West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
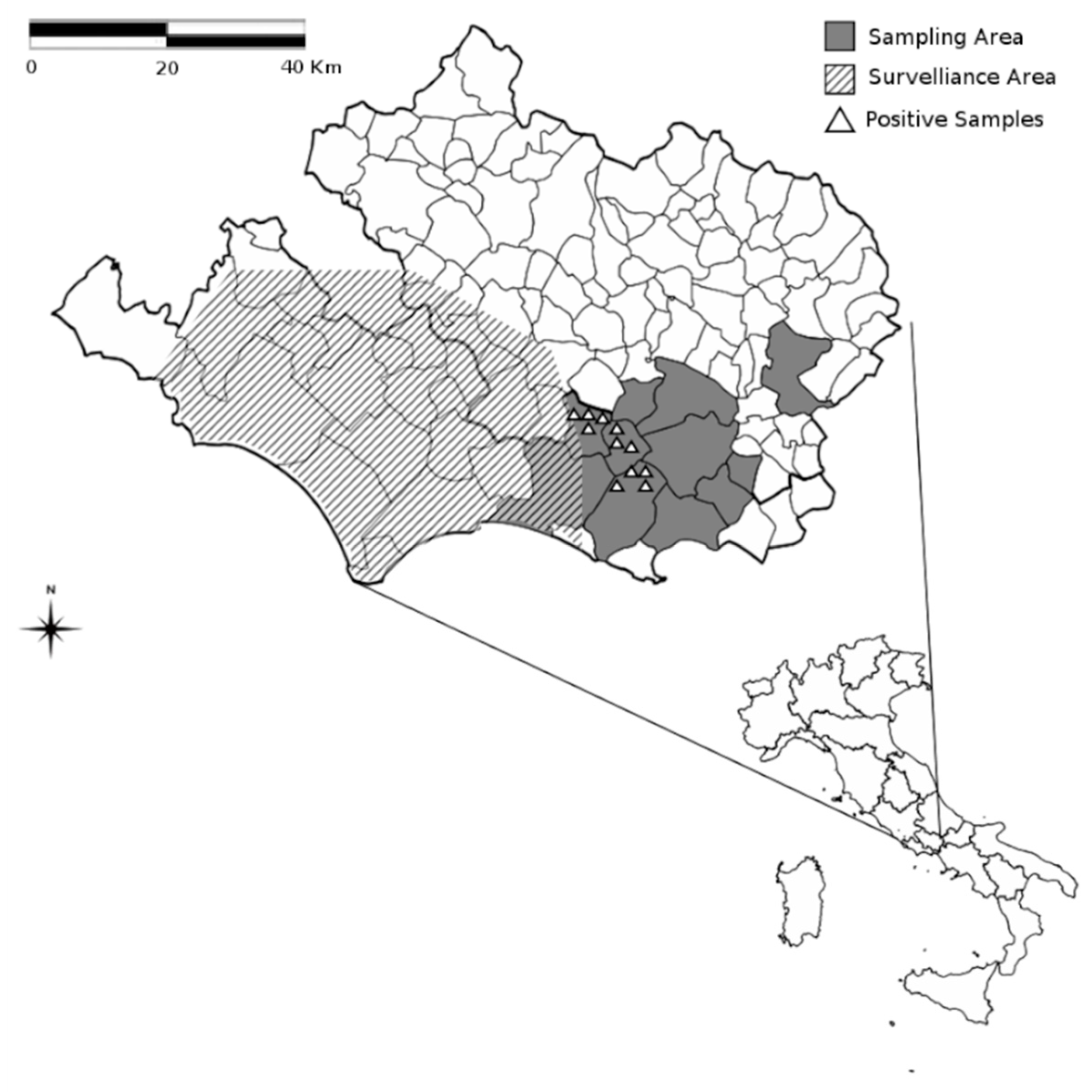
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monath, T.P.; Heinz, F.X. Flaviviruses. In Feilds Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howleym, P.M., Eds.; Lippincott-Ravenvol: Philadelphia, PA, USA, 1996; Volume 1, pp. 961–1034. [Google Scholar]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. 1999 West Nile Outbreak Response Working Group. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Murgue, B.; Murri, S.; Zientara, S.; Durand, B.; Durand, J.P.; Zeller, H. West Nile outbreak in horses in southern France, 2000: The return after 35 years. Emerg. Infect. Dis. 2001, 7, 692–696. [Google Scholar] [CrossRef]
- Napp, S.; Petrić, D.; Busquets, N. West Nile virus and other mosquito-borne viruses present in Eastern Europe. Pathog. Glob. Health 2018, 112, 233–248. [Google Scholar] [CrossRef]
- Macini, P.; Squintani, G.; Finarelli, A.C.; Angelini, P.; Martini, E.; Tamba, M.; Dottori, M.; Bellini, R.; Santi, A.; Loli Piccolomini, L.; et al. Detection of West Nile virus infection in horses, Italy, September 2008. Eur. Surveill. 2008, 13, 18990. [Google Scholar]
- Barzon, L.; Squarzon, L.; Cattai, M.; Franchin, E.; Pagni, S.; Cusinato, R.; Palu, G. West Nile virus infection in Veneto region, Italy, 2008–2009. Eur. Surveill. 2009, 14, 19289. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Healt. Approval of the National Surveillance Plan for West Nile Encephalomyelitis; Ministry of Health: Rome, Italy, 12 February 2008. Available online: http://www.normativasanitaria.it/jsp/dettaglio.jsp?aggiornamenti=&attoCompleto=si&id=25243&page=&anno=null (accessed on 7 January 2020).
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Eur. Surveill. 2010, 15, 19547. [Google Scholar]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef]
- Turell, M.J.; O’Guinn, M.; Oliver, J. Potential for New York mosquitoes to transmit West Nile virus. Am. J. Trop. Med. Hyg. 2000, 62, 413–414. [Google Scholar] [CrossRef]
- Sardelis, M.R.; Turell, M.J.; Dohm, D.J.; O’Guinn, M.L. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 2001, 7, 1018–1022. [Google Scholar] [CrossRef]
- Jeffrey Root, J. West Nile virus associations in wild mammals: A synthesis. Arch. Virol. 2013, 158, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.; Kilpatrick, A.M.; Kramer, L.D.; Dupuis, A.P.; Maffei, J.G.; Goetz, S.J.; Marra, P.P.; Daszak, P.; Aguirre, A.A. Land use and West Nile virus seroprevalence in wild mammals. Emerg. Infect. Dis. 2008, 14, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Juarez, L.I.; Tucker, B.J.; Rowley, W.A.; Platt, K.B. Antibodies to West Nile virus in raccoons and other wild peridomestic mammals in Iowa. J. Wildl. Dis. 2009, 45, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Teehee, M.L.; Bunning, M.L.; Stevens, S.; Bowen, R.A. Experimental infection of pigs with West Nile virus. Brief Report. Arch. Virol. 