Effect of Acrylamide Supplementation on the CART-, VAChT-, and nNOS-Immunoreactive Nervous Structures in the Porcine Stomach
Abstract
Simple Summary
Abstract
1. Introduction
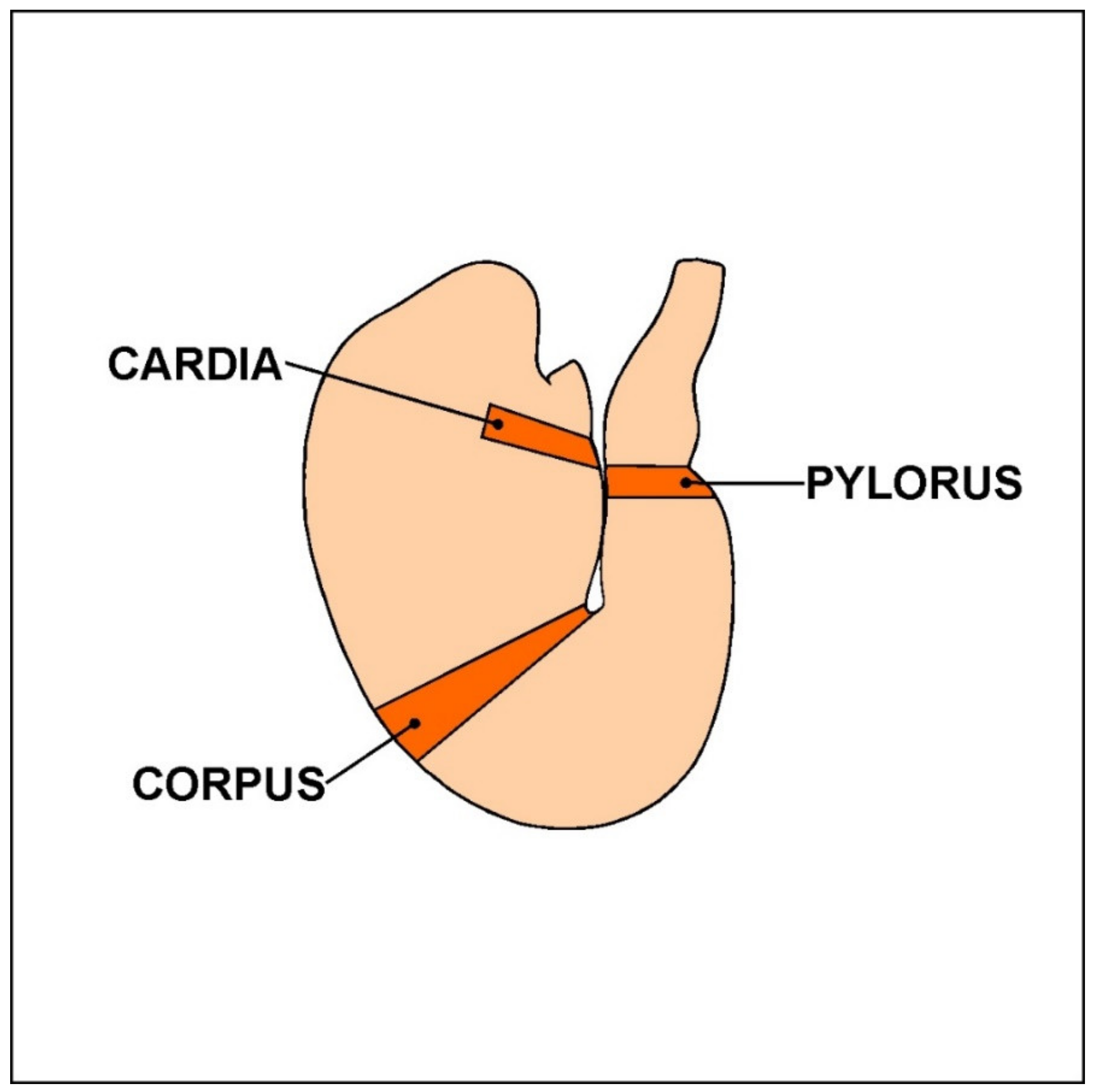
2. Materials and Methods
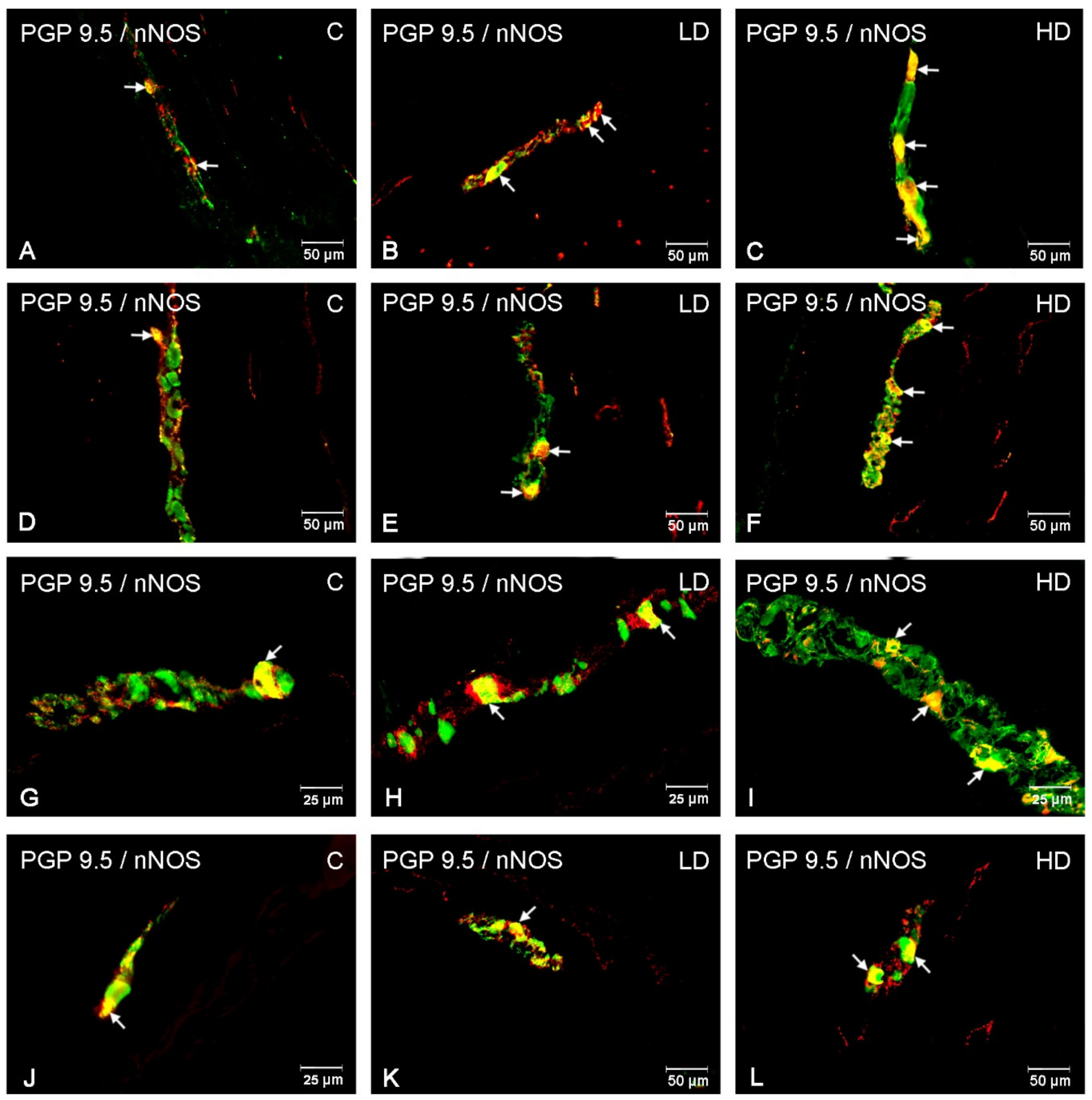
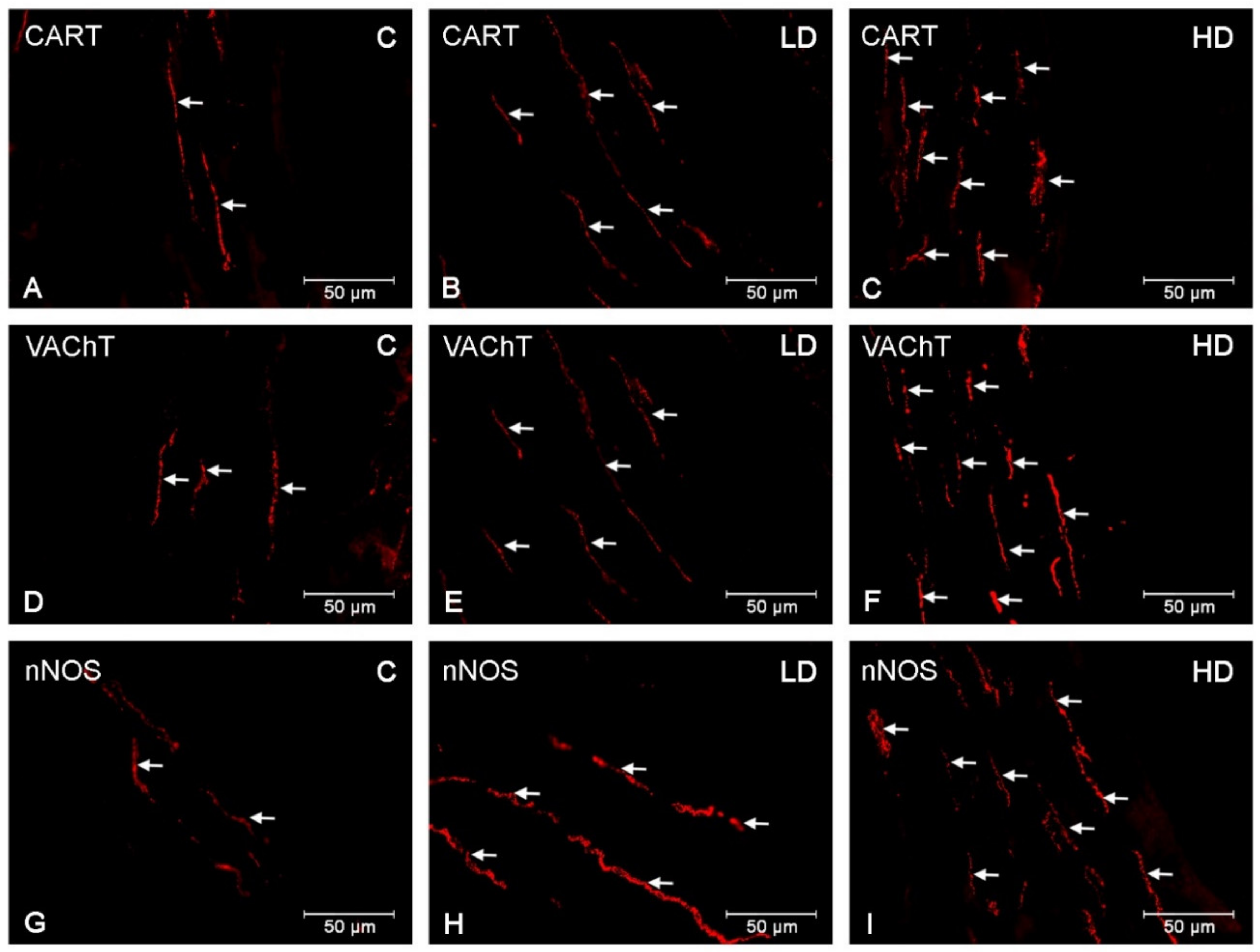
3. Results
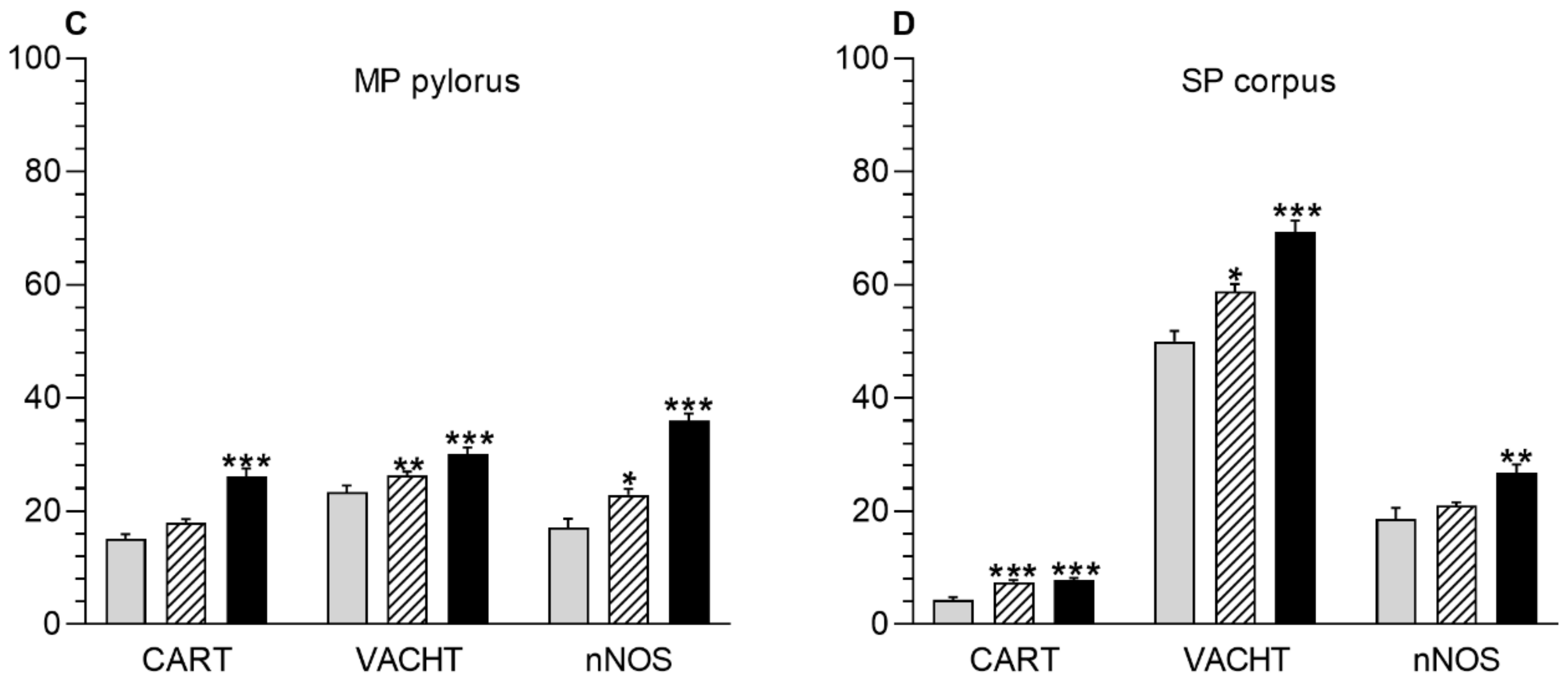
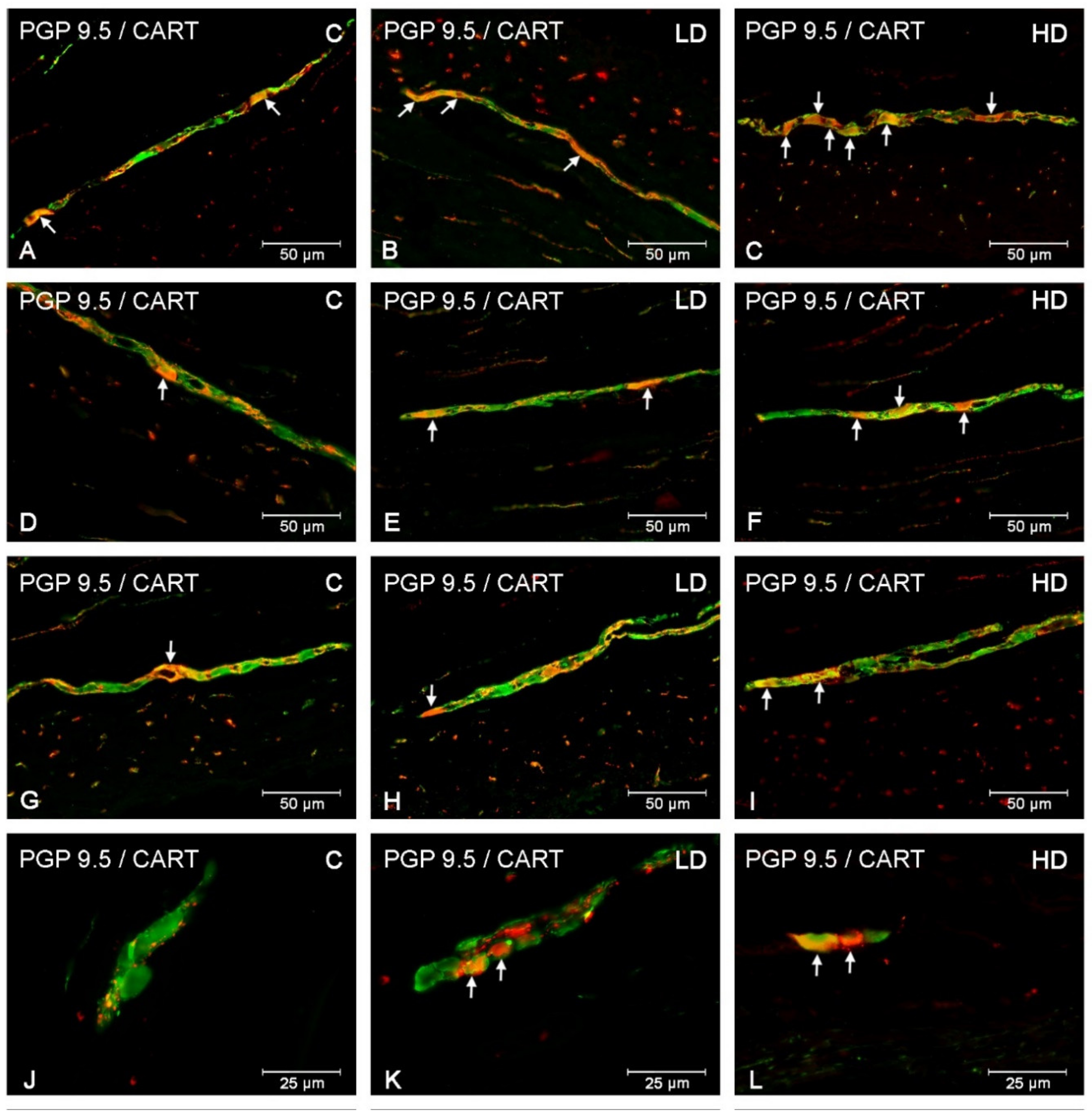
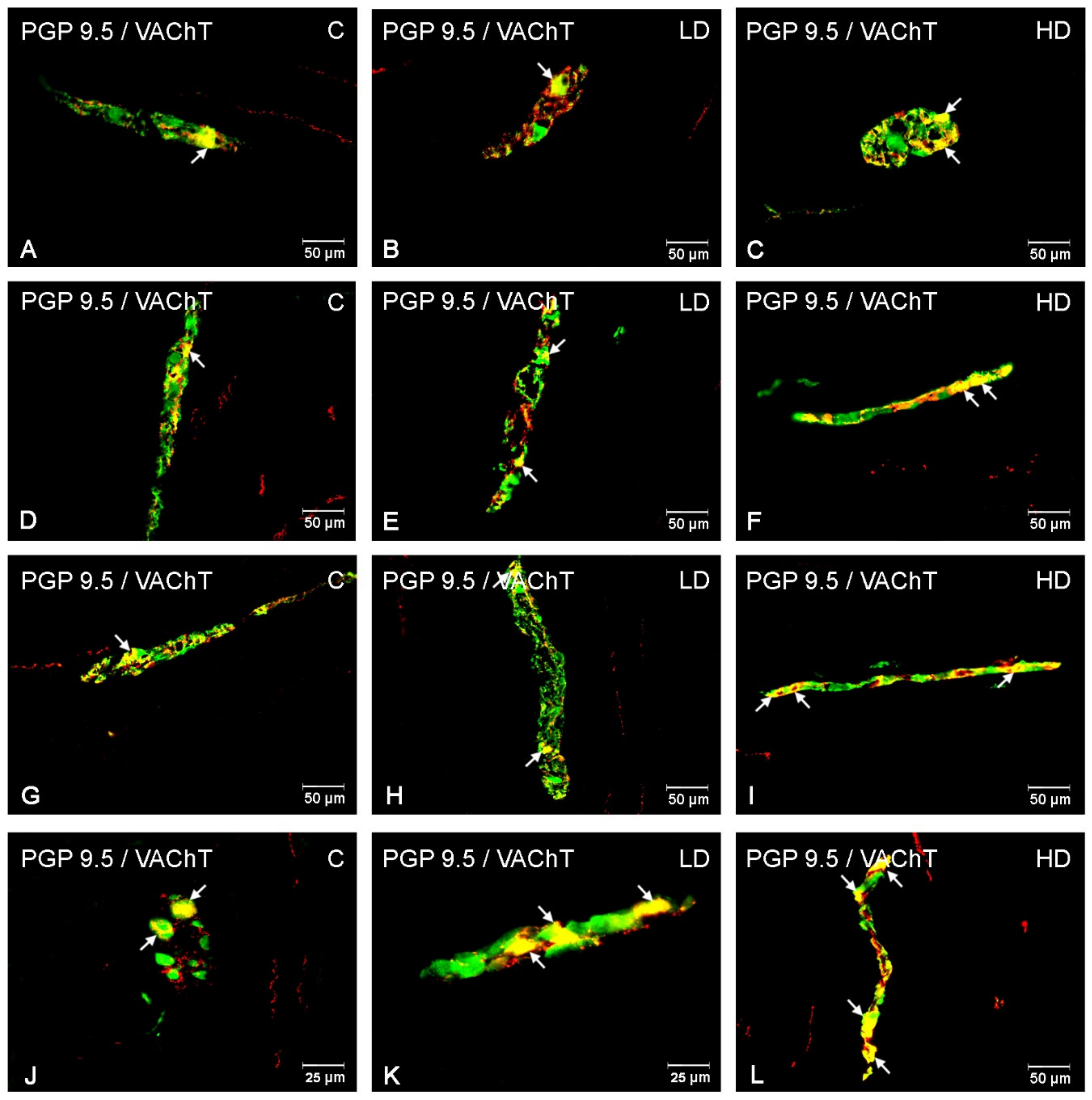
3.1. Myenteric Plexus
3.2. Submucous Plexus
3.3. Nerve Fibres
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 69, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 toxin-induced changes in cocaine- and amphetamine-regulated transcript (CART)-Like immunoreactivity in the enteric nervous system within selected fragments of the porcine digestive tract. Neurotox. Res. 2017, 31, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Makowska, K.; Całka, J. Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. Int. J. Mol. Sci. 2019, 20, 3345. [Google Scholar] [CrossRef]
- Miller, S.M.; Reed, D.; Sarr, M.G.; Farrugia, G.; Szurszewski, J.H. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef]
- Okumura, T.; Yamada, H.; Motomura, W.; Kohgo, Y. Cocaine amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotropin-releasing factor system. Endocrinology 2000, 141, 2854–2860. [Google Scholar] [CrossRef]
- Bulc, M.; Gonkowski, S.; Całka, J. Expression of Cocaine and Amphetamine Regulated Transcript (CART) in the Porcine Intramural Neurons of Stomach in the Course of Experimentally Induced Diabetes Mellitus. J. Mol. Neurosci. 2015, 57, 376–385. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Acrylamide-induced alterations in the cocaine- and amphetamine-regulated peptide transcript (CART)-like immunoreactivity within the enteric nervous system of the porcine small intestines. Ann. Anat. 2018, 219, 94–101. [Google Scholar] [CrossRef]
- Umathe, S.N.; Kochar, N.I.; Jain, N.S.; Dixit, P.V. Gastrointestinal dysfunction in diabetic rats relates with a decline in tissue L-arginine content and consequent low levels of nitric oxide. Nitric Oxide 2009, 20, 129–133. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Dąbrowski, M.; Całka, J. Hyperglycaemia-induced downregulation in expression of nNOS intramural neurons of the small intestine in the pig. Int. J. Mol. Sci. 2019, 20, 1681. [Google