Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Design
2.3. Collection of Samples
2.4. Short Chain Fatty Acids and Lactic Acid Analysis in Feces
2.5. Fecal DNA Extraction, Sequencing, and Taxonomic Annotation
2.6. Quantitative Real-time PCR (qPCR)
2.7. Endocrine Analysis of Saliva and Hair Samples
2.8. Computation and Statistical Analysis
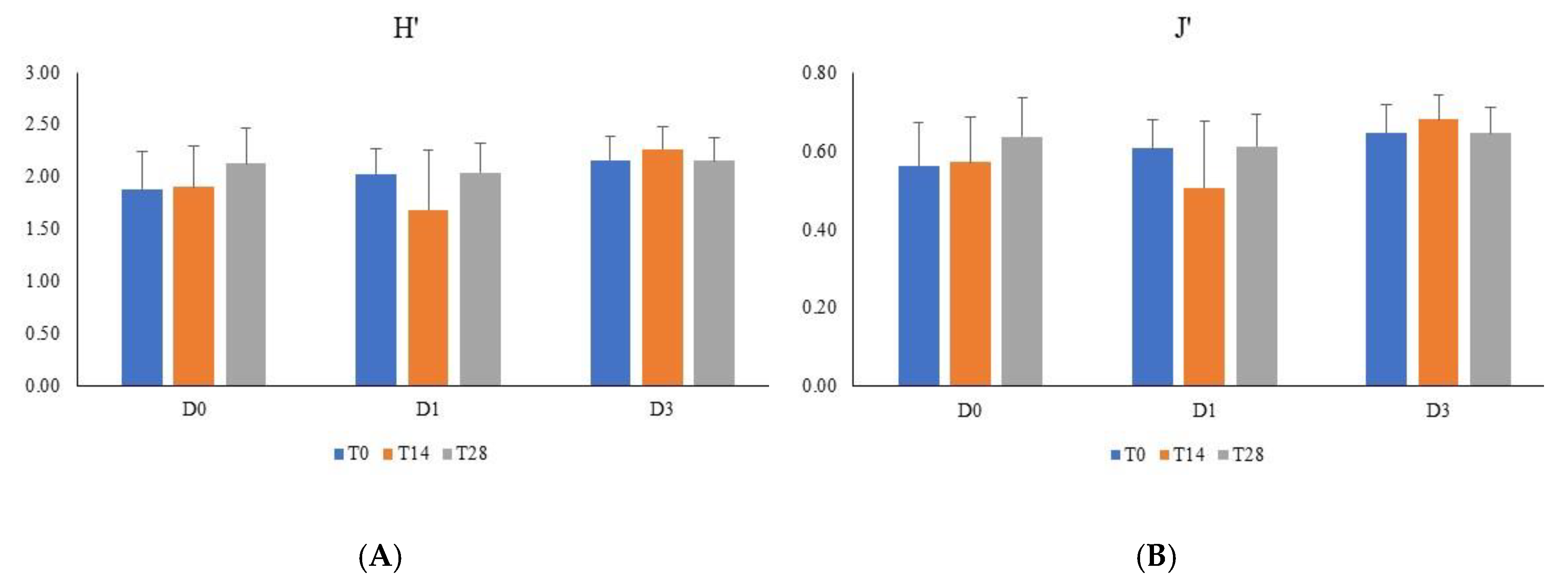
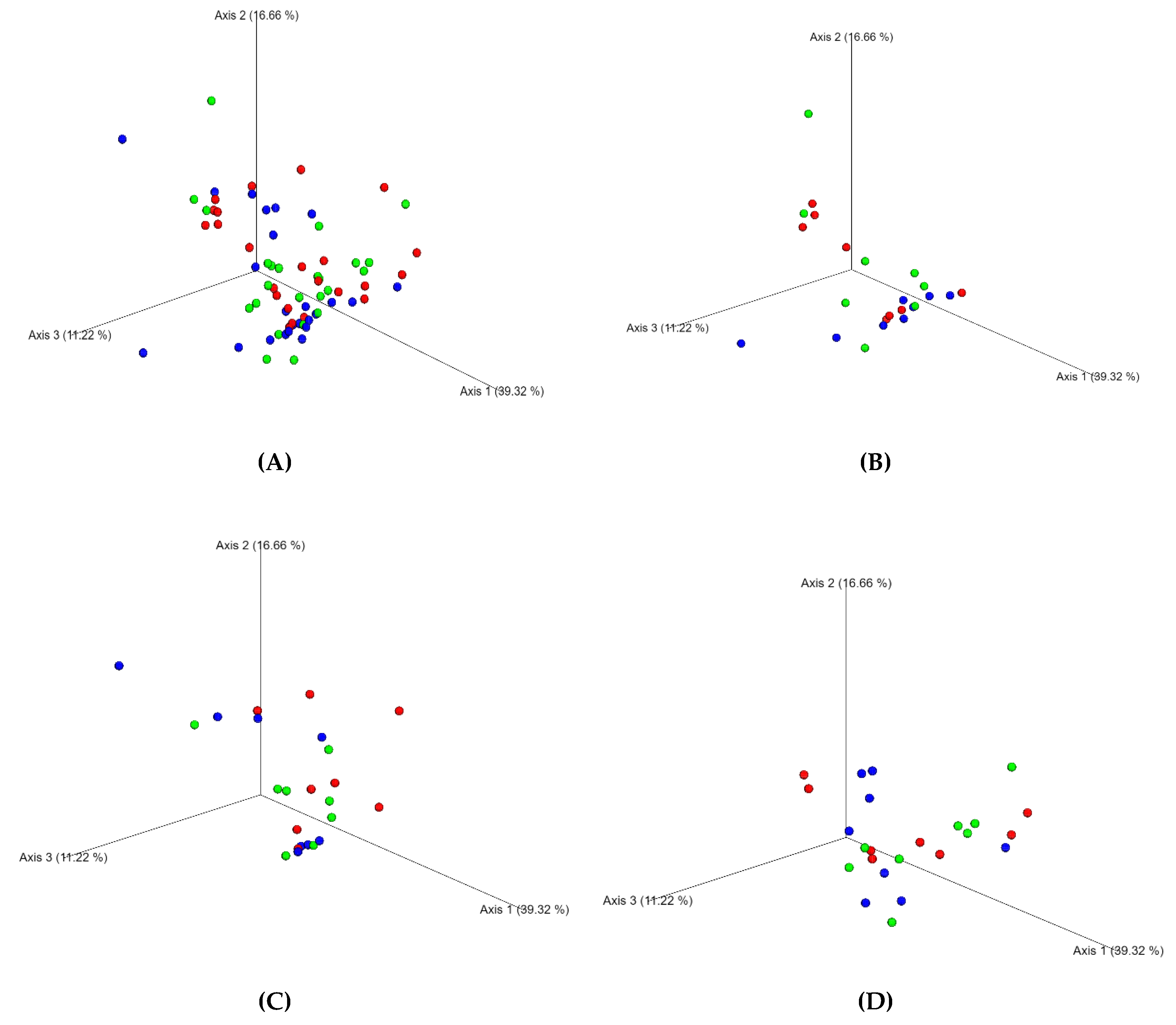
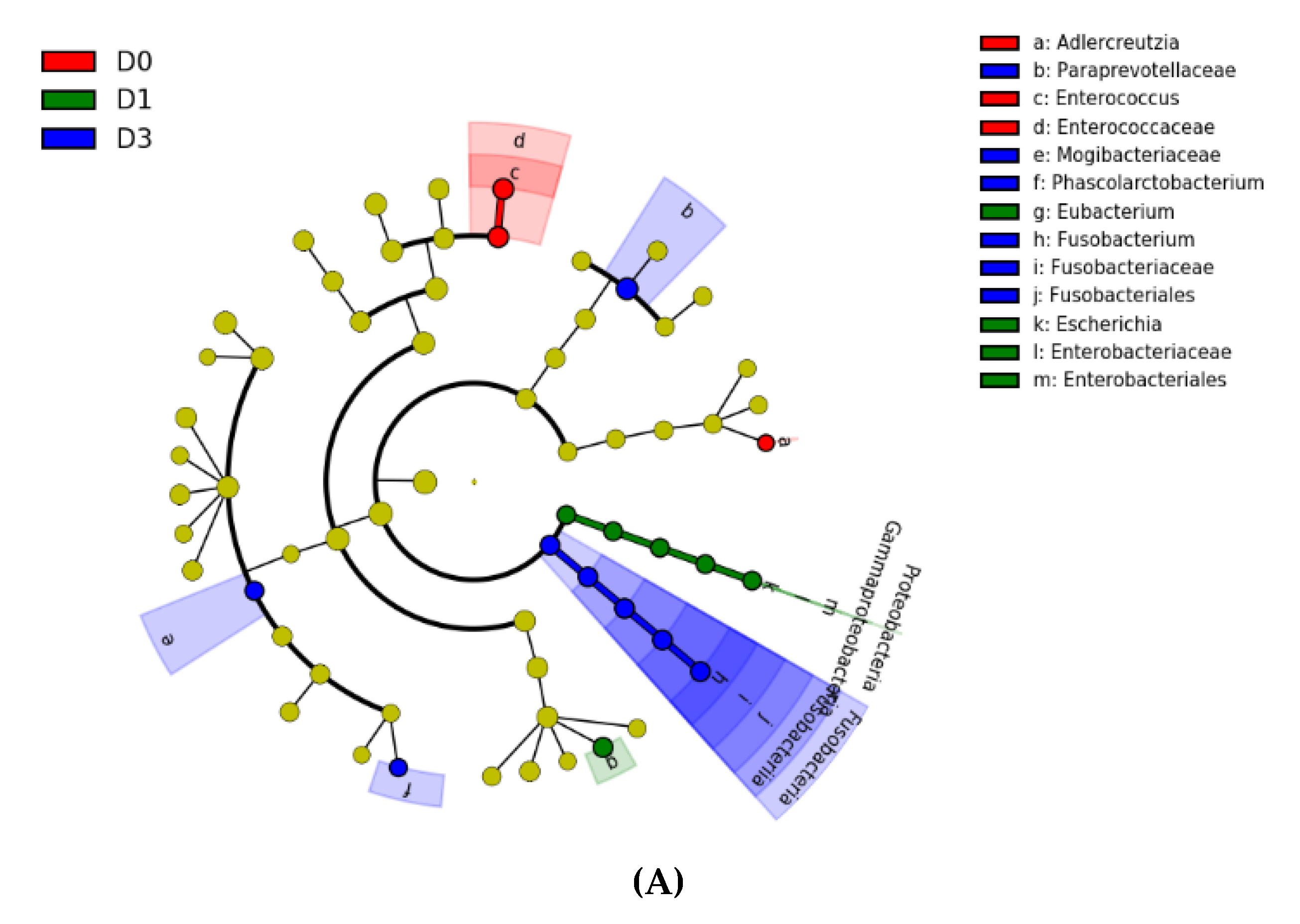
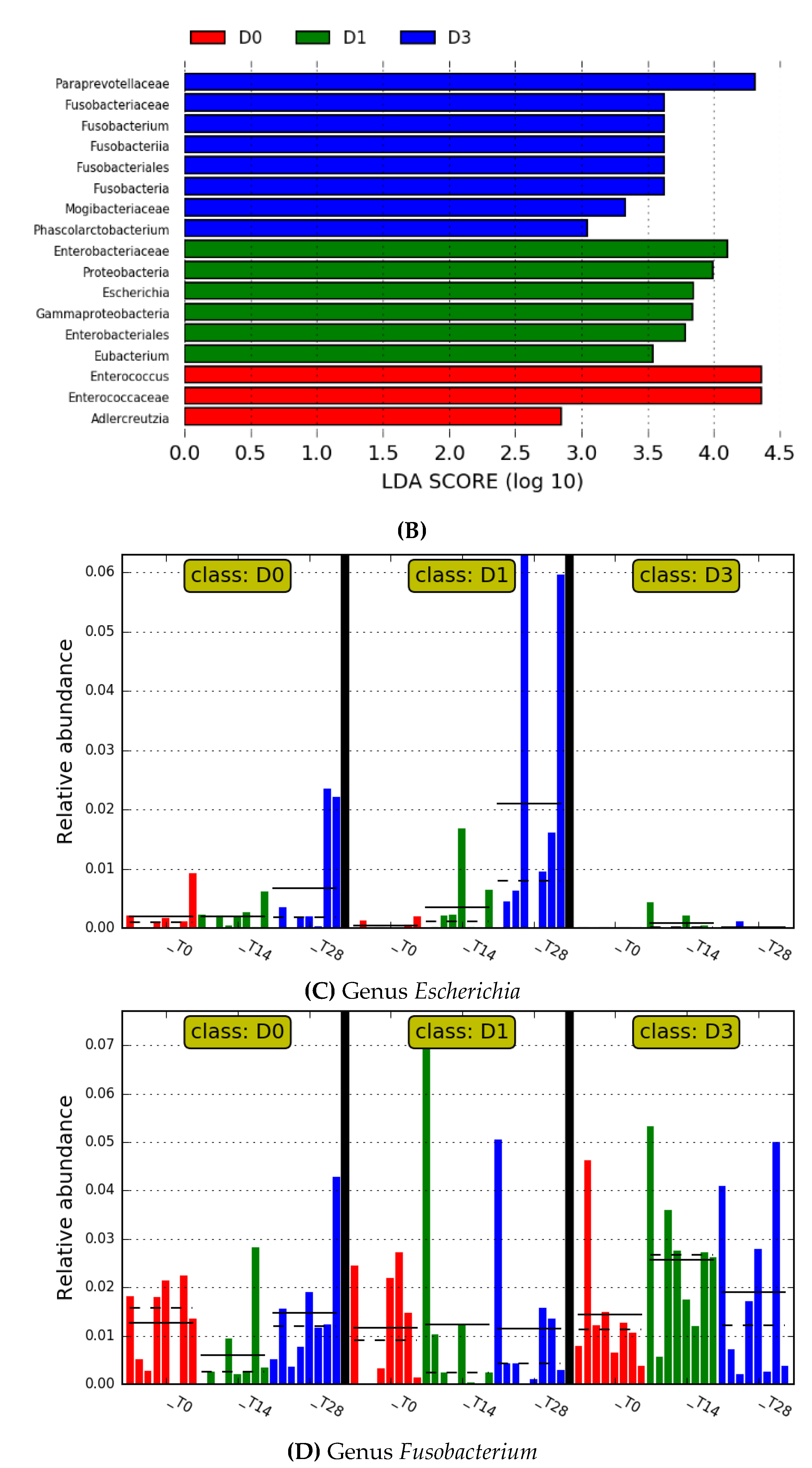
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gilbert, S.F.; Sapp, J.; Tauber, A.I. A symbiotic view of life: We have never been individuals. Q. Rev. Biol. 2012, 87, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroentero. 2014, 20, 14105–14125. [Google Scholar] [CrossRef]
- Forsythe, P.; Kunze, W.; Bienenstock, J. Moody microbes or fecal phrenology: What do we know about the microbiota-gut-brain axis? BMC Med. 2016, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- de Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Russell, W.R.; Scobbie, L.; Labat, A.; Duthie, G.G. Selective bio-availability of phenolic acids from Scottish strawberries. Mol. Nutr. Food Res. 2009, 53, S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandri, M.; Colussi, A.; Perrotta, M.G.; Stefanon, B. Salivary cortisol concentration in healthy dogs is affected by size, sex, and housing context. J. Vet. Behav. 2015, 10, 302–306. [Google Scholar] [CrossRef]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2017, 13, 65. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic. Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [Green Version]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef]