2005, 150, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Halouzka, J.; Juricova, Z.; Jankova, J.; Hubalek, Z. Serologic survey of wild boars for mosquito-borne viruses in South Moravia (Czech Republic). Vet. Med. 2008, 53, 266–271. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Marlenee, N.L.; Romines, J.; Kavanaugh, D.; Corn, J.L.; Stallknecht, D.E. Antibodies to West Nile virus in feral swine from Florida, Georgia, and Texas, USA. Vector Borne Zoonotic Dis. 2006, 6, 261–265. [Google Scholar] [CrossRef]
- Gutiérrez-Guzmán, A.V.; Vicente, J.; Sobrino, R.; Perez-Ramírez, E.; Llorente, F.; Höfle, U. Antibodies to West Nile virus and related Flaviviruses in wild boar, red foxes and other mesomammals from Spain. Vet. Microbiol. 2012, 159, 291–297. [Google Scholar] [CrossRef]
- Cosseddu, G.M.; Sozio, G.; Valleriani, F.; Di Gennaro, A.; Pascucci, I.; Gavaudan, S.; Marianneau, P.; Monaco, F. Serological Survey of Hantavirus and Flavivirus Among Wild Rodents in Central Italy. Vector Borne Zoonotic Dis. 2017, 17, 777–779. [Google Scholar] [CrossRef]
- Calistri, P.; Monaco, F.; Savini, G.; Guercio, A.; Purpari, G.; Vicari, D.; Cascio, S.; Lelli, R. Further spread of West Nile virus in Italy. Vet. Ital. 2010, 46, 471–474. [Google Scholar]
- Monaco, F.; Lelli, R.; Teodori, L.; Pinoni, C.; Di Gennaro, A.; Polci, A.; Calistri, P.; Savini, G. Re-Emergence of West Nile Virus in Italy. Zoonoses Public Health 2010, 57, 476–486. [Google Scholar] [CrossRef]
- Iovane, V.; Ferrara, G.; Petruccelli, A.; Veneziano, V.; D’Alessio, N.; Ciarcia, R.; Fioretti, A.; Pagnini, U.; Montagnaro, S. Prevalence of serum antibodies against the Mycobacterium tuberculosis complex in wild boar in Campania region, Italy. Eur. J. Wildl. Res. 2020, 66, 20. [Google Scholar] [CrossRef]
- Pâslaru, A.; Porea, D.; Savuta, G.; Oşlobanu, L. West Nile Virus Serosurveillance in Wild Boars from the East of Romania. Bull. UASVM Vet. Med. 2016, 73, 144–148. [Google Scholar] [CrossRef]
- Beck, C.; Lowenski, S.; Durand, B.; Bahuon, C.; Zientara, S.; Lecollinet, S. Improved reliability of serological tools for the diagnosis of West Nile fever in horses within Europe. PLoS Negl. Trop. Dis. 2017, 11, e0005936. [Google Scholar] [CrossRef] [PubMed]
- Bournez, L.; Umhang, G.; Faure, E.; Boucher, J.M.; Boué, F.; Jourdain, E.; Sarasa, M.; Llorente, F.; Jiménez-Clavero, M.A.; Moutailler, S.; et al. Exposure of Wild Ungulates to the Usutu and Tick-Borne Encephalitis Viruses in France in 2009–2014: Evidence of Undetected Flavivirus Circulation a Decade Ago. Viruses 2019, 12, 10. [Google Scholar] [CrossRef]