Scholar] [CrossRef]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Calka, J. Increased expression of CART, nNOS, VIP, PACAP, SP and GAL in enteric neurons of the porcine stomach prepyloric region following hydrochloric acid infusion. Folia Histochem. Cytobiol. 2019, 57(4), 179–187. [Google Scholar] [CrossRef]
- Arvidsson, U.; Riedl, M.; Elde, R.; Meister, B. Vesicular acetylcholine transporter (VAChT) protein: A novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 1997, 378, 454–467. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach—The study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Gonkowski, S. Bisphenol A-Induced changes in the enteric nervous system of the porcine duodenum. Neurotoxicology 2018, 66, 78–86. [Google Scholar] [CrossRef]
- Ekblad, E.; Bauer, A.J. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neuro Gastroenterol. Motil. 2004, 16, 123–128. [Google Scholar] [CrossRef]
- Kolgazi, M.; Uslu, U.; Yuksel, M.; Velioglu-Ogunc, A.; Ercan, F.; Alican, I. The role of cholinergic anti-inflammatory pathway in acetic acid-induced colonic inflammation in the rat. Chem. Biol. Interact. 2013, 205, 72–80. [Google Scholar] [CrossRef]
- Czajkowska, M.; Rychlik, A.; Całka, J. Long-term treatment with naproxen changes the chemical coding of the porcine intramural duodenum neurons. Ann Anat. 2020, 227, 151425. [Google Scholar] [CrossRef]
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can. J. Vet. Res. 2017, 81, 129–136. [Google Scholar]
- Shipp, A.; Lawrence, G.; Gentry, R.; McDonald, T.; Bartow, H.; Bounds, J.; Macdonald, N.; Clewell, H.; Allen, B.; van Landingham, C. Acrylamide: Review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit. Rev. Toxicol. 2006, 36, 481–608. [Google Scholar] [CrossRef]
- Lo Pachin, R.M. The changing view of acrylamide neurotoxicity. Toxicol. Vitr. 2010, 25, 573–579. [Google Scholar] [CrossRef]
- Zödl, B.; Schmid, D.; Wassler, G.; Gundacker, C.; Leibetseder, V.; Thalhammer, T.; Ekmekcioglu, C. Intestinal transport and metabolism of acrylamide. Toxicology 2007, 232, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Chemical modifications of peptides and their impact on food properties. Chem. Rev. 2011, 111, 7876–7903. [Google Scholar] [CrossRef] [PubMed]
- WHO. Health Implications of Acrylamide in Food; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2002; Available online: http://apps.who.int/iris/handle/10665/42563 (accessed on 15 November 2016).
