Effects of Acute Hyperthermia on the Thermotolerance of Cow and Sheep Skin-Derived Fibroblasts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
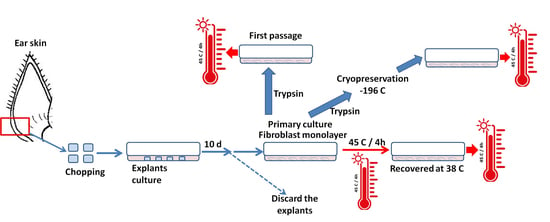
2.2. Skin Tissue Sampling and Culture
2.3. Cryopreservation and Thawing of Fibroblasts
2.4. Measuring Cellular Attachment and Cell Viability
2.5. Assessment of Cell Migration through Wound Healing Assay
2.6. Experimental Design
2.6.1. Effect of Acute Heat Shock on Primary Fibroblast Viability and Migratory Activity
2.6.2. Effects of Acute Heat Shock on the Recovered Primary Fibroblast Viability and Migratory Activity
2.6.3. Impacts of Acute Heat Shock on the First Passage Fibroblast Viability and Migratory Activity
2.6.4. Impact of Acute Heat Shock on the Cryopreserved Fibroblasts Viability and Migratory Activity
2.7. Relative Quantitative Polymerase Chain Reaction (qPCR)
2.8. Statistical Analysis
3. Results
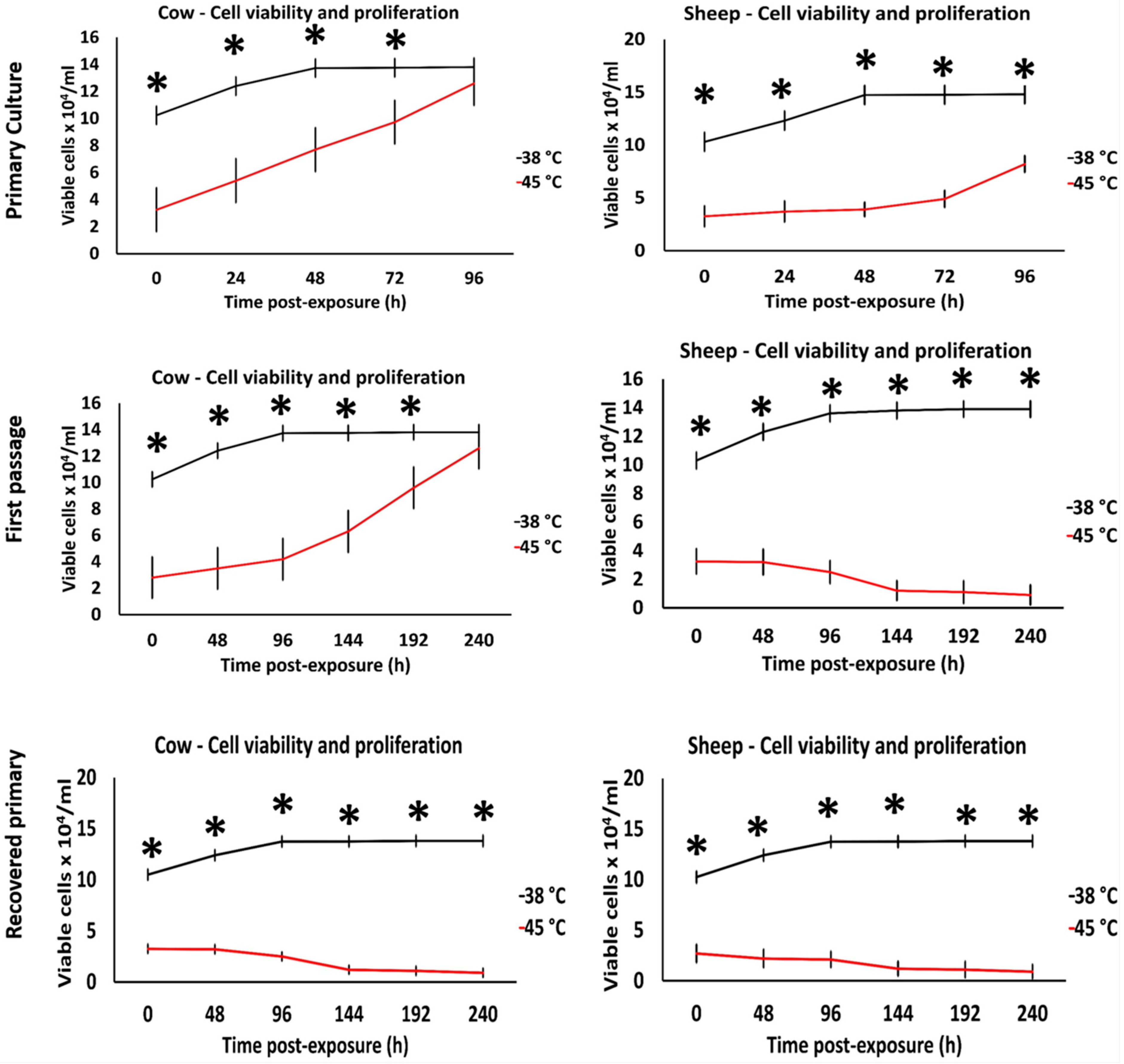
3.1. Effects of Acute Heat Shock on the Viability of Primary Fibroblasts in Cows and Sheep
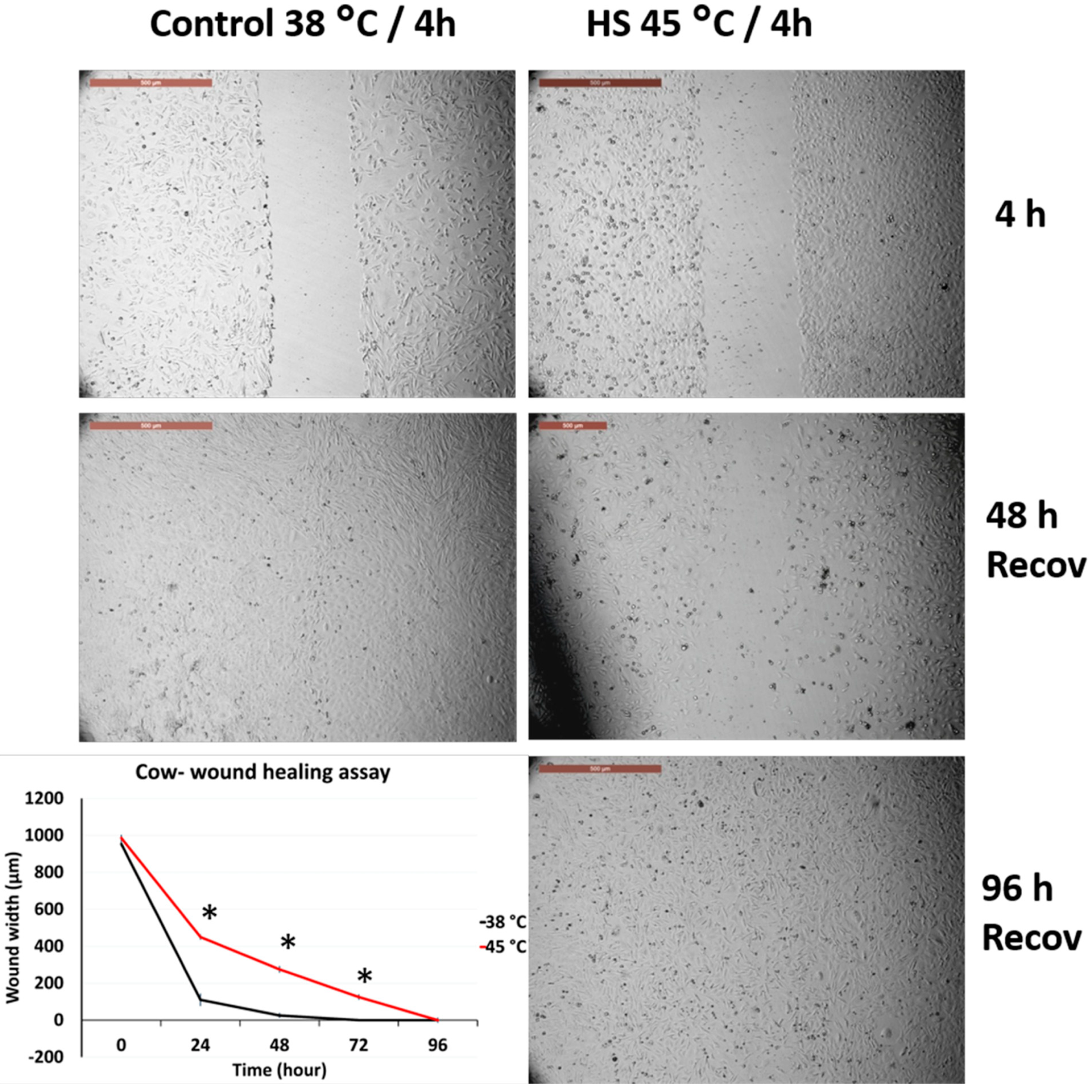
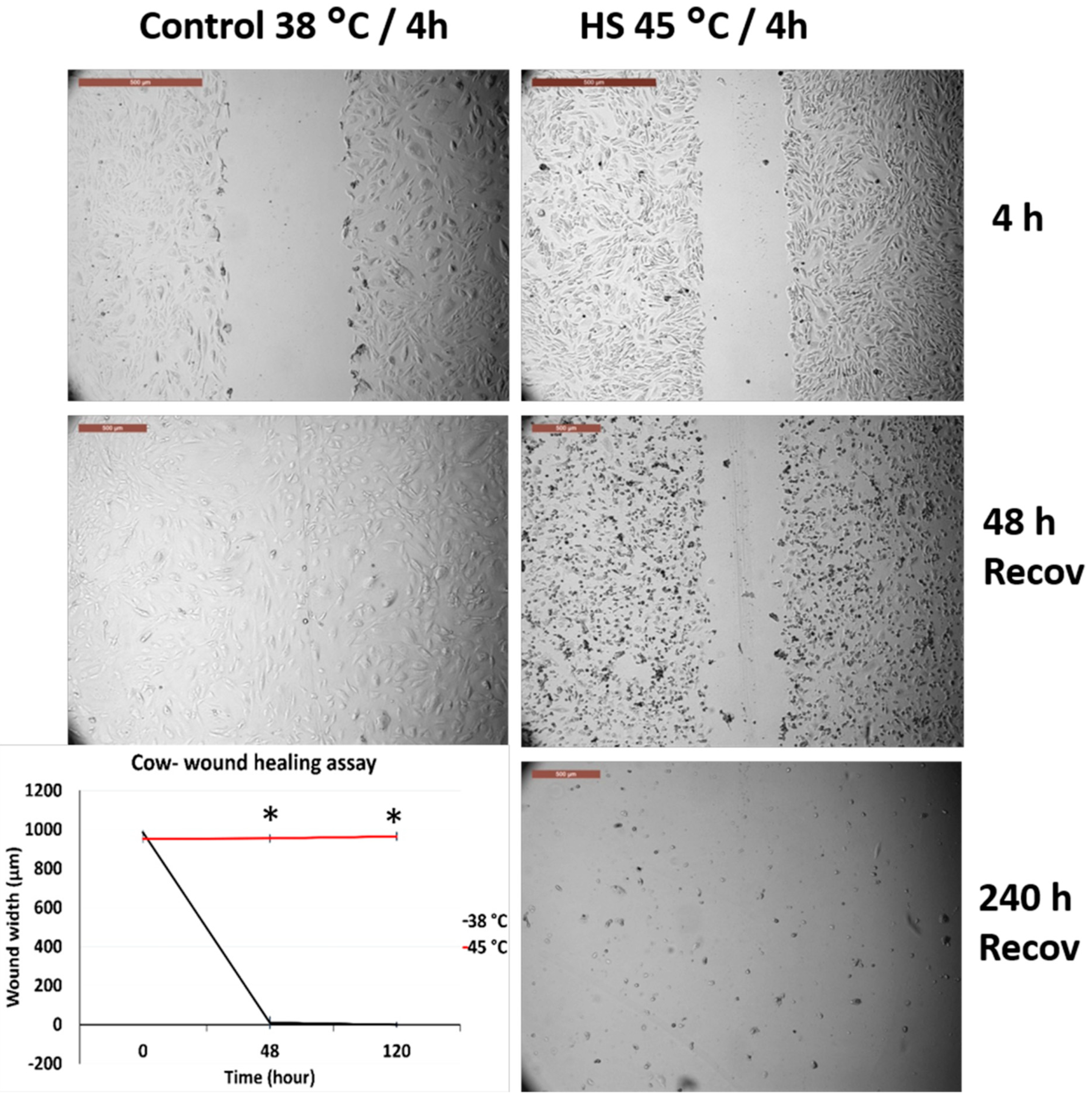
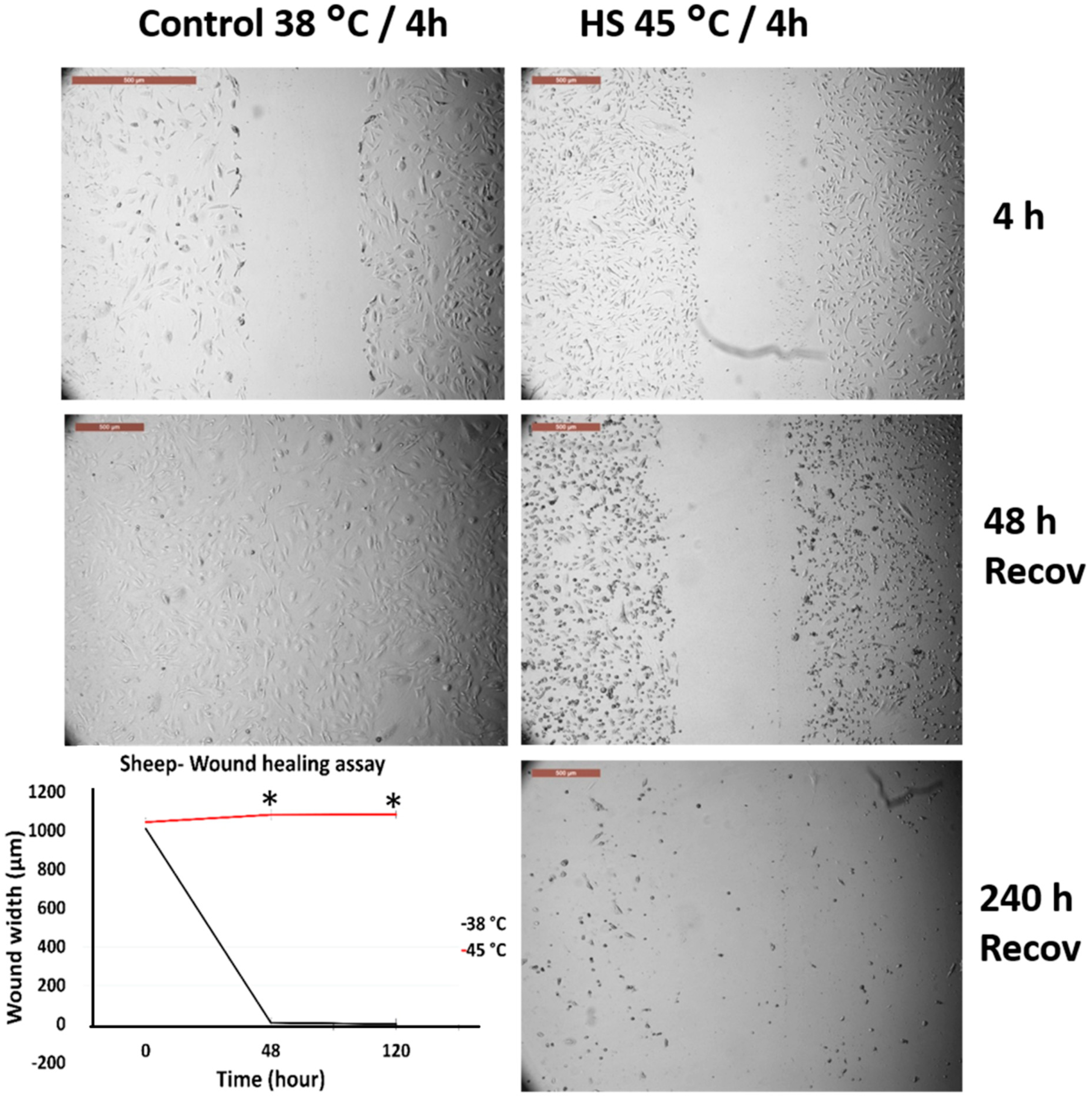
3.2. Effects of Acute Heat Shock on the Migratory Activity of Primary Fibroblasts in Cows and Sheep
3.3. Effects of Acute Heat Shock on the Migratory Activity of the Recovered Primary Fibroblasts in Cows and Sheep
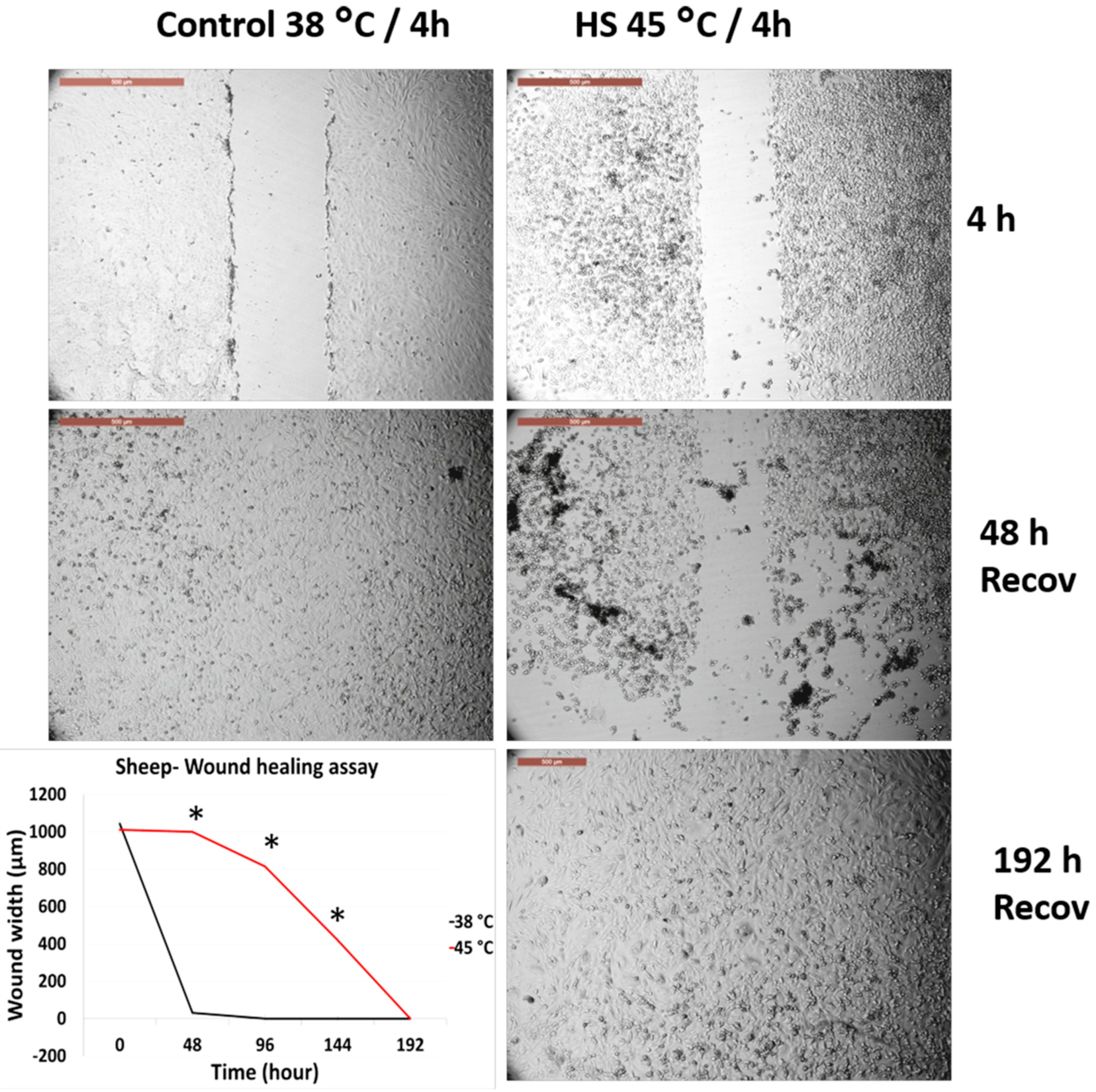
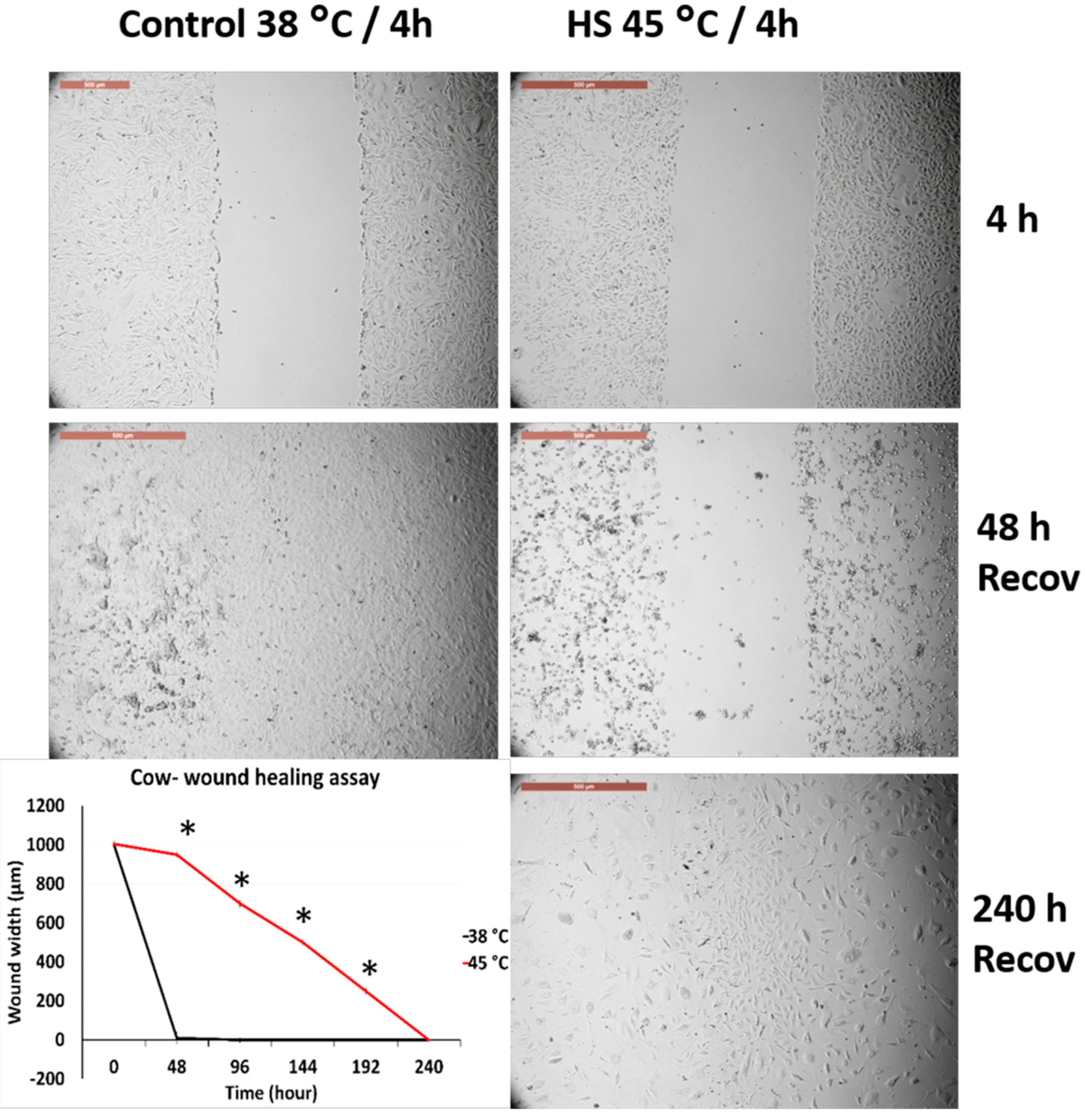
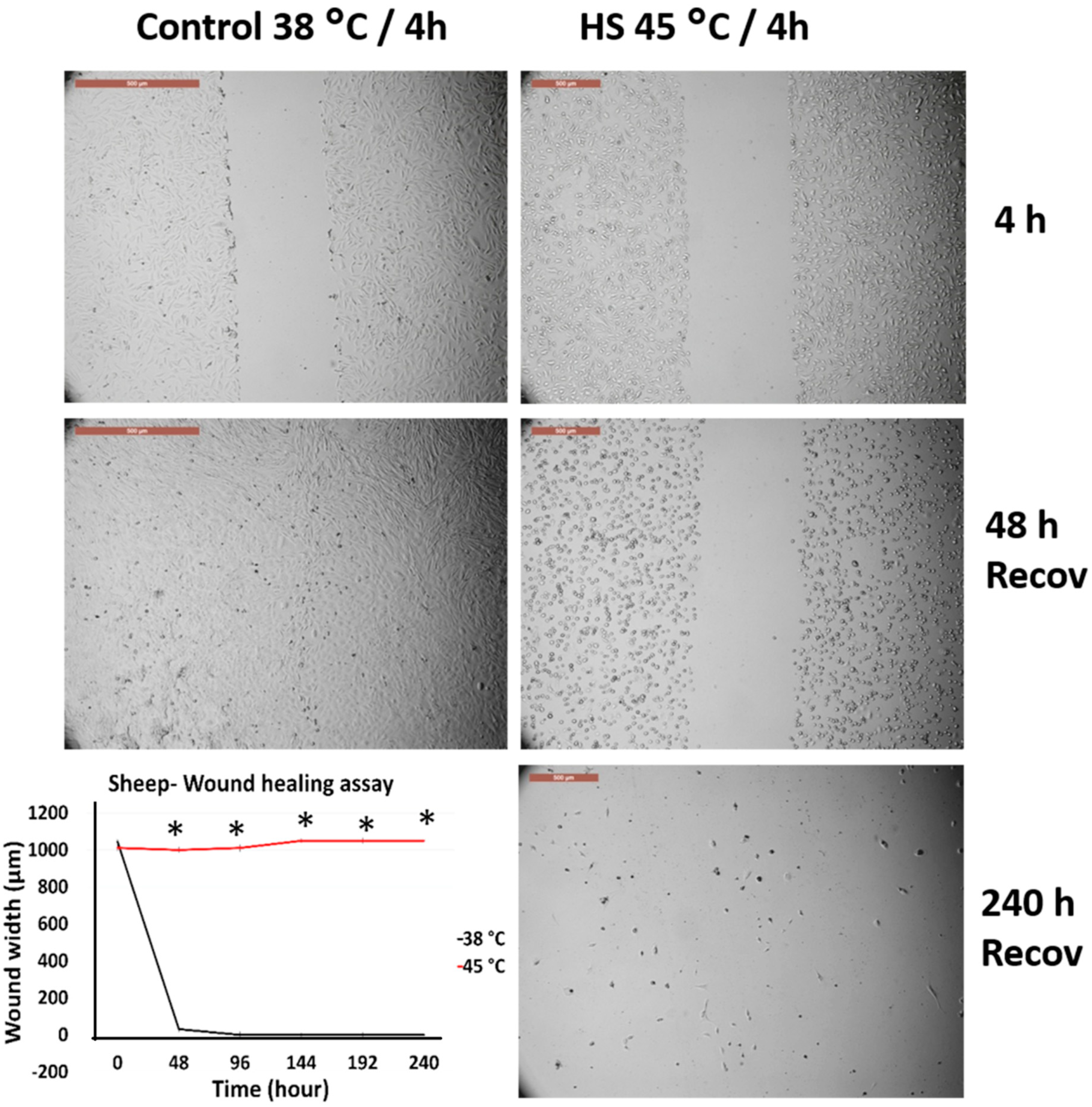
3.4. Effects of Acute Heat Shock on the Migratory Activity of the First Passage Cow and Sheep Fibroblasts