- Johnson, N.; Wakeley, P.R.; Mansfield, K.L.; McCracken, F.; Haxton, B.; Phipps, L.P.; Fooks, A.R. Assessment of a novel real-time pan-flavivirus RT-polymerase chain reaction. Vector Borne Zoonotic Dis. 2010, 10, 665–671. [Google Scholar] [CrossRef]
- Montagnaro, S.; Sasso, S.; De Martino, L.; Longo, M.; Iovane, V.; Ghiurmino, G.; Pisanelli, G.; Nava, D.; Baldi, L.; Pagnini, U. Prevalence of antibodies to selected viral and bacterial pathogens in wild boar (Sus scrofa) in Campania Region, Italy. J. Wildl. Dis. 2010, 46, 316–319. [Google Scholar] [CrossRef]
- Maggi, F.; Mazzetti, P.; Focosi, D.; Macera, L.; Scagnolari, C.; Manzin, A.; Antonelli, G.; Nelli, L.C. Lack of usutu virus RNA in cerebrospinal fluid of patients with encephalitis of unknown etiology, Tuscany, Italy. J. Med. Virol. 2015, 87, 913–916. [Google Scholar] [CrossRef]
- Lelli, R.; Savini, G.; Teodori, L.; Filipponi, G.; Di Gennaro, A.; Leone, A.; Di Gialleonardo, L.; Venturi, L.; Caporale, V. Serological evidence of USUTU virus occurrence in north-eastern Italy. Zoonoses Public Health 2008, 55, 361–367. [Google Scholar] [CrossRef]
- Cavrini, F.; Gaibani, P.; Longo, G.; Pierro, A.M.; Rossini, G.; Bonilauri, P.; Gerundi, G.E.; Di Benedetto, F.; Pasetto, A.; Girardis, M.; et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August–September 2009. Eur. Surveill. 2009, 17, 17–19. [Google Scholar]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.M.T.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.L.; Lelli, R.; et al. First human case of USUTU virus (USUV) neuroinvasive infection. Eurosurveillance, August–September 2009. Eur. Surveill. 2009, 17, 15–17. [Google Scholar]
- Savini, G.; Monaco, F.; Terregino, C.; Di Gennaro, A.; Bano, L.; Pinoni, C.; De Nardi, R.; Bonilauri, P.; Pecorari, M.; Di Gialleonardo, L.; et al. Usutu virus in Italy: An emergence or a silent infection? Vet. Microbiol. 2011, 51, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Romero, E.; Lupulović, D.; Merino-Ramos, T.; Blázquez, A.B.; Lazić, G.; Lazić, S.; Saiz, J.C.; Petrović, T. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet. Microbiol. 2015, 176, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Montagnaro, S.; Piantedosi, D.; Ciarcia, R.; Loponte, R.; Veneziano, V.; Fusco, G.; Amoroso, M.G.; Ferrara, G.; Damiano, S.; Iovane, G.; et al. Serological Evidence of Mosquito-Borne Flaviviruses Circulation in Hunting Dogs in Campania Region, Italy. Vector Borne Zoonotic Dis. 2019, 19, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.A.; Nemeth, N.M. Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, transmission, and human infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Wertheimer, A.M.; Uhrlaub, J.L.; Hirsch, A.; Medigeshi, G.; Sprague, J.; Legasse, A.; Wilk, J.; Wiley, C.A.; Didier, P.; Tesh, R.B.; et al. Immune response to the West Nile virus in aged non-human primates. PLoS ONE 2010, 5, e15514. [Google Scholar] [CrossRef]
