Simple Summary
Horses are frequently transported and exposed to a new environment for sport competition or working tasks and must readapt to their original conditions after a temporary relocation. The objective of this study was to determine if a temporary relocation, and multiple factors associated with a rest period, affect the adrenal response through the analysis of hair cortisol concentrations (HCCs) in horses. Cortisol is a glucocorticoid released after the activation of the hypothalamic–pituitary–adrenal axis and its assessment is being increasingly used as a bioindicator of stress response. Results showed that changes in the daily routine of the animals, including a supposed rest period, increased the HCCs. However, the risk of using low statistical power due to the small sample size cannot be completely ruled out. The elevation in HCCs could be a consequence of the change in the horses’ environmental and routine conditions, which could, in turn, have an impact on their welfare.
Abstract
Horse transportation for temporary relocation during rest periods is a common and widespread practice among horse owners, either from sport competition or working tasks. This study aimed to determine the effect of a relocation period and the multiple factors associated with a rest period on hair cortisol concentrations (HCCs) in horses. Additionally, this study reports the seasonal effect on HCCs and hair growth over a year. Thirteen police horses, Pure Spanish stallions of various ages (5–13 y), were selected to participate in this study. Hair sample collection was carried out approximately every 30 d for seven months (Study 1) and a year (Study 2). Cortisol determinations were performed by enzyme immunoassay. Interestingly, Study 1 revealed that relocated horses (n = 4) exhibited elevated HCCs compared with control horses (n = 4) after the relocation period (p < 0.05). Study 2 (n = 5) showed higher HCCs during summer compared with autumn and winter, and higher hair growth rates in winter compared with the other seasons (p < 0.05). Relocated horses had higher HCCs, suggesting a change in their welfare status, probably related to the sudden change in their surrounding conditions. However, these results should be interpreted cautiously due to the low sample size used. The nature of the relationship between HCCs and horse welfare needs to be further examined.
1. Introduction
Even though machine power has replaced animal force for transport and traction, horses are still used as working animals (e.g., police or carriage horses). Horses are also used in competitive sports like dressage, endurance riding, show jumping, and other disciplines. In the same way that human professional athletes interrupt their competition period, horses are subjected to a compulsory rest period from the competition in endurance disciplines [1]. Nonetheless, the word “rest” is a misnomer as horses continue to train to maintain their athletic performance [2,3]. For logistical reasons, horse owners usually relocate their animals during the rest periods. To the authors’ knowledge, no studies have been performed to assess the effects of a temporary relocation and rest period on horses’ welfare.
Like all mammals, horses are often recurrently exposed to different kinds of stressors and commonly adapt favorably to them. For instance, transport and other stressful situations increase the hypothalamic–pituitary–adrenal (HPA) axis activity releasing cortisol, which produces changes in behavior and energy balance to facilitate coping with the stressor [4]. Because salivary cortisol concentrations correlate well with cortisol concentrations in plasma in horses [5], cortisol in saliva reliably mirrors changes as a result of HPA axis activation. Other matrixes, like feces and urine, have also been used for cortisol determination [6,7,8]. Plasma, salivary, fecal, and urine cortisol concentrations reflect acute stressors, but they are not able to represent a long-term retrospective integrative stress response, as hair cortisol does [9].
Hair cortisol allows reliable monitoring of the accumulation of cortisol during the hair growth cycle [9,10,11]. Because of the lipophilic nature of steroid hormones, these compounds penetrate the hair shaft via passive diffusion from blood during the hair growth cycle [11,12]. In order to obtain a sample with growing hairs, the “shave–reshave” method is frequently used [9]. The method consists on shaving a certain area at the beginning of the study, discarding this hair sample from the analysis. After a period of interest, the regrown hair is reshaved [11,13]. The hair obtained is therefore, representative of the cortisol accumulation and HPA axis activity between the two-time points of interest. However, diverse hair-specific characteristics such as hair growth cycle, the growth rate of the sampling region, seasonal shedding rhythm, and hair color can be a source of variation and should be considered when using hair cortisol concentrations (HCCs) as a presumptive stress indicator [9].
Hair cortisol concentrations can be used as a tool to assess chronic stress or long-term activity of the HPA but not for occasional and sporadic stress events [14]. Experimental repeated stimulations with adrenocorticotropic hormone (ACTH) increased HCCs [15,16,17,18], but single doses of ACTH were insufficient to affect HCCs [14,19], indicating the robustness of HCCs against sporadic stress responses. Additionally, repeated stimulations with corticotropin-releasing hormone (CRH) increased the HCCs in CRH-treated animals compared with non-treated animals [20]. Jointly, the reports suggest that an accumulation of hair cortisol reflects repeated or chronic stimulation of the HPA axis.