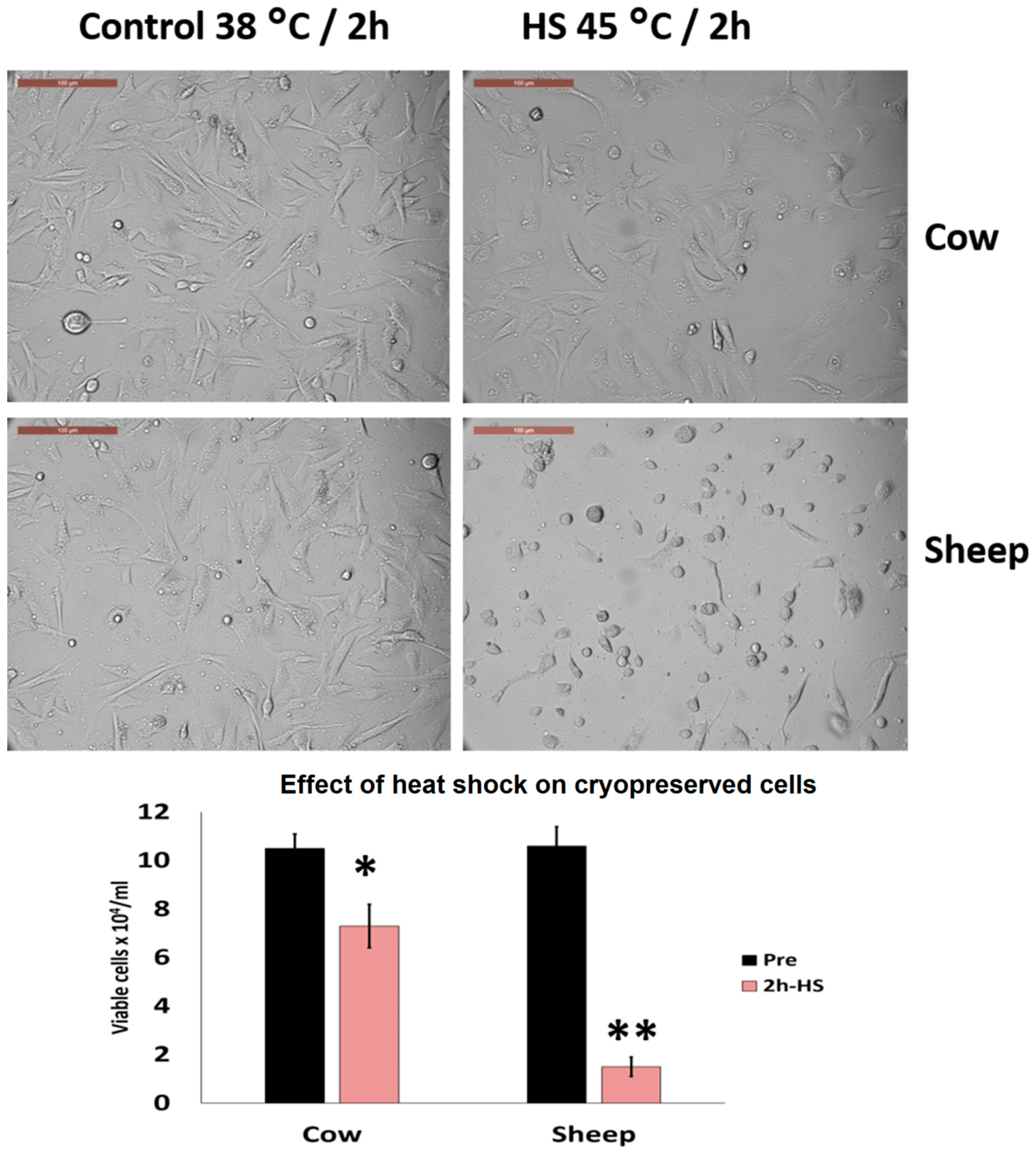
3.5. Effects of Acute Heat Shock on the Viability of Cryopreserved Fibroblasts in Cows and Sheep
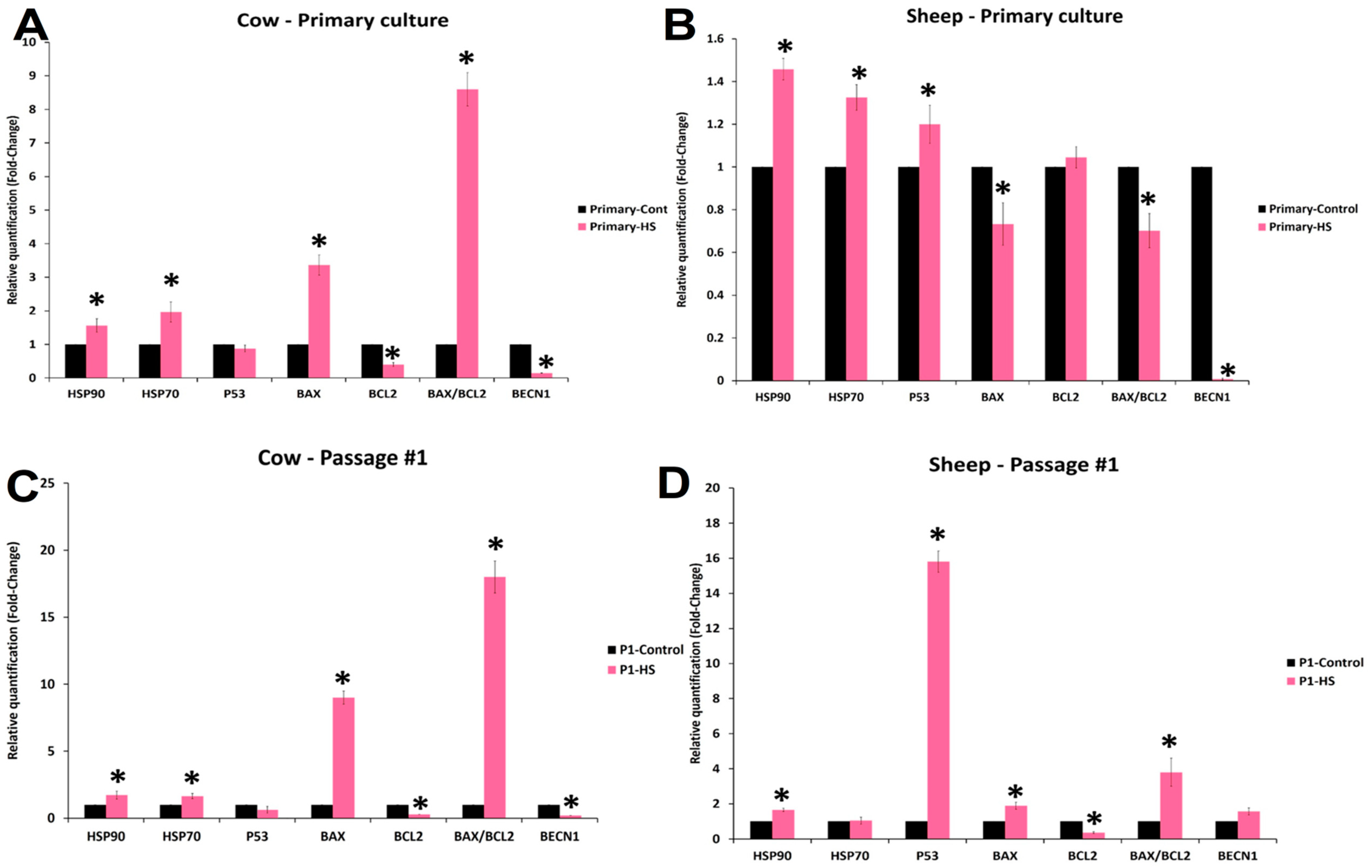
3.6. Effects of Acute Heat Shock on the Expression of mRNA Transcripts in Primary Culture and First Passage Cow and Sheep Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aggarwal, A.; Upadhyay, R. Heat Stress and Milk Production. In Heat Stress and Animal Productivity; Springer: Delhi, India, 2013; pp. 53–77. [Google Scholar] [CrossRef]
- Aggarwal, A.; Upadhyay, R. Heat Stress and Reproduction. In Heat Stress and Animal Productivity; Springer: Delhi, India, 2013; pp. 79–111. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The impact of heat load on cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.T.; Goode, L.; Linnerud, A.C. Effects of high ambient temperature on respiration rate, rectal temperature, fetal development and thyrold gland activity in tropical and temperate breeds of sheep. Theriogenology 1985, 24, 259–269. [Google Scholar] [CrossRef]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.M.; Gaughan, J.B. Physiological responses of Australian Merino wethers exposed to high heat load. J. Anim. Sci. 2012, 90, 212–220. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef]