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–38066. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Hanazono, Y.; Kunita, S. Swine used in the medical university: Overview of 20 years of experience. Exp. Anim. 2018, 67, 7–13. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in Somatostatin-Like Immunoreactivity in the Sympathetic Neurons Projecting to the Prepyloric Area of the Porcine Stomach Induced by Selected Pathological Conditions. Biomed. Res. Int. 2017, 2017, 9037476. [Google Scholar] [CrossRef]
- Smith, E.A.; Oehme, F.W. Acrylamide and polyacrylamide: A review of production, use, environmental fate and neurotoxicity. Rev. Environ. Health 1991, 9, 215–228. [Google Scholar] [CrossRef]
- Abelli, L.; Ferri, G.L.; Astolfi, M.; Conte, B.; Geppetti, P.; Parlani, M.; Dahl, D.; Polak, J.M.; Maggi, C.A. Acrylamide-induced visceral neuropathy: Evidence for the involvement of capsaicin-sensitive nerves of the rat urinary bladder. Neuroscience 1991, 41, 311–321. [Google Scholar] [CrossRef]
- Spencer, P.S.; Schaumburg, H.H. A review of acrylamide neurotoxicity. Part II. Experimental animal neurotoxicity and pathologic mechanisms. Can. J. Neurol. Sci. 1974, 1, 152–169. [Google Scholar] [CrossRef]
- Radad, K.; Al-Shraim, M.; Al-Emam, A.; Moldzio, R.; Rausch, W.D. Neurotoxic effects of acrylamide on dopaminergic neurons in primary mesencephalic cell culture. Folia Neuropathol. 2019, 57, 196–204. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T. Molecular mechanism of acrylamide neurotoxicity: Lessons earned from organic chemistry. Environ. Health Perspect. 2012, 120, 1650. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Baydar, T. Acrylamide neurotoxicity. Nutr. Neurosci. 2014, 17, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Guo, X.; Xiong, F.; Cheng, G.; Lu, Q.; Yan, H. Acrylamide increases dopamine levels by affecting dopamine transport and metabolism related genes in the striatal dopaminergic system. Toxicol. Lett. 2015, 236, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Elblehi, S.S.; El Euony, O.I.; El-Sayed, Y.S. Apoptosis and astrogliosis perturbations and expression of regulatory inflammatory factors and neurotransmitters in acrylamide-induced neurotoxicity under ω3 fatty acids protection in rats. Neurotoxicology 2019, 76, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Komoike, Y.; Matsuoka, M. Endoplasmic reticulum stress-mediated neuronal apoptosis by acrylamide exposure. Toxicol. Appl. Pharmacol. 2016, 310, 68–77. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Z. Evaluation of density and distribution of CART-immunoreactive structures in gastrointestinal tract of hypertensive rats. Biofactors 2012, 38, 407–415. [Google Scholar] [CrossRef]
- Burliński, P.J. Inflammation- and axotomy-induced changes in cocaine- andamphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nervous structures in the porcine descending colon. Pol. J. Vet. Sci. 2012, 15, 517–524. [Google Scholar] [CrossRef]
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.A.; Lima, P.A.; Wonnacott, S. The α7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a calcium ion dependent mechanism. Neuropharmacology 2000, 39, 2799–2807. [Google Scholar] [CrossRef]
- Thompson, S.A.; Smith, O.; Linn, D.M.; Linn, C.L. Acetylcholine neuroprotection against glutamate-induced excitotoxicity in adult pig retinal ganglion cells is partially mediated through alpha4 nAChRs. Exp. Eye. Res. 2006, 83, 1135–1145. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Donald, E.L.; Rahman, A.A.; Campelj, D.G.; Abalo, R.; Rybalka, E.; Bornstein, J.C.; Nurgali, K. Irinotecan-Induced Gastrointestinal Dysfunction Is Associated with Enteric Neuropathy, but Increased Numbers of Cholinergic Myenteric Neurons. Front Physiol. 2017, 8, 391. [Google Scholar] [CrossRef]
- Pennisi, M.; Malaguarnera, G.; Puglisi, V.; Vinciguerra, L.; Vacante, M.; Malaguarnera, M. Neurotoxicity of acrylamide in exposed workers. Int. J. Environ. Res. Public Health. 2013, 10, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Ohba, N.; Nakagomi, S.; Suzuki, Y.; Kiryu-Seo, S.; Namikawa, K.; Kondoh, W.; Tanaka, W.; Kiyama, H. Vesicular acetylcholine transporter can be a morphological marker for the reinnervation to muscle of regenerating motor axons. Neurosci Res. 2004, 48, 305–314. [Google Scholar] [CrossRef]