Recent studies have described the long-term HPA axis activity in horses through the analysis of HCCs [21,22,23]. The assessment of the HPA axis activity has become a common approach to study stress and animal welfare [4] along with measurements of other end-points of the stress response [24].
This study aimed to determine if HCCs in horses could be affected by relocation stress and the multiple factors associated with a rest period. For this purpose, the long-term HPA axis response in a group of horses from the mounted police was evaluated using HCCs during a temporary relocation and rest period. Additionally, we assessed if other factors affect HCCs, such as endogenous factors (age, hair color, and health status), seasonality, and the hair growth rate.
2. Materials and Methods
Horses were managed following the principles and guidelines of the Ethics Committee on Animal and Human Experimentation from the Universitat Autònoma de Barcelona. Informed consent from the staff of the mounted unit of the Municipal Police of Barcelona and the veterinary staff responsible of the care of the animals was obtained prior to the initiation of the study. No other manipulation different to shaving a small area of the abdomen was performed.
2.1. Hair Sampling and Storage
Hair was collected by shaving a 10 × 17 cm area from the ventral abdomen, left of mid-line and caudal to the sternum. Hair was collected at the skin level using single-use razors for each horse to avoid cross-contamination. Care was taken to avoid skin damage. At each sampling, the entire area was shaved again. About 0.3 to 0.4 g of hair were collected per animal and sample. Samples were stored in individually identified envelopes kept in the dark at room temperature until analytical processing.
2.2. Hair Steroid Extraction
Steroid extraction was performed following a methanol-based protocol previously validated for hair samples [25]. Briefly, 250 ± 3 mg of hair from each sample were weighed with a precision scale and placed in 15 mL conical tubes. Three washes were performed with 2.5 mL of isopropanol (2-propanol 99.5%, Scharlab S.L., Sentmenat, Spain) and mixed with a vortex mixer for 2.5 min each. The supernatant of each wash was removed by decantation. After washing, samples were dried for 36 h at room temperature. Once dried, the hair sample was powdered using a ball mill for 4 min at 25 Hz (MM200, Retsch, Haan, Germany; 10 mL stainless-steel grinding jars; two 12 mm stainless-steel grinding balls). A total of 50 ± 0.5 mg of powdered hair was weighed and placed in 2 mL Eppendorf tubes with 1.5 mL of pure methanol. Incubation was performed for 18 h at 30 °C under moderate shaking (G24 Environmental Incubator Shaker, New Brunswick Scientific CO Inc., Edison, NJ, USA). After incubation, samples were centrifuged at 7000× g for 2 min at 25 °C (Z300K Refrigerated Bench Top Centrifuge, Hermle Labortechnik GmbH, Wehingen, Germany). Then, 0.75 mL of supernatant was transferred to a 1.5 mL Eppendorf tube and placed open in an oven (Heraeus model T6; Kendro® Laboratory Products, Langenselbold, Germany) at 38 °C until complete evaporation. Once evaporated, 0.2 mL of buffer solution included in the enzyme immunoassay (EIA) kit (Cortisol ELISA KIT; Neogen Corporation, Ayr, UK) was added to each 1.5 mL Eppendorf tube and vortexed for 30–60 s to reconstitute the dried extracts. Immediately after reconstitution, hormone extracts were stored at −20 °C until their determination.
2.3. Hair Cortisol Detection and Validation Tests
Hair cortisol concentrations were determined using cortisol EIA detection kits (Cortisol ELISA KIT; Neogen Corporation, Ayr, UK) with a sensitivity of 0.32 pg cortisol/mg of hair. The precision within the test was assessed by calculating intra-assay coefficients of variation (CV, where CV = SD/mean × 100) from all duplicate or triplicate samples analyzed. The inter-assay coefficients of variation were calculated from pool samples with markedly different concentrations and analyzed per duplicate in each EIA kit. Linearity under dilution assesses specificity and accuracy, and was calculated by diluting the pool sample at 1:2, 1:5 and 1:8 ratios with the buffer solution included in the EIA kit. The spike-and-recovery test assesses accuracy and was calculated by adding to 50, 100 and 200 μL of pool sample to 200, 100 and 50 μL of pure standard cortisol solution, respectively. Combinations were repeated with three different pure standard cortisol solutions (20, 2, and 0.2 ng/mL) from the initial solution included in the EIA kit. According to the manufacturer, cross-reactivity of the EIA antibody with other steroids is as follows: prednisolone 47.4%, cortisone 15.7%, 11-deoxycortisol 15.0%, prednisone 7.83%, corticosterone 4.81%, 6β-hydroxycortisol 1.37%, 17-hydroxyprogesterone 1.36%, deoxycorticosterone 0.94%. Steroids with a cross-reactivity <0.06% are not presented.