- Kamwanja, L.A.; Chase, C.C.; Gutierrez, J.A.; Guerriero, V.; Olson, T.A.; Hammond, A.C.; Hansen, P.J. Responses of bovine lymphocytes to heat shock as modified by breed and antioxidant status. J. Anim. Sci. 1994, 72, 438–444. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; Al-Katanani, Y.M.; Rivera, R.M.; Tekin, S.; Majewski, A.C.; Ocon, O.M.; Olson, T.A.; Hansen, P.J. Genetic divergence in cellular resistance to heat shock in cattle: Differences between breeds developed in temperate versus hot climates in responses of preimplantation embryos, reproductive tract tissues and lymphocytes to increased culture temperatures. Reproduction 2003, 125, 285–294. [Google Scholar] [CrossRef]
- Deb, R.; Sajjanar, B.; Singh, U.; Kumar, S.; Singh, R.; Sengar, G.; Sharma, A. Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus × Bos taurus) breed of cattle: A comparative study. Gene 2014, 536, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Maibam, U.; Hooda, O.K.; Sharma, P.S.; Upadhyay, R.C.; Mohanty, A.K. Differential level of oxidative stress markers in skin tissue of zebu and crossbreed cattle during thermal stress. Livest. Sci. 2018, 207, 45–50. [Google Scholar] [CrossRef]
- Romero, R.D.; Montero Pardo, A.; Montaldo, H.H.; Rodríguez, A.D.; Hernández Cerón, J. Differences in body temperature, cell viability, and HSP-70 concentrations between Pelibuey and Suffolk sheep under heat stress. Trop. Anim. Health Pro. 2013, 45, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Abdel-Aziz Swelum, A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.; Elsafadi, M.; Mahmood, A.; Alfayez, M.; Alowaimer, A.N. Differences between the tolerance of camel oocytes and cumulus cells to acute and chronic hyperthermia. J. Therm. Biol. 2018, 74, 47–54. [Google Scholar] [CrossRef]
