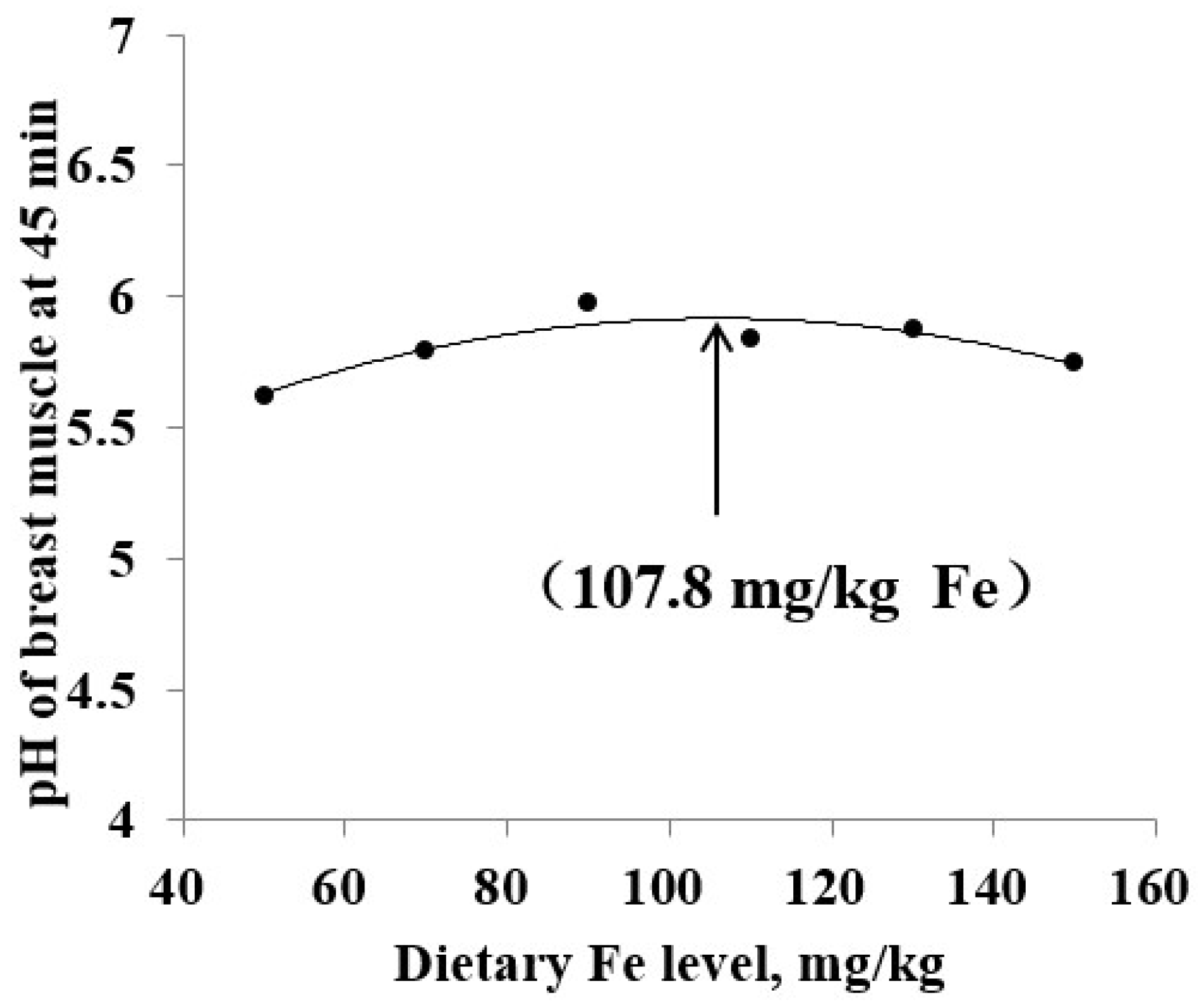
| pH of breast muscle | 45 min | 5.62 b | 5.79 ab | 5.97 a | 5.84 ab | 5.87 ab | 5.75 b | 0.06 | 0.001 | |
| 24 h | 5.72 | 5.83 | 5.89 | 5.85 | 5.82 | 5.85 | 0.04 | 0.477 | ||
| pH of leg muscle | 45 min | 6.12 | 6.13 | 6.17 | 6.21 | 6.19 | 6.24 | 0.05 | 0.342 | |
| 24 h | 6.28 | 6.27 | 6.17 | 6.28 | 6.32 | 6.27 | 0.06 | 0.544 | ||
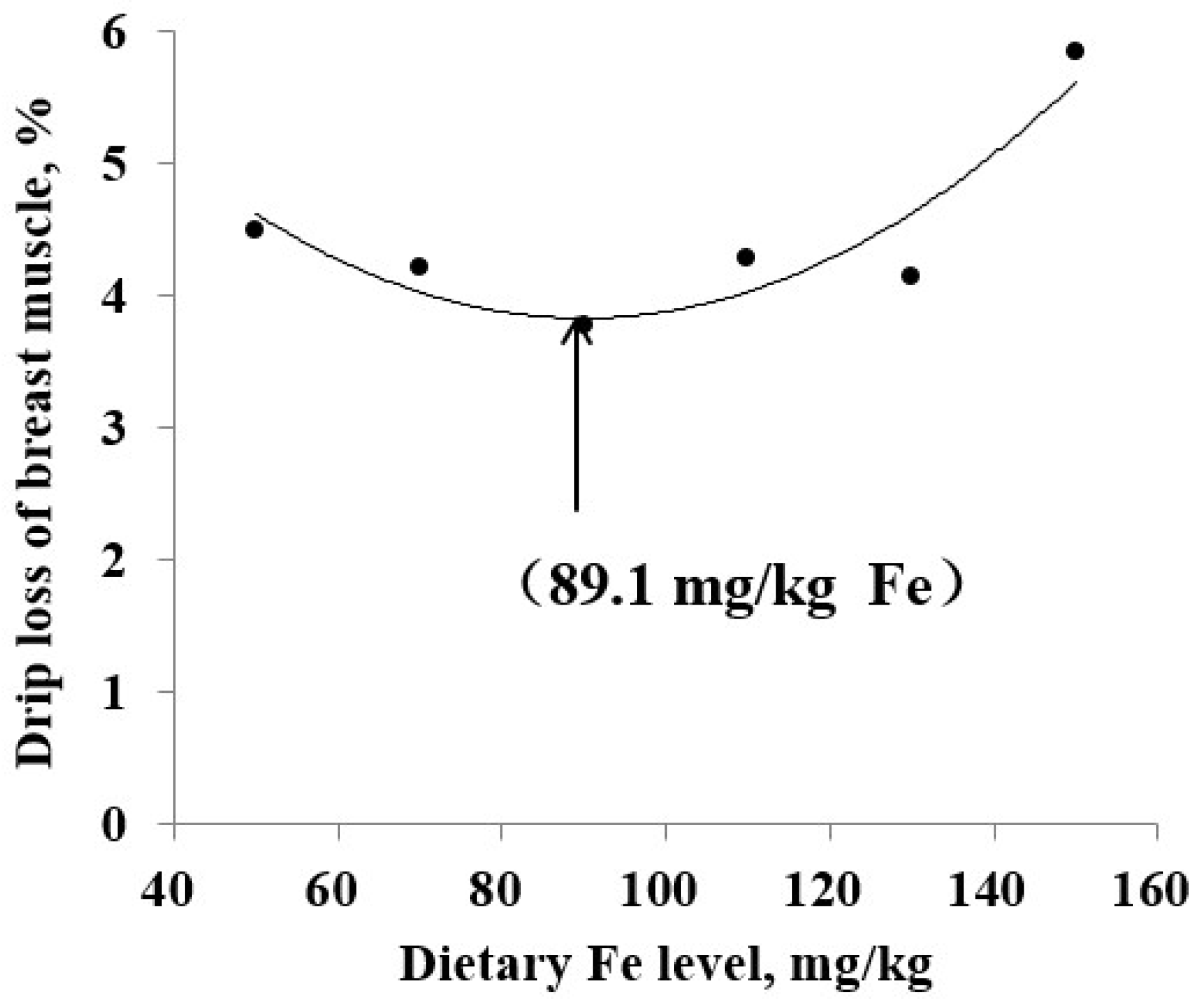
| Drip loss of breast muscle, % | 4.50 b | 4.22 b | 3.77 b | 4.29 ab | 4.14 b | 5.84 a | 0.45 | 0.024 | ||
| Shear force of breast muscle, N | 388.46 | 381.06 | 385.50 | 356.61 | 382.06 | 373.28 | 14.48 | 0.861 | ||
| Variable | Model | Regression Equation 1 | Dietary Fe Response, mg/kg | p-Value | R 2 |
|---|---|---|---|---|---|
| pH of breast muscle (45 min) | QP 2 | y = −0.0009x2 + 0.0194x + 4.8897 | 107.8 | 0.064 | 0.831 |
| Drip loss of breast muscle | QP 2 | y = 0.0005x2 − 0.0891x + 7.8401 | 89.1 | 0.069 | 0.841 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Gou, Z.; Wang, Y.; Li, L.; Fan, Q.; Ding, F.; Zheng, C.; Jiang, S. Effects