- Sgorlon, S.; Mattiello, A.; Ronutti, L.; Sandri, M.; Stefanon, B. Concentration of elements in the hair of growing and adult dogs. It. J. Anim. Sci. 2019, 18, 1126–1134. [Google Scholar] [CrossRef]
- Sgorlon, S.; Fanzago, M.; Guiatti, D.; Gabai, G.; Stradaioli, G.; Stefanon, B. Factors affecting milk cortisol in mid lactating dairy cows. BMC Vet. Res. 2015, 11, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandri, M.; Sgorlon, S.; Conte, G.; Serra, A.; Dal Monego, S.; Stefanon, B. Substitution of a commercial diet with raw meat complemented with vegetable foods containing chickpeas or peas affects faecal microbiome in healthy dogs. Ital. J. Anim. Sci. 2019, 18, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, J.A. Microbial community structure and its functional implications. Nature 2009, 459, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.P.S.; He, F.; Mangian, H.F.; Oba, P.M.; De Godoy, M.R.C. Dietary supplementation of a fiber-prebiotic and saccharin-eugenol blend in extruded diets fed to dogs. J. Anim. Sci. 2019, 97, 4519–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knigh, R. Quantitative and qualitative diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome. Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: Boston, MA, USA, 2020. [Google Scholar]
- Redfern, A.; Suchodolski, J.; Jergens, A. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Rec. 2017, 181, 370. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tang, L.; Zhou, H.; Zhou, J.; Glenn, T.C.; Shen, C.L.; Wang, J.S. Long-term treatment with green tea polyphenols modifies the gut microbiome of female sprague-dawley rats. J. Nutr. Biochem. 