- Hansen, P.J. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 2004, 82–83, 349–360. [Google Scholar] [CrossRef]
- Hassan, F.-u.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.R.; Yang, C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef]
- Kerr, S.R.; Katz, S.G. Focus: Death: Activation of the Unfolded Protein Response Pathway in Cytotoxic T Cells: A Comparison Between in vitro Stimulation, Infection, and the Tumor Microenvironment. Yale J. Biol. Med. 2019, 92, 675–685. [Google Scholar]
- Mayer, M.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
- Schlesinger, M.J. Heat shock proteins. J. Biol. Chem. 1990, 265, 12111–12114. [Google Scholar]
- Jindal, S. Heat shock proteins: Applications in health and disease. Trends Biotechnol. 1996, 14, 17–20. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.; Tukur, H.A.; Alowaimer, A.N. Thermotolerance of camel (Camelus dromedarius) somatic cells affected by the cell type and the dissociation method. Environ. Sci. Pollut. Res. Int. 2019, 26, 29490–29496. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Gurbuxani, S.; Ravagnan, L.; Kroemer, G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001, 286, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Urano, M. Kinetics of thermotolerance in normal and tumor tissues: A review. Cancer Res. 1986, 46, 474–482. [Google Scholar]
- Ohtsuka, K. Thermotolerance in normal and tumor tissues. Gan no rinsho. Jpn. J. Cancer Clin. 1986, 32, 1671–1677. [Google Scholar]
