Effect of Artesunate on Leishmania Amazonesis Induced Neuroinflammation and Nociceptive Behavior in Male Balb/C Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Parasites and Mouse Infections
2.3. Experimental Groups and Treatment
2.4. Paw Edema
2.5. Mechanical Allodynia
2.6. Thermal Hyperalgesia (Paw Withdrawal Test)
2.7. Behaviors
2.7.1. Open Field (OF) Test
2.7.2. Elevated Plus Maze (EPM)
2.8. Histological Analysis and Myeloperoxidase (MPO) Activity
2.9. Cytokine Determination
2.10. Western Blot Analysis
2.11. Parasite Quantification
2.12. Materials
2.13. Statistical Analysis
3. Results
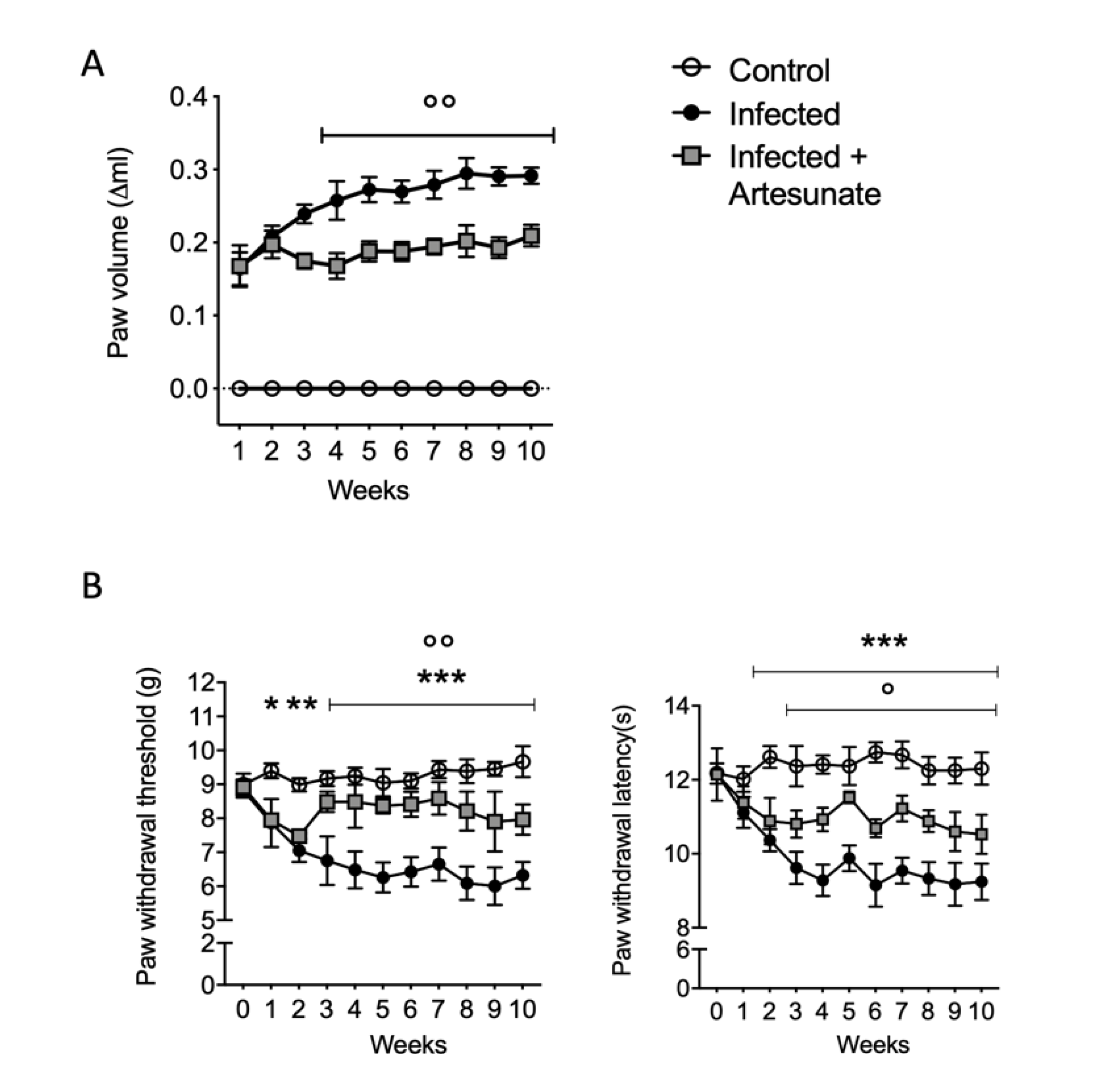
3.1. Effect of Artesunate on L. amazonensis Induced Paw Edema and Pain Behavior
3.2. Effect of Artesunate on L. amazonensis-Induced Sickness Behavior
3.3. Effect of Artesunate on L. amazonensis-Induced MPO and Histological Damage
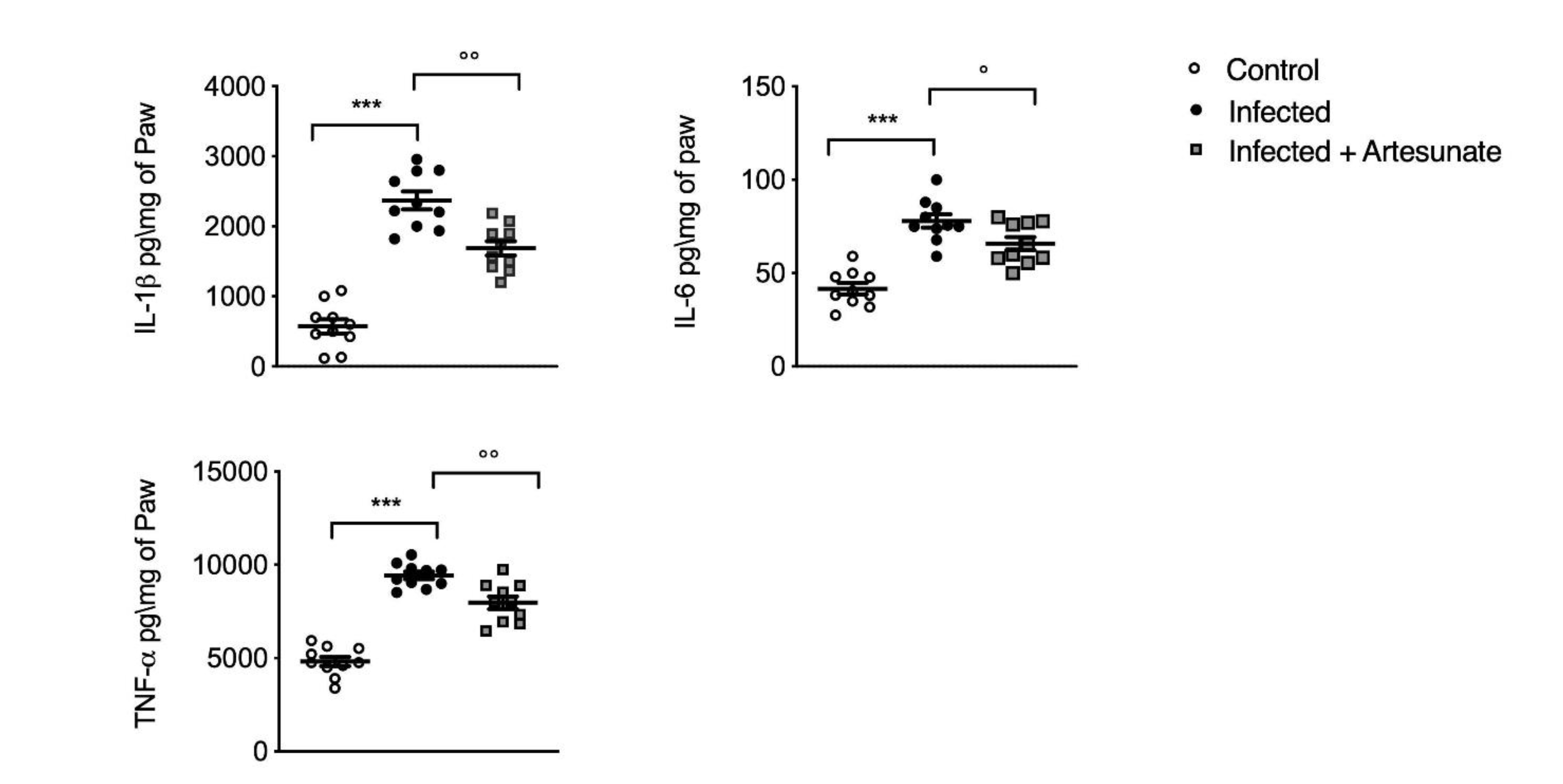
3.4. Effect of Artesunate on L. amazonensis-Induced IL-1β, IL-6, and TNF-α in Paw Tissue
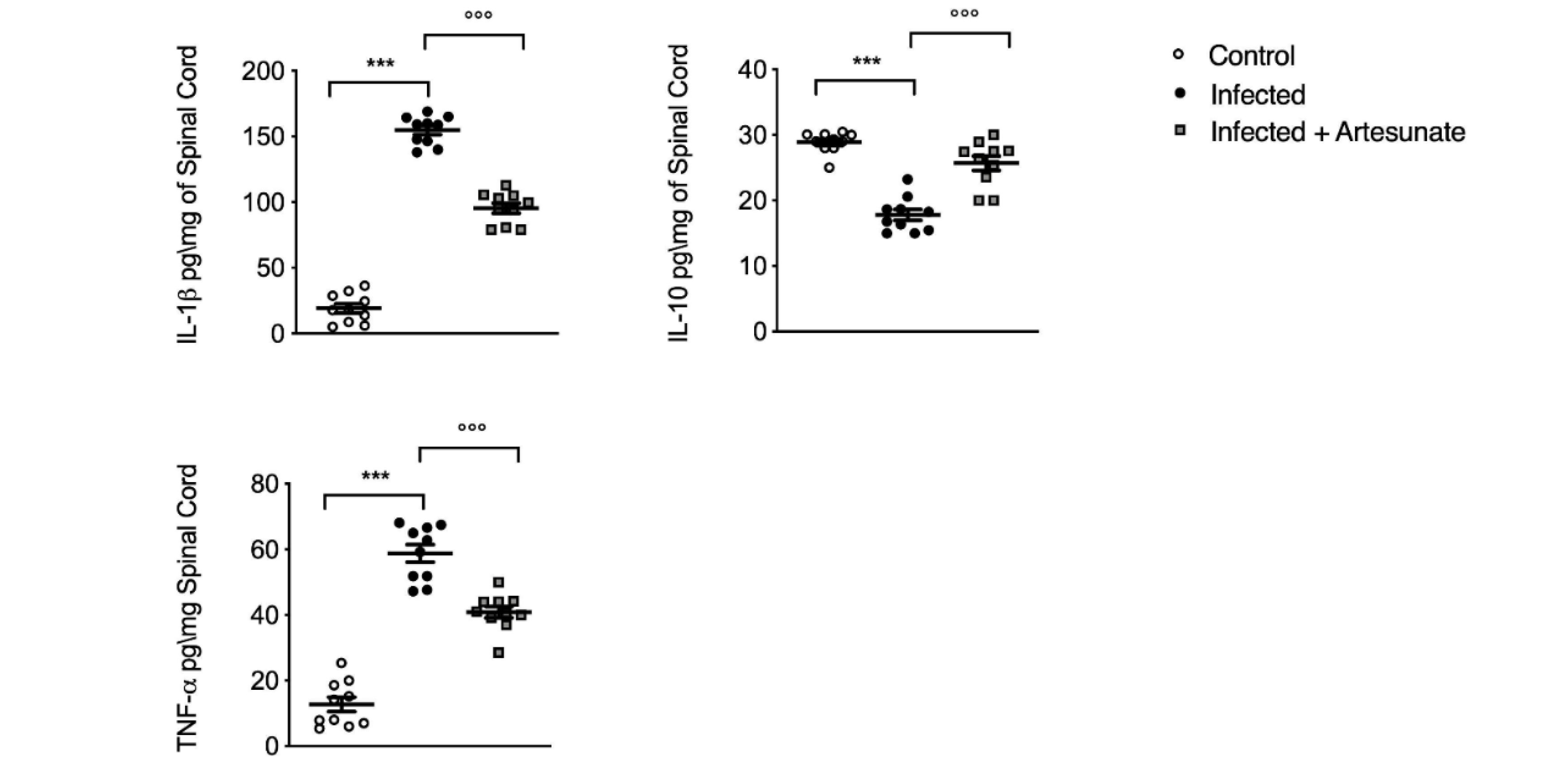
3.5. Effect of Artesunate on L. amazonensis-Induced IL-1β, IL-10, and TNF-α in Spinal Cord Tissue
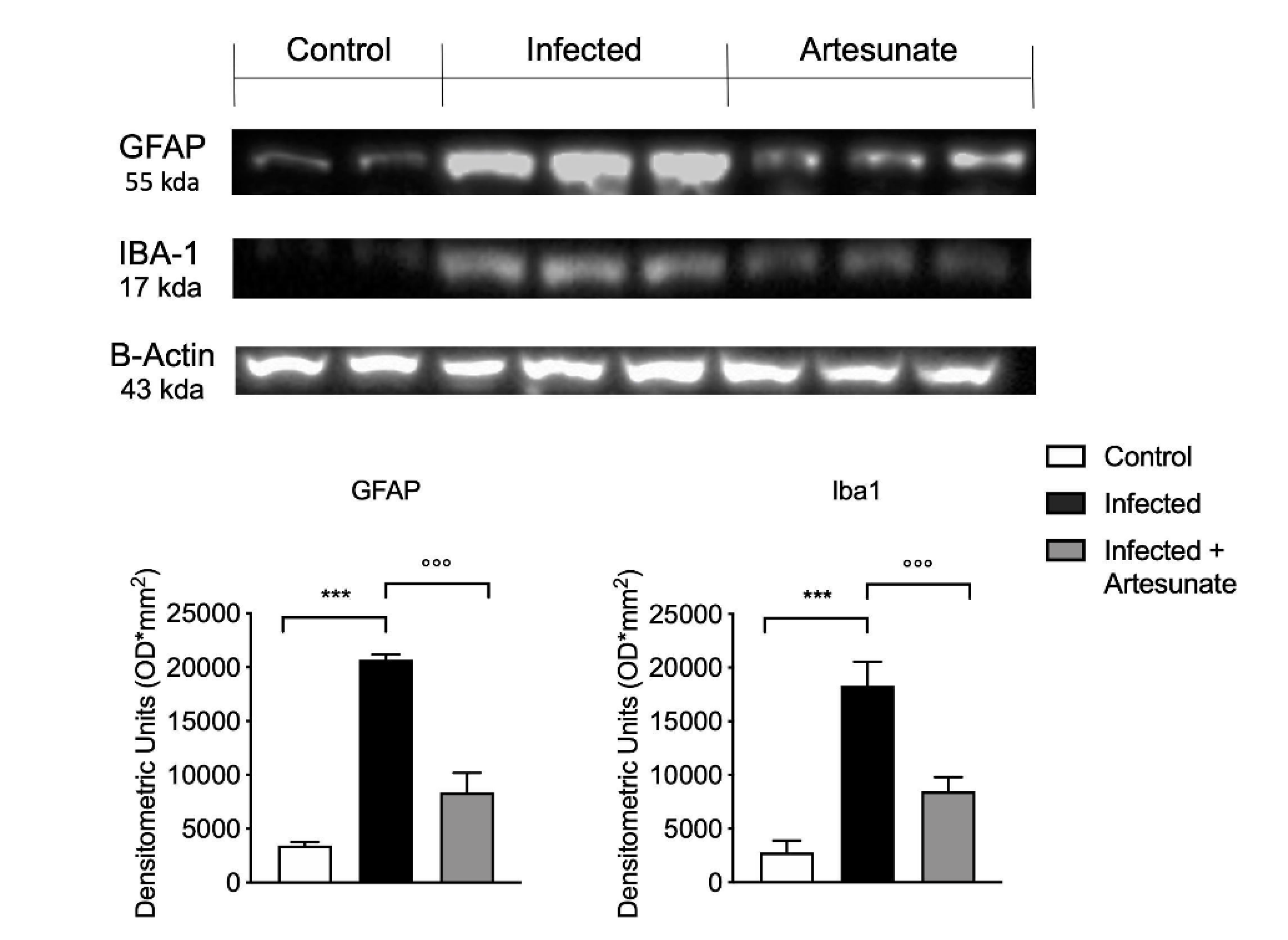
3.6. Effect of Artesunate on L. amazonensis-Induced Astrocyte and Microglia Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashford, R.W. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000, 30, 1269–1281. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis emergence in Europe. EuroSurveillance 2010, 15, 19505. [Google Scholar] [PubMed]