2.4. Study 1: Relocation Effect on HCCs
A total of 8 Pure Spanish stallions of the Municipal Police of Barcelona, Spain, aged between 5 and 13 y (8.7 ± 2.6 y on average ±SD), were included in this study. The body condition score (BCS) was evaluated using the 0 (emaciated) to 5 (extremely fat) BCS scale [26]. For all the animals included in the study, BCS was 3 (moderate good body condition). Four horses on duty during the entire study period were used as the control group (2 bay and 2 gray), whereas four individuals (relocated horses; 2 bay and 2 gray) were temporally relocated during a specified period in the summer of 2016.
Hair samples (n = 56) were collected approximately every 30 d (33.7 ± 7.1 d, mean ±SD) between August 2016 and February 2017 by shaving the same anatomical area. The hair collected at each sampling was only the new hair grown after the previous collection.
During on-duty periods, all horses were housed in the same building, in indoor individual conventional stalls (2 × 2 m) with wood-chip bedding, with ad libitum access to water, and fed eight times a day (combination of forage, pelleted ration, bran, and fresh grass). Generally, horses trained daily either on the treadmill, patrolling peripheral areas of the city, or performing exercises in the outdoor arena. Transportation to peripheral areas lasted approximately 20–25 min/trip.
Relocated horses were driven to a new location 39.5 km away and 45 min drive in a horse truck from the main stables for a rest period of 22 d (12–31 August). During this period, horses were housed individually in outdoor paddocks (3 × 4 m) with ad libitum access to water, fed three times a day (combination of forage and pelleted ration), with no routine work or training, unknown stable staff, and visual and olfactory contact with unknown male and female horses. After this period, the horses were returned to the main stables of the municipal police under the same conditions as during on-duty periods.
The vehicle used for transportation in both situations was a commercial two-horse van, and each animal was loaded into the truck without the use of force and within 5 min.
Clinical examination findings were recorded routinely by the veterinary staff. Horses’ health status was classified depending on the absence (healthy) or presence (non-healthy) of at least one clinical condition or disease in the clinical history during the 30 d before the hair sampling. Horses with the presence of at least one clinical condition or disease during the hair growth period of a sample were classified as non-healthy for that month. Clinical conditions and disease status included wound/abrasion (4 episodes affecting 3 horses), coronitis (3 episodes affecting 1 horse), lameness (1 episode affecting 1 horse) and back pain (1 episode affecting 1 horse).
2.5. Study 2: Seasonal Effect on HCCs and Hair Growth
Five Pure Spanish stallions of the Municipal Police of Barcelona, Spain, aged between 7 and 13 y (8.8 ± 2.7 y on average ±SD), were included in this study. Their BCS was 3 (moderate good body condition). Hair samples (n = 53) were collected approximately every 30 d (34.4 ± 3.6 d, mean ±SD) between November 2016 and October 2017 by shaving the same anatomical area. The hair collected on each occasion was only the hair that had grown since the previous collection. Additionally, the length of 25 randomly selected hair samples was measured with precision calipers (±0.05 mm; Metric, Spain).
All horses were housed in the same building, in indoor individual conventional stalls (2 × 2 m) with wood-chip bedding, with ad libitum access to water, and fed eight times a day (combination of forage, pelleted ration, bran, and fresh grass). The routine work of horses was the same as the daily training of Study 1 horses. Transportation to peripheral areas lasted approximately 20–25 min/trip.
Hair samplings were classified per month and according to the season as spring, April to June; summer, July to September; autumn, October to December; and winter, January to March.
2.6. Statistical Analyses
Statistical analyses were performed using GraphPad software (version 8.0.2) and RStudio software (R version 3.4.4). All data were checked for outliers and normal distribution using graphic tests (QQ-plot and box-plot) and the numeric test Shapiro–Wilk. The hair cortisol concentration data were not normally distributed. Transformed data restored normality using the inverse distribution (1/x). The significance level for all data was set at p < 0.05. Data are presented as median (25% percentile, 75% percentile) unless otherwise stated.
Cortisol biochemical validations were analyzed using Pearson’s product moment correlation to evaluate the correlation between obtained and expected values from serial dilutions and spiked pool extracts with cortisol standard.
Study 1 data were analyzed using a repeated measures ANOVA. Post hoc comparisons over time and groups were performed using the Sidak’s multiple comparisons test. An additional analysis was performed using a linear mixed model, including age, hair color, and health status as dependent factors and individuals as independent factors. Post hoc comparisons over time were performed using Tukey’s multiple comparisons test.
Study 2 data were analyzed using a mixed-effects model to analyze HCCs among months and a one-way ANOVA to analyze HCCs among seasons. Post hoc comparisons over time were performed using Tukey’s multiple comparisons test. Hair growth was analyzed by mixed-effects model. Post hoc comparisons over time were performed using Tukey’s multiple comparisons test.