- Carper, S.W.; Duffy, J.J.; Gerner, E.W. Heat shock proteins in thermotolerance and other cellular processes. Cancer Res. 1987, 47, 5249–5255. [Google Scholar]
- Van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperthermia 2016, 32, 446–454. [Google Scholar] [CrossRef]
- Kosaka, M.; Othman, T.; Matsumoto, T.; Ohwatari, N. Heat Shock Proteins: Roles in Thermotolerance and as Molecular Targets for Cancer Therapy. Thermal Med. (Jap. J. Hyperthermic Oncol.) 1998, 14, 170–188. [Google Scholar] [CrossRef]
- Dings, R.P.; Loren, M.L.; Zhang, Y.; Mikkelson, S.; Mayo, K.H.; Corry, P.; Griffin, R.J. Tumour thermotolerance, a physiological phenomenon involving vessel normalisation. Int. J. Hyperthermia 2011, 27, 42–52. [Google Scholar] [CrossRef]
- Staples, J.F. Metabolic Flexibility: Hibernation, Torpor, and Estivation. Compr. Physiol. 2016, 6, 737–771. [Google Scholar] [CrossRef]
- Geiser, F. Aestivation in Mammals and Birds. In Aestivation. Progress in Molecular and Subcellular Biology; Arturo Navas, C., Carvalho, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 95–111. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Noreldin, A.E.; Tukur, H.A.; Abdelazim, A.M.; Abomughaid, M.M.; Alowaimer, A.N. Isolation and culture of skin-derived differentiated and stem-like cells obtained from the Arabian camel (Camelus dromedarius). Animals 2019, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Song, A.S.; Najjar, A.M.; Diller, K.R. Thermally induced apoptosis, necrosis, and heat shock protein expression in three-dimensional culture. J. Biomech. Eng. 2014, 136. [Google Scholar] [CrossRef]