- Petersen, C.A. Leishmaniasis, an emerging disease found in companion animals in the United States. Top. Companion Anim. Med. 2009, 24, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Fattori, V.; Conchon-Costa, I.; Pinge-Filho, P.; Pavanelli, W.R.; Verri, W.A., Jr. Leishmania infection: Painful or painless? Parasitol. Res. 2017, 116, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Jose-Lopez, R.; de la Fuente, C.; Pumarola, M.; Anor, S. Intramedullary spinal cord mass presumptively associated with leishmaniasis in a dog. J. Am. Vet. Med. Assoc. 2014, 244, 200–204. [Google Scholar] [CrossRef]
- Borghi, S.M.; Fattori, V.; Ruiz-Miyazawa, K.W.; Miranda-Sapla, M.M.; Casagrande, R.; Pinge-Filho, P.; Pavanelli, W.R.; Verri, W.A., Jr. Leishmania (L). amazonensis induces hyperalgesia in balb/c mice: Contribution of endogenous spinal cord TNFalpha and NFkappaB activation. Chem. Biol. Interact. 2017, 268, 1–12. [Google Scholar] [CrossRef]
- Portes, A.; Giestal-de-Araujo, E.; Fagundes, A.; Pandolfo, P.; de Sa Geraldo, A.; Lira, M.L.; Amaral, V.F.; Lagrota-Candido, J. Leishmania amazonensis infection induces behavioral alterations and modulates cytokine and neurotrophin production in the murine cerebral cortex. J. Neuroimmunol. 2016, 301, 65–73. [Google Scholar] [CrossRef]
- Croft, S.L.; Coombs, G.H. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003, 19, 502–508. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miro, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Amorim, I.F.; Ribeiro, R.R.; Azevedo, E.G.; Demicheli, C.; Melo, M.N.; Tafuri, W.L.; Gontijo, N.F.; Michalick, M.S.; Frezard, F. Efficacy of combined therapy with liposome-encapsulated meglumine antimoniate and allopurinol in treatment of canine visceral leishmaniasis. Antimicrob. Agents Chemother. 