2018, 56, 55–64. [Google Scholar] [CrossRef]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Fragua, V.; Lepoudère, A.; Leray, V.; Baron, C.; Araujo, J.A.; Nguyen, P.; Milgram, N.W. Effects of dietary supplementation with a mixed blueberry and grape extract on working memory in aged beagle dogs. J. Nutr. Sci. 2017, 6, e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beloshapka, A.N.; Duclos, L.M.; Vester Boler, B.M.; Swanson, K.S. Effects of inulin or yeast cell-wall extract on nutrient digestibility, fecal fermentative end-product concentrations, and blood metabolite concentrations in adult dogs fed raw meat-based diets. Am. J. Vet. Res. 2012, 73, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.E.; Hume, I.D. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol. Rev. 1998, 78, 393–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panasevich, M.R.; Rossoni Serao, M.C.; de Godoy, M.R.; Swanson, K.S.; Guerin-Deremaux, L.; Lynch, G.L.; Wils, D.; Fahey, G.C., Jr.; Dilger, R.N. Potato fiber as a dietary fiber source in dog foods. J. Anim. Sci. 2013, 91, 5344–5352. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 2014, 36, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.S.; Leray, V.; Lepoudere, A.; Blanchard, G.; Bensalem, J.; Gaudout, D.; Ouguerram, K.; Nguyen, P. Neurophenols Consortium. A mixed grape and blueberry extract is safe for dogs to consume. BMC Vet. Res. 2016, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Comblain, F.; Barthélémy, N.; Lefèbvre, M.; Schwartz, C.; Lesponne, I.; Serisier, S.; Feugier, A.; Balligand, M.; Henrotin, Y. A