3. Results
3.1. Biochemical Validation of the Enzyme Immunoassay
Intra-assay variation computed for the mean of 11 replicate tests ranged from 2.07 to 0.07 ng cortisol/mL. Intra-assay and inter-assay CV were 6.52% and 6.82%, respectively. The linearity under dilution study provided R2 of 0.99 (p < 0.001) and the spike-and-recovery test provided R2 of 0.869 (p = 0.005). Replicates used in the spike-and-recovery test ranged from 2.07 to 0.62 ng cortisol/mL. The average recovery percentage from the spike-and-recovery test was 116.23 ± 25.15%.
3.2. Study 1: Relocation Effect on HCCs
Relocated horses and control horses had similar HCCs during the study (p > 0.9) except for the third sampling point (October 2016), when relocated horses had higher HCCs compared to control horses (5.34 (3.67, 9.33) and 3.09 (1.94, 4.85), respectively; p = 0.02), approximately a month after the end of the relocation period (Figure 1). The linear mixed model confirmed no effect of age, hair color (bay vs. gray), and health status on HCCs (p > 0.05).
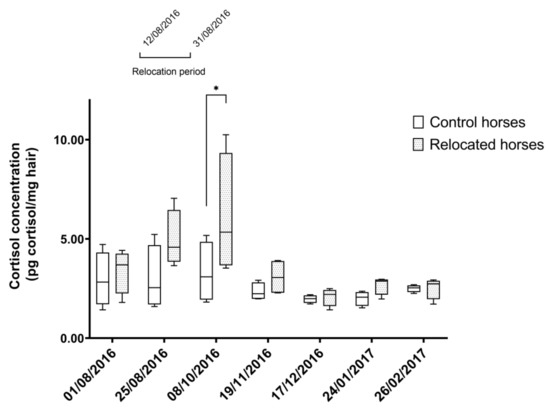
Figure 1.
Hair cortisol concentrations in control and relocated horses during the study period. Hair cortisol concentrations (HCCs) (pg cortisol/mg hair) in control horses (N = 4, n = 28) and HCCs (pg cortisol/mg hair) in relocated horses (N = 4, n = 28) over the time of the study. Relocation period took place between 12th of August 2016 and 31st of August 2016. Asterisks represent significant differences between groups (p < 0.05). Median (25% percentile, 75% percentile). N = number of horses per group; n = number of determinations of HCCs.
3.3. Study 2: Seasonal Effect on HCCs and Hair Growth
The HCC was higher in summer (5.10 (3.67, 5.70) pg cortisol/ mg hair) compared to autumn and winter (2.33 (1.97, 3.50) and 2.36 (1.83, 3.02) pg cortisol/mg hair, p = 0.0006 and p = 0.0002, respectively) (Figure 2). The HCC was similar in spring (4.06 (2.76, 4.41) pg cortisol/mg hair) and the rest of the seasons.
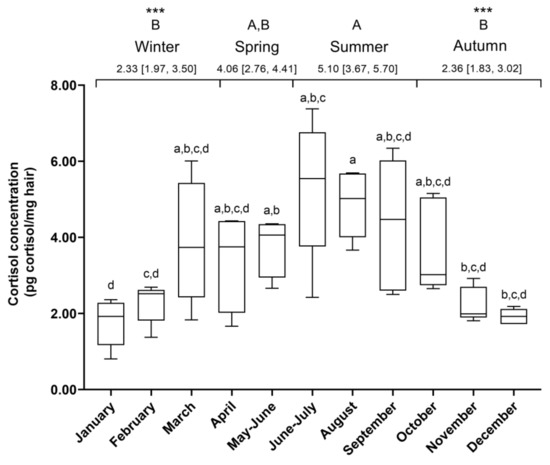
Figure 2.
Fluctuation of hair cortisol concentrations over a year. Representation of hair cortisol concentrations (HCCs) (pg cortisol/mg hair) in horses (N = 5, n = 53) during a year. Different lowercase letters represent significant differences between months. Different uppercase letters represent significant differences between seasons (*** p < 0.001). Median (25% percentile, 75% percentile). N = number of horses per group; n = number of determinations of HCCs.
The mean hair growth rate on the ventral abdomen, left of mid-line and caudal to the sternum was 0.66 ± 0.11 cm/month (mean ±SD). Figure 3 shows the hair growth rate over the year of the study. The mixed-model analysis showed differences between January and March (0.90 ± 0.18 and 0.67 ± 0.17 cm, p = 0.0487), and between February and December (0.86 ± 0.15 and 0.64 ± 0.10 cm, p = 0.0372). Hair growth was faster in winter (0.80 ± 0.24 cm) compared to the rest of the seasons (p < 0.0001). Growth rates were similar in spring, autumn, and summer (0.62 ± 0.27, 0.61 ± 0.12 and 0.58 ± 0.10 cm, respectively, p > 0.05).