- Filpa, V.; Carpanese, E.; Marchet, S.; Pirrone, C.; Conti, A.; Rainero, A.; Moro, E.; Chiaravalli, A.M.; Zucchi, I.; Moriondo, A.; et al. Nitric oxide regulates homeoprotein OTX1 and OTX2 expression in the rat myenteric plexus after intestinal ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G374–G389. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, K.; Lin, Z.; Fex Svenningsen, A.; Ekblad, E. Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J. Neurosci. Res. 2003, 72, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.H.; Li, Q.; Sarna, S.K. Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn’s colitis and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G295–G302. [Google Scholar] [CrossRef]
- Palus, K.; Obremski, K.; Bulc, M.; Całka, J. The impact of low and high doses of acrylamide on the intramural neurons of the porcine ileum. Food Chem. Toxicol. 2019, 132, 110673. [Google Scholar] [CrossRef]
- Gonkowski, S.; Burlinski, P.; Szwajca, P.; Całka, J. Changes in cocaine- andamphetamine-regulated transcript-like immunoreactive (CART-LI) nerve structures of the porcine descending colon during proliferative enteropathy. Bull. Vet. Inst. Pulawy 2012, 56, 199–203. [Google Scholar] [CrossRef]
- Hao, J.; Simard, A.R.; Turner, G.H.; Wu, J.; Whiteaker, P.; Lukas, R.J.; Shi, F.D. Attenuation of CNS inflammatory responses by nicotine involves α7 and non-α7 nicotinic receptors. Exp. Neurol. 2011, 227, 110–119. [Google Scholar] [CrossRef]
- Lebda, M.A.; El-Neweshy, M.S.; El-Sayed, Y.S. Neurohepatic toxicity of subacute manganese chloride exposure and potential chemoprotective effects of lycopene. Neurotoxicology 2012, 33, 98–104. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Wallace, J.L. Nitric oxide as a mediator of gastrointestinal mucosal injury?—Say it ain’t so. Mediat. Inflamm. 1995, 4, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Całka, J. The Influence of Prolonged Acetylsalicylic Acid Supplementation-Induced Gastritis on the Neurochemistry of the Sympathetic Neurons Supplying Prepyloric Region of the Porcine Stomach. PLoS ONE 2015, 10, e0143661. [Google Scholar] [CrossRef]
- Barrachina, M.D.; Panés, J.; Esplugues, J.V. Role of nitric oxide in gastrointestinal inflammatory and ulcerative diseases: Perspective for drugs development. Curr. Pharm. Des. 2001, 7, 31–48. [Google Scholar] [CrossRef] [PubMed]


| Antigen | Host Species | Code | Dilution | Manufacturer/Supplier |
|---|---|---|---|---|
| Primary antibodies | ||||
| PGP 9.5 | Mouse | 7863-2004 | 1:1000 | Bio-Rad, Hercules, CA, USA |
| CART | Rabbit | H-003-61 | 1:16000 | Phoenix Pharmaceuticals, Burlingame, CA, USA |
| VAChT | Rabbit | H-V007 | 1:2000 | Phoenix Pharmaceuticals, Burlingame, CA, USA |
| nNOS | Rabbit | AB5380 | 1:2000 | Sigma–Aldrich, Saint Louis, MO, USA |
| Secondary antibodies | ||||
| Alexa Fluor 488 nm donkey anti-mouse IgG | A21202 | 1:1000 | Thermo Fisher Scientific, Waltham, MA, USA | |
| Alexa Fluor 546 nm goat anti-rabbit IgG | A11010 | 1:1000 | Thermo Fisher Scientific, Waltham, MA, USA | |
| Part of the Stomach | Cardia | Corpus | Pylorus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C Group | LD Group | HD Group | C Group | LD Group | HD Group | C Group | LD Group | HD Group | |
| CART | |||||||||
| CML | ++ | ++ | +++ | +++ | +++ | ++++ | ++ | ++ | +++ |
| S/ML | + | + | ++ | + | ++ | +++ | ++ | ++ | +++ |
| VACHT | |||||||||
| CML | + | + | ++ | + | + | ++ | + | ++ | ++ |
| S/ML | + | + | + | + | ++ | ++ | + | ++ | ++ |
| nNOS | |||||||||
| CML | + | + | ++ | + | ++ | ++ | + | ++ | +++ |
| S/ML | + | + | ++ | + | + | ++ | + | ++ | ++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palus, K.; Bulc, M.; Całka, J. Effect of Acrylamide Supplementation on the CART-, VAChT-, and nNOS-Immunoreactive Nervous Structures in the Porcine Stomach. Animals 2020, 10, 555. https://doi.org/10.3390/ani10040555
Palus K, Bulc M, Całka J. Effect of Acrylamide Supplementation on the CART-, VAChT-, and nNOS-Immunoreactive Nervous Structures in the Porcine Stomach. Animals. 2020; 10(4):555. https://doi.org/10.3390/ani10040555
Chicago/Turabian StylePalus, Katarzyna, Michał Bulc, and Jarosław Całka. 2020. "Effect of Acrylamide Supplementation on the CART-, VAChT-, and nNOS-Immunoreactive Nervous Structures in the Porcine Stomach" Animals 10, no. 4: 555. https://doi.org/10.3390/ani10040555
APA StylePalus, K., Bulc, M., & Całka, J. (2020). Effect of Acrylamide Supplementation on the CART-, VAChT-, and nNOS-Immunoreactive Nervous Structures in the Porcine Stomach. Animals, 10(4), 555. https://doi.org/10.3390/ani10040555