- Gong, Y.N.; Crawford, J.C.; Heckmann, B.L.; Green, D.R. To the edge of cell death and back. FEBS J. 2018, 286, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Guzman, E.; Balasanyan, V.; Conner, C.M.; Wong, K.; Zhou, H.R.; Kosik, K.S.; Montell, D.J. A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J. Cell Biol. 2017, 216, 3355–3368. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Tang, H.M.; Mak, K.H.; Hu, S.; Wang, S.S.; Wong, K.M.; Wong, C.S.T.; Wu, H.Y.; Law, H.T.; Liu, K.; et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell 2012, 23, 2240–2252. [Google Scholar] [CrossRef]
- Tang, H.M.; Tang, H.L. Anastasis: Recovery from the brink of cell death. R. Soc. Open Sci. 2018, 5, 180442. [Google Scholar] [CrossRef]
- Raj, A.T.; Kheur, S.; Bhonde, R.; Gupta, A.A.; Patil, V.R.; Kharat, A. Potential role of anastasis in cancer initiation and progression. Apoptosis 2019, 24, 383–384. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Moumen, A.F.; Alzahrani, F.A.; Mahmood, A.; Alfayez, M.; Alowaimer, A.N. Isolation and characterization of the trophectoderm from the Arabian camel (Camelus dromedarius). Placenta 2017, 57, 113–122. [Google Scholar] [CrossRef]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hagai, T.; Chen, X.; Miragaia, R.J.; Rostom, R.; Gomes, T.; Kunowska, N.; Henriksson, J.; Park, J.-E.; Proserpio, V.; Donati, G.; et al. Gene expression variability across cells and species shapes innate immunity. Nature 2018, 563, 197–202. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperon. 2016, 21, 467–475. [Google Scholar] [CrossRef]
