Validation of Loop-Mediated Isothermal Amplification (LAMP) Field Tool for Rapid and Sensitive Diagnosis of Contagious Agalactia in Small Ruminants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
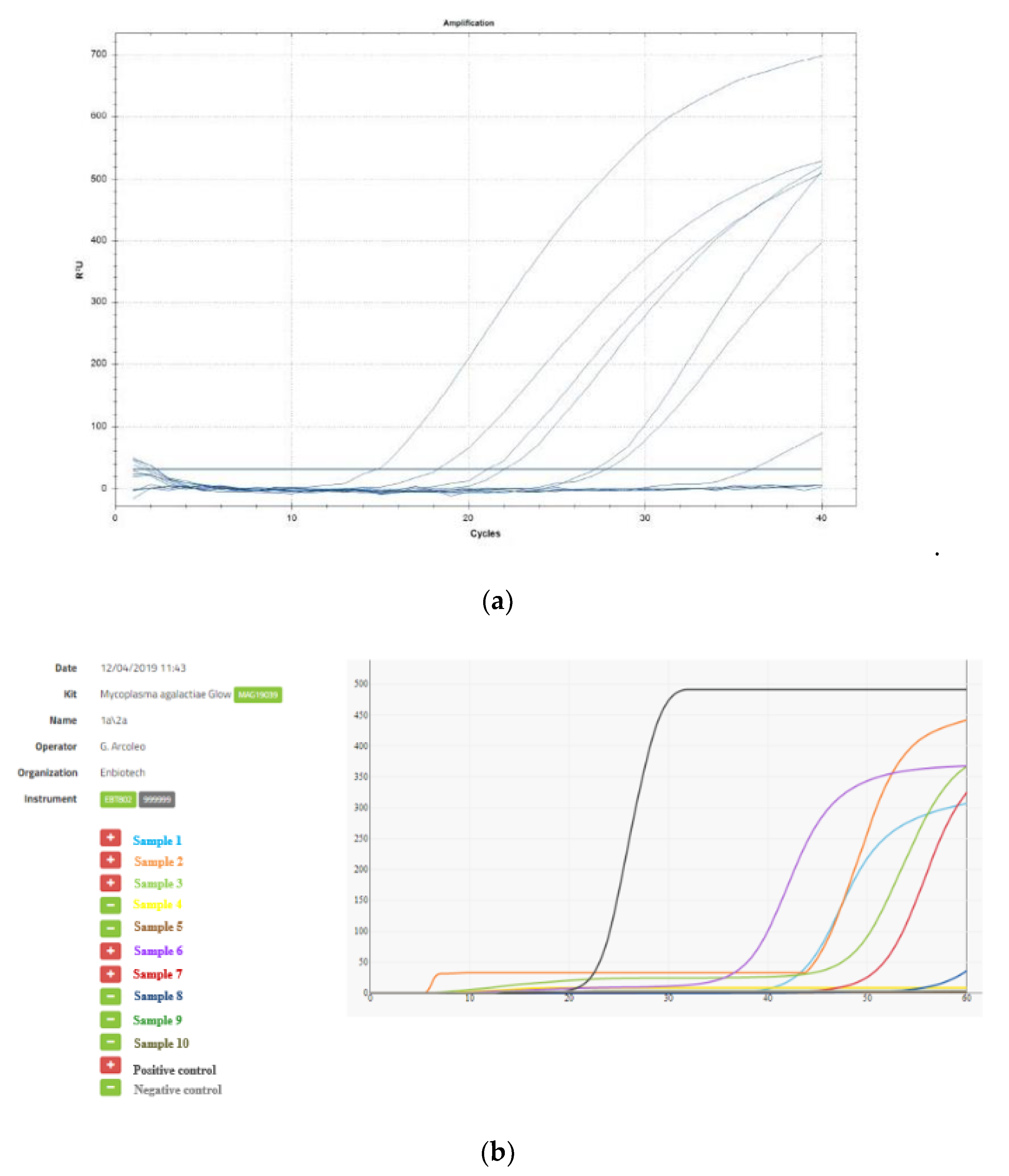
2.2. TaqMan Real-Time-PCR
2.3. Lamp Assay
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Organization for Animal Health (OIE). Chapter 3.7.3 Contagious agalactia. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Terrestrial Manual), 8th ed.; OIE: Paris, France, 2018; Volume 1, pp. 1430–1440. [Google Scholar]
- Nicholas, R.A.J.; Ayling, R.D.; Loria, G.R. Ovine mycoplasmal infections. Small Rumin. Res. 2008, 76, 92–98. [Google Scholar] [CrossRef]
- Tolone, M.; Sutera, A.M.; Borrello, S.; Tumino, S.; Scatassa, M.L.; Portolano, B.; Puleio, R.; Nicholas, R.A.J.; Loria, G.R. Effect of Mycoplasma agalactiae mastitis on milk production and composition in Valle dell Belice dairy sheep. Ital. J. Anim. Sci. 2019, 18, 1067–1072. [Google Scholar] [CrossRef]
- Buonavoglia, D.; Fasanella, A.; Greco, G.; Pratelli, A. A study on an experimental infection of sheep with Mycoplasma agalactiae. New Microbiol. 1999, 22, 27–30. [Google Scholar]
- McAuliffe, L.; Ellis, R.; Lawes, J.; Ayling, R.D.; Nicholas, R.A.J. 16S rDNA and DGGE: A single generic test for detecting and differentiating Mycoplasma species. J. Med. Microbiol. 2005, 54, 1–9. [Google Scholar] [CrossRef]
- Oravcova, K.; Lopez-Enrıquez, L.; Rodrıguez-Lazaro, D.; Hernandez, M. Mycoplasma agalactiae p40 gene, a novel marker for diagnosis of contagious agalactia in sheep by real-time PCR: Assessment of analytical performance and in-house validation using naturally contaminated milk samples. J. Clin. Microbiol. 2009, 47, 445–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Becker, C.A.; Ramos, F.; Sellal, E.; Moine, S.; Poumarat, F.; Tardy, F. Development of a multiplex real-time PCR for contagious agalactia diagnosis in small ruminants. J. Microbiol. Meth. 2012, 90, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loopmediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Hill, J.; Beriwal, S.; Chandra, I.; Paul, V.K.; Kapil, A.; Singh, T.; Wadowsky, R.M.; Singh, V.; Goyal, A.; Jahnukainen, T.; et al. Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Common Strains of Escherichia coli. J. Clin. Microbiol. 2008, 46, 2800–2804. [Google Scholar] [CrossRef]
- Trangoni, M.D.; Gioffre, A.K.; Ceron Cucchi, M.E.; Caimi, K.C.; Ruybal, P.; Zumarraga, M.J.; Cravero, S.L. LAMP technology: Rapid identification of Brucella and Mycobacterium avium subsp. paratuberculosis. Braz. J. Microbiol. 2015, 46, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Sheet, O.H.; Grabowski, N.T.; Klein, G.; Abdulmawjood, A. Development and validation of a loop mediated isothermal amplification (LAMP) assay for the detection of Staphylococcus aureus in bovine mastitis milk samples. Mol. Cell. Probes 2016, 30, 320–325. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M.; Yaqub, T.; Tayyab, M.; Shehzad, W.; Mingala, C.N.; Chang, Y.F. Development and validation of a loop-mediated isothermal amplification assay for the detection of Mycoplasma bovis in mastitic milk. Folia Microbiol. 2018, 63, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Rekha, V.; Rana, R.; Thomas, P.; Viswas, K.N.; Singh, V.P.; Agarwal, R.K.; Arun, T.R.; Karthik, K.; Sophia, I. Development of loop-mediated isothermal amplification test for the diagnosis of contagious agalactia in goats. Trop. Anim. Health Prod. 2015, 47, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Loria, G.R.; Puleio, R.; Nicholas, R.A.J.; Manno, C.; Arcoleo, G.; Drago, C. A novel LAMP field test for the diagnosis of contagious agalactia caused by Mycoplasma agalactiae. Anim. Husb. Dairy Vet. Sci. 2018, 2. [Google Scholar] [CrossRef]
- Rodwell, A.W.; Whitcomb, R.F. Methods for direct and indirect measurement of mycoplasma growth. In Methods in Mycoplasmology, Mycoplasma Characterization; Razin, S., Tully, J.G., Eds.; Academic Press: New York, NY, USA, 1983; Volume 1, pp. 185–196. [Google Scholar]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Jaÿ, M.; Tardy, F. Contagious Agalactia in Sheep and Goats: Current Perspectives. Vet. Med. 2019, 10, 229–247. [Google Scholar] [CrossRef]
- Tola, S.; Angioi, A.; Rocchigiani, A.M.; Idini, G.; Manunta, D.; Galleri, G.; Leori, G. Detection of Mycoplasma agalactiae in sheep milk samples by polymerase chain reaction. Vet. Microbiol. 1997, 54, 17–22. [Google Scholar] [CrossRef]
- Lorusso, A.; Decaro, N.; Greco, C.; Fasanella, A.; Buonavoglia, D. A real-time PCR assay for detection and quantification of Mycoplasma agalactiae. J. Appl. Microbiol. 2007, 103, 918–923. [Google Scholar] [CrossRef]
- Kursa, O.; Woźniakowski, G.; Tomczyk, G.; Sawicka, A.; Minta, Z. Rapid detection of Mycoplasma synoviae by loop-mediated isothermal amplification. Arch. Microbiol. 2015, 197, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Zhu, M.; Xu, M.; Shi, F. Loop-mediated isothermal amplification-lateral-flow dipstick (LAMPLFD) to detect Mycoplasma ovipneumoniae. World J. Microbiol. Biotechnol. 2019, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Tatay-Dualde, J.; Sanchez, A.; Prats-van der Ham, M.; Gomez-Martin, A.; Paterna, A.; Corrales, J.C.; de la Fe, C.; Contreras, A.; Amores, J. Sensitivity of two methods to detect Mycoplasma agalactiae in goat milk. Ir. Vet. J. 2015, 68, 21. [Google Scholar] [CrossRef] [PubMed]
- Tardy, F.; Treilles, M.; Gay, E.; Ambroset, C.; Tricot, A.; Maingourd, C.; Vialard, J.; Le Grand, D. Contagious agalactia monitoring in caprine herds through regular bulk tank milk sampling. J. Dairy Sci. 2019, 102, 5379–5388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, P.; Zhou, S.; Zhang, L. Loop-mediated isothermal amplification (LAMP): A novel rapid detection platform for pathogens. Microb. Pathog. 2017, 107, 54–61. [Google Scholar] [CrossRef]