randomized, double-blind, prospective, placebo-controlled study of the efficacy of a diet supplemented with curcuminoids extract, hydrolyzed collagen and green tea extract in owner’s dogs with osteoarthritis. BMC Vet. Res. 2017, 13, 395. [Google Scholar] [CrossRef] [Green Version]
- Colitti, M.; Gaspardo, B.; Della Pria, A.; Scaini, C.; Stefanon, B. Transcriptome modification of white blood cells after dietary administration of curcumin and non-steroidal anti-inflammatory drug in osteoarthritic affected dogs. Vet. Immunol. Immunopathol. 2012, 147, 136–146. [Google Scholar] [CrossRef]
- Sgorlon, S.; Stefanon, B.; Sandri, M.; Colitti, M. Nutrigenomic activity of plant derived compounds in health and disease: Results of a dietary intervention study in dog. Res. Vet. Sci. 2016, 109, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Jose, T.; Pattanaik, A.K.; Jadhav, S.E.; Dutta, N.; Sharma, S. Nutrient digestibility, hindgut metabolites and antioxidant status of dogs supplemented with pomegranate peel extract. J. Nutr. Sci. 2017, 6, e36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Barcenas-Walls, J.R.; Suchodolski, J.S.; Steiner, J.M. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ 2017, 5, e3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.E.; Kim, H.R.; Jeong, J.Y.; So, K.M.; Lee, S.; Ji, S.Y.; Kim, M.; Lee, H.J.; Lee, S.; Kim, K.H.; et al. Impact of Breed on the Fecal Microbiome of Dogs under the Same Dietary Condition. J. Microbiol. Biotechnol. 2019, 29, 1947–1956. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Davenport, E.R.; Clark, A.G.; Ley, R.E. The Relationship Between the Human Genome and Microbiome Comes into View. Annu. Rev. Genet. 2017, 51, 413–433. [Google Scholar] [CrossRef] [Green Version]