- Luo, M.; Li, L.; Xiao, C.; Sun, Y.; Wang, G.-L. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell Biochem. 2015, 412, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2017, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Hagn, F.; Lagleder, S.; Retzlaff, M.; Rohrberg, J.; Demmer, O.; Richter, K.; Buchner, J.; Kessler, H. Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nat. Struct. Mol. Biol. 2011, 18, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xu, X.; Qin, H.; Liu, A.; Fan, Z.; Kang, L.; Fu, J.; Liu, J.; Ye, Q. The molecular mechanism and potential role of heat shock-induced p53 protein accumulation. Mol. Cell Biochem. 2013, 378, 161–169. [Google Scholar] [CrossRef]
- White, E. Autophagy and p53. Cold Spring Harb. Perspect. Med. 2016, 6, a026120. [Google Scholar] [CrossRef]
- Highfield, D.P.; Holahan, E.V.; Holahan, P.K.; Dewey, W.C. Hyperthermic survival of Chinese hamster ovary cells as a function of cellular population density at the time of plating. Radiat. Res. 1984, 97, 139. [Google Scholar] [CrossRef]
- Warters, R.L.; Henle, K.J. DNA degradation in chinese hamster ovary cells after exposure to hyperthermia. Cancer Res. 1982, 42, 4427–4432. [Google Scholar]
- Corver, W.E.; Cornelisse, C.J.; Hermans, J.; Fleuren, G.J. Limited loss of nine tumor-associated surface antigenic determinants after tryptic cell dissociation. Cytometry 1995, 19, 267–272. [Google Scholar] [CrossRef]