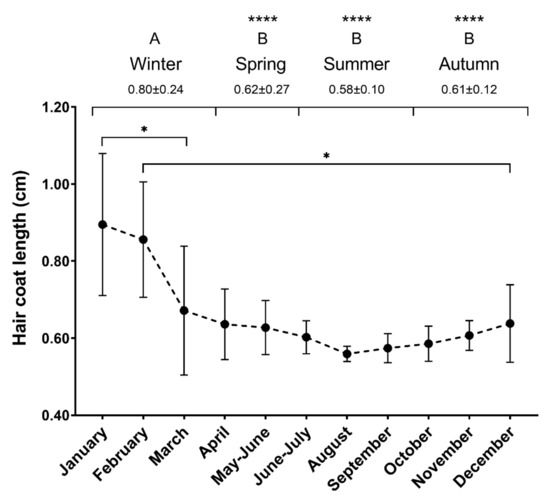
Figure 3.
Hair growth rates over a year. Hair growth (cm) for each month and season in the horses included in the study (n = 5). Different uppercase letters represent significant differences between seasons. Different lowercase letters represent significant differences between months (* p < 0.05, **** p < 0.0001). Mean ±SD.
4. Discussion
To our knowledge, this is the first study to analyze the effect of a rest period and temporary relocation on HCCs in horses. The effects of seasonality, health status, age, hair growth, and coat color (bay vs. gray) were also analyzed.
Interestingly, the results of our study showed that the temporary relocation and rest period experienced by the police horses included in this study increased the HCCs, probably indicating increased HPA axis activity. However, the sample number was small and not representative of all sexes and breeds. Increasing the sample size and including more breeds and both sexes, will probably aid in increasing the statistical power of both studies.
Animal welfare is affected by a complex interrelation of several factors [27]. Thus, our results suggest that an unexpected change in the multiple factors associated with a temporary relocation and resting period such as environment condition, housing system, habitual workload, nutrition, changes in staff and support, and social novelty may result in a wide range of stressors that increase the HPA axis activity in a long-term manner.
Housing systems and management practices affect horses’ behavior [28]. Horses are usually housed in single stalls, but this type of housing restricts some natural behaviors and social interactions [28]. However, police horses spend most of their time training or patrolling with other horses, thus facilitating social interaction. During the relocation period, horses were subjected to solitary free exercise, which seems to be related to a higher degree of stress [28]. However, the external environment provided by the paddocks could ensure important behaviors and physiological needs [29]. Diet composition and changes in feeding frequency can produce fluctuations in horse hormone patterns, including cortisol [30,31]. Interestingly, cows relocated from their habitual environment, and nutrition conditions had higher HCCs [32]. In addition, staff turnover in a laboratory animal facility seems enough to produce an increase of HCCs in rabbits [33]. Taken together, all these potential stressors may have been reflected in the HCCs of the horses included in our study.
The circannual rhythm of cortisol concentrations has been well described previously in healthy and sick horses [34]. Higher plasma cortisol concentrations were found between summer and autumn season compared with the rest of the year [35,36]. Our study reported higher HCC in summer compared with autumn and winter. Nevertheless, the risk of a small sample size with not enough statistical power to identify differences in HCCs between seasons cannot be completely discarded. Slightly different range of cortisol concentrations were detected between our study and the one performed by Duran and colleagues [22], probably related to the different location of the hair sample.
The body region and the type of hair could influence HCCs [9]. Our study showed a hair growth rate of 0.66 ± 0.11 cm/month on average in the ventral abdominal area. Different factors affect the seasonal coat shedding, including the season, genetic background, type of feeding, and housing systems [37,38,39]. This process aims to adjust the body temperature to the environmental conditions. A complete change of the summer coat to a winter coat should take place during late autumn [39]. From August until January, the hair growth rate increased, indicating a greater overall length during late autumn and early winter. In the same way, the highest hair growth rate was found in winter (0.80 ± 0.24 cm/season). Taken together, we observed a normal transition from a summer to a winter coat that usually starts in September in our latitude.
In the present study, the health status did not have a significant influence on horse HCCs. The absence of an effect of the clinical episodes could be explained by the broad range of painless and painful disorders that were included in the health status assessment. In the study performed by Duran et al. [22], HCCs were higher one and two months after a very stressful acute procedure. In that study, horses were submitted to surgical intervention (castration) with complete anesthesia and analgesia protocols. However, lower HCCs have been reported in horses with severe squamous gastric disease when compared to horses without gastric ulcers [40]. Our study included a broad range of disorders that could mask a negative feedback produced by a high concentration of circulating glucocorticoids [41]. In other species such as cows, contradictory results concerning illness and HCCs have been reported [42,43].