- Soleimani, M.; Shams, S.; Majidzadeh, A.K. Developing a real-time quantitative loop-mediated isothermal amplification assay as a rapid and accurate method for detection of Brucellosis. J. Appl. Microbiol. 2013, 115, 828–834. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.I. Colorimetric detection of loop mediated isothermal amplification reaction by hydroxy napthol blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef]
- Iwamoto, T.; Sonobe, T.; Hayashi, K. Loop-mediated isothermal amplifcation for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J. Clin. Microbiol. 2003, 41, 2616–2622. [Google Scholar] [CrossRef]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.V.L.; Morita, K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef]
- Lau, Y.L.; Meganathan, P.; Sonaimuthu, P.; Thiruvengadam, G.; Nissapatorn, V.; Chen, Y. Specific, sensitive, and rapid diagnosis of active toxoplasmosis by a loop-mediated isothermal amplification method using blood samples from patients. J. Clin. Microbiol. 2010, 48, 3698–3702. [Google Scholar] [CrossRef]
| Primers | Length (bp) | Primer Sequence (5′-3′) |
|---|---|---|
| F3 | 21 | GGTTTATTAACTGCGTCATCA |
| B3 | 19 | CAACAGTTGCATTCGTCTT |
| FIP | 46 | ACCTTATCACCATTATCTTGTGGATCAGTGCCTTTATTAGCTCGTA |
| BIP | 46 | AGCATTAGGTGAAGTTGTCAAAAATATTGAGCTTGCTTCAGGAATT |
| LF | 24 | GTGAATTTTCGTTCTTATCATCAC |
| LB | 26 | ACAAATCTAGGTGAAATAGTATTACC |
| Test | TP | FP | FN | TN | P (%) | Se (95% CI) | Sp (95% CI) | PPV | NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Real-time PCR | 69 | 0 | 21 | 20 | 0.82 | 0.77 (0.59–0.95) | 1 | 1 | 0.49 (0.44–0.54) |
| LAMP | 81 | 0 | 9 | 20 | 0.82 | 0.90 (0.84–0.96) | 1 | 1 | 0.69 (0.65–0.73) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumino, S.; Tolone, M.; Parco, A.; Puleio, R.; Arcoleo, G.; Manno, C.; Nicholas, R.A.J.; Loria, G.R. Validation of Loop-Mediated Isothermal Amplification (LAMP) Field Tool for Rapid and Sensitive Diagnosis of Contagious Agalactia in Small Ruminants. Animals 2020, 10, 509. https://doi.org/10.3390/ani10030509
Tumino S, Tolone M, Parco A, Puleio R, Arcoleo G, Manno C, Nicholas RAJ, Loria GR. Validation of Loop-Mediated Isothermal Amplification (LAMP) Field Tool for Rapid and Sensitive Diagnosis of Contagious Agalactia in Small Ruminants. Animals. 2020; 10(3):509. https://doi.org/10.3390/ani10030509
Chicago/Turabian StyleTumino, Serena, Marco Tolone, Alessio Parco, Roberto Puleio, Giuseppe Arcoleo, Claudia Manno, Robin A.J. Nicholas, and Guido Ruggero Loria. 2020. "Validation of Loop-Mediated Isothermal Amplification (LAMP) Field Tool for Rapid and Sensitive Diagnosis of Contagious Agalactia in Small Ruminants" Animals 10, no. 3: 509. https://doi.org/10.3390/ani10030509
APA StyleTumino, S., Tolone, M., Parco, A., Puleio, R., Arcoleo, G., Manno, C., Nicholas, R. A. J., & Loria, G. R. (2020). Validation of Loop-Mediated Isothermal Amplification (LAMP) Field Tool for Rapid and Sensitive Diagnosis of Contagious Agalactia in Small Ruminants. Animals, 10(3), 509. https://doi.org/10.3390/ani10030509