2012, 56, 2858–2867. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; da Silva, M.E.; Dos Santos, C.C.P.; Frezard, F.J.G.; da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. Biomed Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef]
- Barbosa, J.F.; de Figueiredo, S.M.; Monteiro, F.M.; Rocha-Silva, F.; Gaciele-Melo, C.; Coelho, S.S.; Lyon, S.; Caligiorne, R.B. New Approaches on Leishmaniasis Treatment and Prevention: A Review of Recent Patents. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, C.J.; Haynes, R.K.; Krishna, S. Artemisinins. Postgrad. Med. J. 2005, 81, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Nosten, F.; White, N.J. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 2007, 77, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, J.; Pan, X.; Ding, G.; Cao, H.; Jiang, W.; Zheng, J.; Zhou, H. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-alpha release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-kappaB activation. Int. Immunopharmacol. 2010, 10, 344–350. [Google Scholar] [CrossRef]
- Miranda, A.S.; Brant, F.; Rocha, N.P.; Cisalpino, D.; Rodrigues, D.H.; Souza, D.G.; Machado, F.S.; Rachid, M.A.; Teixeira, A.L., Jr.; Campos, A.C. Further evidence for an anti-inflammatory role of artesunate in experimental cerebral malaria. Malar. J. 2013, 12, 388. [Google Scholar] [CrossRef]
- Zuo, S.; Li, Q.; Liu, X.; Feng, H.; Chen, Y. The Potential Therapeutic Effects of Artesunate on Stroke and Other Central Nervous System Diseases. Biomed Res. Int. 2016, 2016, 1489050. [Google Scholar] [CrossRef]