- Roehe, R.; Dewhurst, R.J.; Duthie, C.A.; Rooke, J.A.; McKain, N.; Ross, D.W.; Hyslop, J.J.; Waterhouse, A.; Freeman, T.C.; Watson, M.; et al. Bovine Host Genetic Variation Influences Rumen Microbial Methane Production with Best Selection Criterion for Low Methane Emitting and Efficiently Feed Converting Hosts Based on Metagenomic Gene Abundance. PLoS Genet. 2016, 12, e1005846. [Google Scholar] [CrossRef]
- Colussi, A.; Stefanon, B.; Adorini, C.; Sandri, M. Variations of salivary cortisol in dogs exposed to different cognitive and physical activities. Ital. J. Anim. Sci. 2018, 17, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.; Selvage, C.; Bosdet, T.; Branov, J.; Rosen-Heath, A.; Bishop, C.; Sirrs, S.; Horvath, G. Salivary serotonin does not correlate with central serotonin turnover in adult phenylketonuria (PKU) patients. Mol. Genet. Metab. Rep. 2018, 15, 100–105. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Guzik, A.C.; van der Meulen, J.; Dekker, R.; Kogut, J.; Kerr, B.J.; Southern, L.L. Effects of supplemental L-tryptophan on serotonin, cortisol, intestinal integrity, and behavior in weanling piglets. J. Anim. Sci. 2006, 84, 963–971. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondo, E.; Barone, M.; Soverini, M.; D’Amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P.A. Gut microbiome structure and adrenocortical activity in dogs with aggressive and phobic behavioral disorders. Heliyon 2020, 1, e03311. [Google Scholar] [CrossRef] [PubMed]