No significant differences in HCCs between bay vs. gray hair color were detected in our study. In the same way, hair color had no significant influence on HCCs in bears and wolves [44,45]. However, some authors reported contradictory results regarding this issue in cows [10,25].
Within the range of ages of this study, we did not detect an effect on HCC. This result follows other reports that analyzed cortisol concentrations in other matrixes of the adult horse, such as salivary [46] and plasma cortisol [47]. However, higher HCCs have been found in new-born foals and 30–60-day-old foals compared with adult horses [21,22,23].
5. Conclusions
Our results suggest that HCC can be used to monitor the adaptation of horses to environmental and management variations, although the accurate nature of the relationship between HCC and welfare needs to be further investigated. Results of the present study could be applied to improve the management and environment of horses, which in turn could potentially improve the animals’ welfare. However, future studies with a larger sample size are needed to confirm these implications.
Author Contributions
Conceptualization, E.J.-C. and M.L.-B.; Methodology, J.G., S.O.-M.; Software, J.G. and M.Á.-R.; Validation, J.G., A.C. and O.T.-P.; Formal analysis, J.G., A.C., O.T.-P. and M.Á.-R.; Investigation, J.G. and S.O.-M.; Resources, E.J.-C. and M.L.-B.; Data curation, J.G. and M.Á.-R.; Writing—Original draft preparation, J.G.; Writing—Review and editing, J.G., A.C., O.T.-P., S.O.-M., M.Á.-R., E.J.-C., M.L.-B.; Visualization, J.G.; Supervision, E.J.-C. and M.L.-B.; Project administration, E.J.-C. and M.L.-B.; Funding acquisition, E.J.-C. and M.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the staff of the mounted unit of the Municipal Police of Barcelona and their veterinary staff for the information provided regarding the management and clinical history of the horses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FEI Endurance Rules. Available online: https://inside.fei.org/node/3835/ (accessed on 3 February 2020).
- Mukai, K.; Ohmura, H.; Hiraga, A.; Eto, D.; Takahashi, T.; Asai, Y.; Jones, J.H. Effect of detraining on cardiorespiratory variables in young Thoroughbred horses. Equine Vet. J. Suppl. 2006, 36, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Hiraga, A.; Takahashi, T.; Matsui, A.; Ohmura, H.; Aida, H.; Jones, J.H. Effects of maintaining different exercise intensities during detraining on aerobic capacity in Thoroughbreds. Am. J. Vet. Res. 2017, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Bohák, Z.; Szabó, F.; Beckers, J.F.; Melo de Sousa, N.; Kutasi, O.; Nagy, K.; Szenci, O. Monitoring the circadian rhythm of serum and salivary cortisol concentrations in the horse. Domest. Anim. Endocrinol. 2013, 45, 38–42. [Google Scholar] [CrossRef]
- Pawluski, J.; Jego, P.; Henry, S.; Bruchet, A.; Palme, R.; Coste, C.; Hausberger, M. Low plasma cortisol and fecal cortisol metabolite measures as indicators of compromised welfare in domestic horses (Equus caballus). PLoS ONE 2017, 12, e0182257. [Google Scholar] [CrossRef] [PubMed]
- Mercer-Bowyer, S.; Kersey, D.C.; Bertone, J.J. Use of fecal glucocorticoid and salivary cortisol concentrations as a measure of well-being of New York City carriage horses. J. Am. Vet. Med. Assoc. 2017, 250, 316–321. [Google Scholar] [CrossRef]
- Van Der Kolk, J.H.; Kalsbeek, H.C.; Wensing, T.; Breukink, H.J. Urinary concentration of corticoids in normal horses and horses with hyperadrenocorticism. Res. Vet. Sci. 1994, 56, 126–128. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Ghassemi, J.; Kim, B.W.; Lee, B.H.; Sung, K. Coat and hair color: Hair cortisol and serotonin levels in lactating Holstein cows under heat stress conditions. Anim. Sci. J. 2017, 88, 190–194. [Google Scholar] [CrossRef]
- Meyer, J.S.; Novak, M.A. Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar] [CrossRef]
- Henderson, G.L. Mechanisms of drug incorporation into hair. Forensic Sci. Int. 1993, 63, 19–29. [Google Scholar] [CrossRef]