- Loo, C.S.; Lam, N.S.; Yu, D.; Su, X.Z.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Neuroprotective Effect of Artesunate in Experimental Model of Traumatic Brain Injury. Front. Neurol. 2018, 9, 590. [Google Scholar] [CrossRef]
- Mutiso, J.M.; Macharia, J.C.; Barasa, M.; Taracha, E.; Bourdichon, A.J.; Gicheru, M.M. In vitro and in vivo antileishmanial efficacy of a combination therapy of diminazene and artesunate against Leishmania donovani in BALB/c mice. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 129–132. [Google Scholar] [CrossRef]
- Rutteman, G.R.; Erich, S.A.; Mol, J.A.; Spee, B.; Grinwis, G.C.; Fleckenstein, L.; London, C.A.; Efferth, T. Safety and efficacy field study of artesunate for dogs with non-resectable tumours. Anticancer. Res. 2013, 33, 1819–1827. [Google Scholar] [PubMed]
- da Silva, S.S.; Mizokami, S.S.; Fanti, J.R.; Costa, I.N.; Bordignon, J.; Felipe, I.; Pavanelli, W.R.; Verri, W.A., Jr.; Conchon Costa, I. Glucantime reduces mechanical hyperalgesia in cutaneous leishmaniasis and complete Freund’s adjuvant models of chronic inflammatory pain. J. Pharm. Pharmacol. 2018, 70, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Cortes, D.F.; Carneiro, M.B.; Santos, L.M.; Souza, T.C.; Maioli, T.U.; Duz, A.L.; Ramos-Jorge, M.L.; Afonso, L.C.; Carneiro, C.; Vieira, L.Q. Low and high-dose intradermal infection with Leishmania major and Leishmania amazonensis in C57BL/6 mice. Mem. Inst. Oswaldo Cruz 2010, 105, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Esposito, E.; Muia, C.; Abdelrahman, M.; Di Paola, R.; Crisafulli, C.; Bramanti, P.; Thiemermann, C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007, 33, 880–893. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Militi, A.; Oteri, G.; Wallace, J.L.; Di Paola, R.; Cuzzocrea, S. Anti-inflammatory effect of ATB-352, a H2S-releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharmacol. Res. 2018, 132, 220–231. [Google Scholar] [CrossRef]