- Kirchoff, N.S.; Udell, M.A.R.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, C.; Prykhodko, O.; Alminger, M.; Fak Hallenius, F.; Nyman, M. Barley Products of Different Fiber Composition Selectively Change Microbiota Composition in Rats. Mol. Nutr. Food Res. 2018, 62, e1701023. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Situ, C. Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J. Clin. Nutr. Metab. 2017, 1, 1–12. [Google Scholar]

| Item | D0 | D1 | D3 | Effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T14 | T28 | T0 | T14 | T28 | T0 | T14 | T28 | SEM 1 | Diet | Time | D × T | |
| Lactate (μmol/g) | 4.7 ab | 3.8 ab | 1.4 ab | 0.9 b | 7.5 ab | 2.1 ab | 15.3 a | 2.5 ab | 2.9 ab | 1.02 | NS | NS | NS |
| Acetate | 143.3 | 128.8 | 113.7 | 137.9 | 123.4 | 124.4 | 143.7 | 139.6 | 150.5 | 4.09 | NS | NS | NS |
| Propionate | 39.3 b | 35.9 b | 38.0 b | 39.0 b | 49.5 ab | 45.5 ab | 38.9 b | 48.0 ab | 61.0 a | 1.67 | * | * | * |
| Isobutirate | 96.8 | 61.9 | 90.5 | 82.7 | 58.4 | 71.0 | 55.6 | 72.5 | 82.2 | 4.12 | NS | NS | NS |
| Butirate | 8.1 | 8.0 | 9.8 | 8.7 | 11.6 | 10.6 | 11.3 | 11.3 | 11.1 | 0.46 | NS | NS | NS |
| Isovalerate | 4.2 | 12.4 | 4.2 | 4.5 | 4.2 | 4.0 | 5.3 | 3.7 | 5.7 | 1.06 | NS | NS | NS |
| Total | 296.3 | 250.7 | 257.6 | 273.7 | 254.6 | 257.6 | 270.1 | 277.6 | 313.3 | 6.63 | NS | NS | NS |
| Lactate (molar %) | 1.8 ab | 2.5 ab | 0.6 b | 0.3 b | 6.4 ab | 0.8 ab | 7.9 a | 0.9 ab | 1.0 ab | 0.75 | NS | NS | * |
| Acetate | 48.3 | 50.8 | 44.8 | 50.5 | 48.4 | 48.7 | 52.2 | 50.2 | 47.9 | 0.89 | NS | NS | NS |
| Propionate | 13.2 b | 14.4 ab | 15.0 ab | 14.2 ab | 18.6 ab | 17.8 ab | 14.4 ab | 17.4 ab | 19.6 a | 0.52 | NS | ** | NS |
| Isobutirate | 32.4 ab | 24.5 abc | 34.0 ab | 30.3 ab | 19.8 bc | 26.9 abc | 19.1 c | 26.2 abc | 26.2 abc | 1.19 | NS | NS | NS |