- Davenport, M.D.; Lutz, C.K.; Tiefenbacher, S.; Novak, M.A.; Meyer, J.S. A Rhesus Monkey Model of Self-Injury: Effects of Relocation Stress on Behavior and Neuroendocrine Function. Biol. Psychiatry 2008, 63, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Tallo-Parra, O.; Lopez-Bejar, M.; Carbajal, A.; Monclús, L.; Manteca, X.; Devant, M. Acute ACTH-induced elevations of circulating cortisol do not affect hair cortisol concentrations in calves. Gen. Comp. Endocrinol. 2017, 240, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, G.D.L.V.; Lemus-Ramirez, V.; Vázquez-Chagoyán, J.C.; Villa-Godoy, A.; Romano, M.C. Effects of adrenocorticotropic hormone challenge and age on hair cortisol concentrations in dairy cattle. Can. J. Vet. Res. 2011, 75, 216–221. [Google Scholar]
- Terwissen, C.V.; Mastromonaco, G.F.; Murray, D.L. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef]
- Mastromonaco, G.F.; Gunn, K.; McCurdy-Adams, H.; Edwards, D.B.; Schulte-Hostedde, A.I. Validation and use of hair cortisol as a measure of chronic stress in eastern chipmunks (Tamias striatus). Conserv. Physiol. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Endo, N.; Yamane, H.; Rahayu, L.P.; Tanaka, T. Effect of repeated adrenocorticotropic hormone administration on reproductive function and hair cortisol concentration during the estrous cycle in goats. Gen. Comp. Endocrinol. 2018, 259, 207–212. [Google Scholar] [CrossRef]
- Ashley, N.T.; Barboza, P.S.; Macbeth, B.J.; Janz, D.M.; Cattet, M.R.L.; Booth, R.K.; Wasser, S.K. Glucocorticosteroid concentrations in feces and hair of captive caribou and reindeer following adrenocorticotropic hormone challenge. Gen. Comp. Endocrinol. 2011, 172, 382–391. [Google Scholar] [CrossRef]
- Schubach, K.M.; Cooke, R.F.; Brandao, A.P.; Lippolis, K.; Hinchliff, M.T.; Bohnert, D.W.; Cerri, R.L.A. Using hair cortisol concentrations to assess the adrenocortical stress response in beef cattle administered corticotrophin-release hormone. J. Anim. Sci. 2016, 94, 109. [Google Scholar] [CrossRef]
- Comin, A.; Veronesi, M.C.; Montillo, M.; Faustini, M.; Valentini, S.; Cairoli, F.; Prandi, A. Hair cortisol level as a retrospective marker of hypothalamic–pituitary–adrenal axis activity in horse foals. Vet. J. 2012, 194, 131–132. [Google Scholar] [CrossRef]
- Duran, M.C.; Janz, D.M.; Waldner, C.L.; Campbell, J.R.; Marques, F.J. Hair Cortisol Concentration as a Stress Biomarker in Horses: Associations with Body Location and Surgical Castration. J. Equine Vet. Sci. 2017, 55, 27–33. [Google Scholar] [CrossRef]
- Montillo, M.; Comin, A.; Corazzin, M.; Peric, T.; Faustini, M.; Veronesi, M.C.; Valentini, S.; Bustaffa, M.; Prandi, A. The Effect of temperature, rainfall, and light conditions on hair cortisol concentrations in newborn foals. J. Equine Vet. Sci. 2014, 34, 774–778. [Google Scholar] [CrossRef]
- MacDougall-Shackleton, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “Stress” Are Not Synonymous. Integr. Org. Biol. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Tallo-Parra, O.; Manteca, X.; Sabes-Alsina, M.; Carbajal, A.; Lopez-Bejar, M. Hair cortisol detection in dairy cattle by using EIA: Protocol validation and correlation with faecal cortisol metabolites. Animal 2015, 9, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Huntington, P.J. Body condition scoring and weight estimation of horses. Equine Vet. J. 1988, 20, 41–45. [Google Scholar] [CrossRef]
- Popescu, S.; Diugan, E.A.; Spinu, M. The interrelations of good welfare indicators assessed in working horses and their relationships with the type of work. Res. Vet. Sci. 2014, 96, 406–414. [Google Scholar] [CrossRef]
- Werhahn, H.; Hessel, E.F.; Van den Weghe, H.F.A. Competition Horses Housed in Single Stalls (II): Effects of Free Exercise on the Behavior in the Stable, the Behavior during Training, and the Degree of Stress. J. Equine Vet. Sci. 2012, 32, 22–31. [Google Scholar] [CrossRef]
- Ruet, A.; Lemarchand, J.; Parias, C.; Mach, N.; Moisan, M.P.; Foury, A.; Briant, C.; Lansade, L. Housing horses in individual boxes is a challenge with regard to welfare. Animals 2019, 9, 621. [Google Scholar] [CrossRef]