- Swiergiel, A.H.; Dunn, A.J. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol. Biochem. Behav. 2007, 86, 651–659. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- de Vries, H.J.; Reedijk, S.H.; Schallig, H.D. Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef]
- Buracco, P.; Abate, O.; Guglielmino, R.; Morello, E. Osteomyelitis and arthrosynovitis associated with Leishmania donovani infection in a dog. J. Small Anim. Pract. 1997, 38, 29–30. [Google Scholar] [CrossRef]
- Bukirwa, H.; Unnikrishnan, B.; Kramer, C.V.; Sinclair, D.; Nair, S.; Tharyan, P. Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Bruneel, F. Human cerebral malaria: 2019 mini review. Rev. Neurol. 2019, 175, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Clemmer, L.; Martins, Y.C.; Zanini, G.M.; Frangos, J.A.; Carvalho, L.J. Artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria and rapidly decrease leukocyte accumulation in the brain. Antimicrob. Agents Chemother. 2011, 55, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, J.; Lv, S.; Xu, W.; Li, J.; Ge, W.; Xiao, C.; Geng, D.; Liu, Y. Artesunate Attenuates Lipopolysaccharide-Stimulated Proinflammatory Responses by Suppressing TLR4, MyD88 Expression, and NF-kappaB Activation in Microglial Cells. Inflammation 2015, 38, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, A.K.; Pal, Y.; Dwivedi, S.K. In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. J. Vet. Med. Sci. 2003, 65, 1171–1177. [Google Scholar] [CrossRef]