| Butirate | 2.8 | 3.2 | 3.9 | 3.1 | 4.3 | 4.2 | 4.4 | 4.0 | 3.5 | 0.17 | NS | NS | NS |
| Isovalerate | 1.5 | 4.7 | 1.7 | 1.6 | 2.5 | 1.6 | 2.1 | 1.3 | 1.8 | 0.42 | NS | NS | NS |
| Item | D0 | D1 | D3 | Effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T28 | T0 | T28 | T0 | T28 | SEM 1 | Diet | Time | D × T | |
| HCS (ng/mL) | 1.23 a | 4.80 a | 1.88 ab | 6.26 b | 1.37 a | 2.88 b | 0.44 | NS | ** | * |
| HCH (ng/g) | 6.80 | 6.89 | 6.96 | 6.56 | 6.91 | 7.10 | 0.15 | NS | NS | NS |
| SES (ng/mL) | 32.47 b | 34.97 b | 42.37 ab | 77.64 a | 44.31 ab | 75.41 a | 5.77 | NS | ** | * |
| HCS:SES | 0.21 ab | 0.31 a | 0.08 b | 0.09 ab | 0.07 b | 0.05 b | 0.03 | * | * | NS |
| HCS:HCH | 0.18 b | 0.68 a | 0.27 b | 0.96 a | 0.20 b | 0.44 ab | 0.06 | NS | ** | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarsella, E.; Cintio, M.; Iacumin, L.; Ginaldi, F.; Stefanon, B. Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study. Animals 2020, 10, 531. https://doi.org/10.3390/ani10030531
Scarsella E, Cintio M, Iacumin L, Ginaldi F, Stefanon B. Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study. Animals. 2020; 10(3):531. https://doi.org/10.3390/ani10030531
Chicago/Turabian StyleScarsella, Elisa, Michela Cintio, Lucilla Iacumin, Federica Ginaldi, and Bruno Stefanon. 2020. "Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study" Animals 10, no. 3: 531. https://doi.org/10.3390/ani10030531
APA StyleScarsella, E., Cintio, M., Iacumin, L., Ginaldi, F., & Stefanon, B. (2020). Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study. Animals, 10(3), 531. https://doi.org/10.3390/ani10030531