of Dietary Iron Level on Growth Performance, Immune Organ Indices and Meat Quality in Chinese Yellow Broilers. Animals 2020, 10, 670. https://doi.org/10.3390/ani10040670
Lin X, Gou Z, Wang Y, Li L, Fan Q, Ding F, Zheng C, Jiang S. Effects of Dietary Iron Level on Growth Performance, Immune Organ Indices and Meat Quality in Chinese Yellow Broilers. Animals. 2020; 10(4):670. https://doi.org/10.3390/ani10040670
Chicago/Turabian StyleLin, Xiajing, Zhongyong Gou, Yibing Wang, Long Li, Qiuli Fan, Fayuan Ding, Chuntian Zheng, and Shouqun Jiang. 2020. "Effects of Dietary Iron Level on Growth Performance, Immune Organ Indices and Meat Quality in Chinese Yellow Broilers" Animals 10, no. 4: 670. https://doi.org/10.3390/ani10040670
APA StyleLin, X., Gou, Z., Wang, Y., Li, L., Fan, Q., Ding, F., Zheng, C., & Jiang, S. (2020). Effects of Dietary Iron Level on Growth Performance, Immune Organ Indices and Meat Quality in Chinese Yellow Broilers. Animals, 10(4), 670. https://doi.org/10.3390/ani10040670