- Adam, I.; Hagelnur, A.A. Artesunate plus sulfamethoxypyrazine/pyrimethamine for the treatment of cutaneous leishmaniasis: A double-blind, placebo-controlled clinical trial. Int. J. Antimicrob. Agents 2009, 34, 380–381. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Krishna, S.; Greten, H.J.; Kremsner, P.G.; Efferth, T. Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res. 2016, 110, 216–226. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Liu, Z.; Hu, L.; Xu, P.; Xuan, Y.; Liu, Y.; Liu, X.; Fan, J. Studies on prophylactic effect of artesunate on schistosomiasis japonica. Chin. Med. J. 1996, 109, 848–853. [Google Scholar]
- Ceron, J.J.; Pardo-Marin, L.; Caldin, M.; Furlanello, T.; Solano-Gallego, L.; Tecles, F.; Bernal, L.; Baneth, G.; Martinez-Subiela, S. Use of acute phase proteins for the clinical assessment and management of canine leishmaniosis: General recommendations. BMC Vet. Res. 2018, 14, 196. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Stelow, E. Behavior as Illness Indicator. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, ix–x. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004, 500, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Xu, H.Y.; Yang, H.J. Effect of proinflammatory factors TNF-alpha,IL-1beta, IL-6 on neuropathic pain. Zhongguo Zhong Yao Za Zhi 2017, 42, 3709–3712. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.S.; Park, D. Anti-allodynic effect of interleukin 10 in a mouse model of complex regional pain syndrome through reduction of NK1 receptor expression of microglia in the spinal cord. J. Pain Res. 2018, 11, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.M.; Fattori, V.; Pinho-Ribeiro, F.A.; Domiciano, T.P.; Miranda-Sapla, M.M.; Zaninelli, T.H.; Casagrande, R.; Pinge-Filho, P.; Pavanelli, W.R.; Alves-Filho, J.C.; et al. Contribution of spinal cord glial cells to L. amazonensis experimental infection-induced pain in BALB/c mice. J. Neuroinflamm. 2019, 16, 113. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, E.; Palma, E.; Peritore, A.F.; Siracusa, R.; D’Amico, R.; Fusco, R.; Licata, P.; Crupi, R. Effect of Artesunate on Leishmania Amazonesis Induced Neuroinflammation and Nociceptive Behavior in Male Balb/C Mice. Animals 2020, 10, 557. https://doi.org/10.3390/ani10040557
Gugliandolo E, Palma E, Peritore AF, Siracusa R, D’Amico R, Fusco R, Licata P, Crupi R. Effect of Artesunate on Leishmania Amazonesis Induced Neuroinflammation and Nociceptive Behavior in Male Balb/C Mice. Animals. 2020; 10(4):557. https://doi.org/10.3390/ani10040557
Chicago/Turabian StyleGugliandolo, Enrico, Ernesto Palma, Alessio Filippo Peritore, Rosalba Siracusa, Ramona D’Amico, Roberta Fusco, Patrizia Licata, and Rosalia Crupi. 2020. "Effect of Artesunate on Leishmania Amazonesis Induced Neuroinflammation and Nociceptive Behavior in Male Balb/C Mice" Animals 10, no. 4: 557. https://doi.org/10.3390/ani10040557
APA StyleGugliandolo, E., Palma, E., Peritore, A. F., Siracusa, R., D’Amico, R., Fusco, R., Licata, P., & Crupi, R. (2020). Effect of Artesunate on Leishmania Amazonesis Induced Neuroinflammation and Nociceptive Behavior in Male Balb/C Mice. Animals, 10(4), 557. https://doi.org/10.3390/ani10040557