- Stull, C.L.; Rodiek, A.V. Responses of Blood Glucose, Insulin and Cortisol Concentrations to Common Equine Diets. J. Nutr. 1988, 118, 206–213. [Google Scholar] [CrossRef]
- Jacob, S.I.; Geor, R.J.; Weber, P.S.D.; Harris, P.A.; McCue, M.E. Effect of dietary carbohydrates and time of year on ACTH and cortisol concentrations in adult and aged horses. Domest. Anim. Endocrinol. 2018, 63, 15–22. [Google Scholar] [CrossRef]
- Comin, A.; Prandi, A.; Peric, T.; Corazzin, M.; Dovier, S.; Bovolenta, S. Hair cortisol levels in dairy cows from winter housing to summer highland grazing. Livest. Sci. 2011, 138, 69–73. [Google Scholar] [CrossRef]
- Peric, T.; Comin, A.; Corazzin, M.; Montillo, M.; Canavese, F.; Stebel, M.; Prandi, A. Relocation and Hair Cortisol Concentrations in New Zealand White Rabbits. J. Appl. Anim. Welf. Sci. 2017, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toribio, R.E. Disorders of the Endocrine System. In Equine Internal Medicine; Reed, S.M., Bayly, W.M., Sellon, D.C., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 1029–1138. [Google Scholar]
- Donaldson, M.T.; McDonnell, S.M.; Schanbacher, B.J.; Lamb, S.V.; McFarlane, D.; Beech, J. Variation in Plasma Adrenocorticotropic Hormone Concentration and Dexamethasone Suppression Test Results with Season, Age, and Sex in Healthy Ponies and Horses. J. Vet. Intern. Med. 2005, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.; Brorsen, B.W.; McFarlane, D. Circadian and circannual rhythms of cortisol, ACTH, and α-melanocyte-stimulating hormone in healthy horses. Domest. Anim. Endocrinol. 2012, 43, 317–324. [Google Scholar] [CrossRef]
- Cymbaluk, N.F.; Christison, G.I. Effects of Diet and Climate on Growing Horses. J. Anim. Sci. 1989, 67, 48. [Google Scholar] [CrossRef]
- Stachurska, A.; Robovský, J.; Bocian, K.; Janczarek, I. Changes of coat cover in primitive horses living on a reserve. J. Anim. Sci. 2011, 93, 1411–1417. [Google Scholar] [CrossRef]
- Bocian, K.; Strzelec, K.; Janczarek, I.; Jabłecki, Z.; Kolstrung, R. Length of winter coat in horses depending on husbandry conditions. Anim. Sci. J. 2017, 88, 339–346. [Google Scholar] [CrossRef]
- Prinsloo, M.; Hynd, P.; Franklin, S.; Weaver, S.; van den Boom, R. Hair cortisol concentration is inversely related to the severity of equine squamous gastric disease. Vet. J. 2019, 249, 58–59. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic–pituitary–adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar]
- Tallo-Parra, O.; Carbajal, A.; Monclús, L.; Manteca, X.; Lopez-Bejar, M. Hair cortisol and progesterone detection in dairy cattle: Interrelation with physiological status and milk production. Domest. Anim. Endocrinol. 2018, 64, 1–8. [Google Scholar] [CrossRef]
- Burnett, T.A.; Madureira, A.M.L.; Silper, B.F.; Tahmasbi, A.; Nadalin, A.; Veira, D.M.; Cerri, R.L.A. Relationship of concentrations of cortisol in hair with health, biomarkers in blood, and reproductive status in dairy cows. J. Dairy Sci. 2015, 98, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, B.J.; Cattet, M.R.L.; Stenhouse, G.B.; Gibeau, M.L.; Janz, D.M. Hair cortisol concentration as a noninvasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): Considerations with implications for other wildlife. Can. J. Zool. 2010, 88, 935–949. [Google Scholar] [CrossRef]
- Bryan, H.M.; Adams, A.G.; Invik, R.M.; Wynne-Edwards, K.E.; Smits, J.E. Hair as a Meaningful Measure of Baseline Cortisol Levels over Time in Dogs. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 189–196. [Google Scholar] [PubMed]
- Aurich, J.; Wulf, M.; Ille, N.; Erber, R.; von Lewinski, M.; Palme, R.; Aurich, C. Effects of season, age, sex, and housing on salivary cortisol concentrations in horses. Domest. Anim. Endocrinol. 2015, 52, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.A.; Wochele, D.M.; Norton, N.A.; Mcfarlane, D.; Wooldridge, A.A.; Frank, N. Effect of Age, Season, Body Condition, and Endocrine Status on Serum Free Cortisol Fraction and Insulin Concentration in Horses. J. Vet. Intern. Med. 2016, 30, 653–663. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).