- Danhier, P.; Copetti, T.; De Preter, G.; Leveque, P.; Feron, O.; Jordan, B.F.; Sonveaux, P.; Gallez, B. Influence of Cell Detachment on the Respiration Rate of Tumor and Endothelial Cells. PLoS ONE 2013, 8, e53324. [Google Scholar] [CrossRef]
- Lin, C.; Tsai, S. The effect of cryopreservation on DNA damage, gene expression and protein abundance in vertebrate. Ital. J. Anim. Sci. 2016, 11, e21. [Google Scholar] [CrossRef]
- Štětina, T.; Hůla, P.; Moos, M.; Šimek, P.; Šmilauer, P.; Koštál, V. Recovery from supercooling, freezing, and cryopreservation stress in larvae of the drosophilid fly, Chymomyza costata. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadeldin, I.M.; Swelum, A.A.-A.; Zakri, A.M.; Tukur, H.A.; N. Alowaimer, A. Effects of Acute Hyperthermia on the Thermotolerance of Cow and Sheep Skin-Derived Fibroblasts. Animals 2020, 10, 545. https://doi.org/10.3390/ani10040545
Saadeldin IM, Swelum AA-A, Zakri AM, Tukur HA, N. Alowaimer A. Effects of Acute Hyperthermia on the Thermotolerance of Cow and Sheep Skin-Derived Fibroblasts. Animals. 2020; 10(4):545. https://doi.org/10.3390/ani10040545
Chicago/Turabian StyleSaadeldin, Islam M., Ayman Abdel-Aziz Swelum, Adel M. Zakri, Hammed A. Tukur, and Abdullah N. Alowaimer. 2020. "Effects of Acute Hyperthermia on the Thermotolerance of Cow and Sheep Skin-Derived Fibroblasts" Animals 10, no. 4: 545. https://doi.org/10.3390/ani10040545
APA StyleSaadeldin, I. M., Swelum, A. A.-A., Zakri, A. M., Tukur, H. A., & N. Alowaimer, A. (2020). Effects of Acute Hyperthermia on the Thermotolerance of Cow and Sheep Skin-Derived Fibroblasts. Animals, 10(4), 545. https://doi.org/10.3390/ani10040545