- Beck, C.; Jimenez-Clavero, M.A.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int. J. Environ. Res. Public Health 2013, 12, 6049–6083. [Google Scholar] [CrossRef]
- Zottola, T.; Montagnaro, S.; Magnapera, C.; Sasso, S.; De Martino, L.; Bragagnolo, A.; D’Amici, L.; Condoleo, R.; Pisanelli, G.; Iovane, G.; et al. Prevalence and antimicrobial susceptibilityof salmonella in European wild boar (Sus scrofa); Latium Region—Italy. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 161–168. [Google Scholar] [CrossRef]
- Geevarghese, G.; Shaikh, B.H.; Jacob, P.G.; Bhat, H.R. Persistence of haemagglutination-inhibition antibodies to JE and WN viruses in naturally infected domestic pigs in Karnataka State, India. Acta Virol. 1994, 38, 235–237. [Google Scholar]
- Blitvich, B.J.; Bowen, R.A.; Marlenee, N.L.; Hall, R.A.; Bunning, M.L.; Beaty, B.J. Epitope-blocking enzyme-linked immunosorbent assays for detection of west nile virus antibodies in domestic mammals. J. Clin. Microbiol. 2003, 41, 2676–2679. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology, 2nd ed.; Blackwell Science: Oxford, UK, 1995; pp. 138–188. [Google Scholar]
- Boadella, M.; Díez-Delgado, I.; Gutiérrez-Guzmán, A.V.; Höfle, U.; Gortázar, C. Do wild ungulates allow improved monitoring of flavivirus circulation in Spain? Vector Borne Zoonotic Dis. 2012, 12, 490–495. [Google Scholar] [CrossRef] [PubMed]
| Factor | n | Flavivirus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | SE% | 95% CI | χ2 | p-Value | OR | 95% CI | ||
| Total | 168 | 13 | 7.73 | 4.03 | 3.70–11.76 | ||||
| Gender | |||||||||
| Female | 79 | 4 | 5.06 | 4.83 | 0.23–9.89 | ||||
| 0.871 | p = 0.3507 | 0.47 | 0.140–1.604 | ||||||
| Male | 89 | 9 | 10.11 | 6.26 | 3.85–16.37 | ||||
| Age | |||||||||
| 0–24 mounth (juvenile) | 76 | 10 | 13.15 | 7.33 | 5.28–20.48 | Ref. | |||
| 25–36 mounth (sub-adults) | 52 | 1 | 1.92 | 3.77 | 0–5.69 | 4.248 | p = 0.0495 | 7.72 | 0.957–62.34 |
| >36 mounth (adults) | 40 | 2 | 5.0 | 6.75 | 0–11.75 | 2.87 | 0.599–13.83 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruccelli, A.; Zottola, T.; Ferrara, G.; Iovane, V.; Di Russo, C.; Pagnini, U.; Montagnaro, S. West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study. Animals 2020, 10, 494. https://doi.org/10.3390/ani10030494
Petruccelli A, Zottola T, Ferrara G, Iovane V, Di Russo C, Pagnini U, Montagnaro S. West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study. Animals. 2020; 10(3):494. https://doi.org/10.3390/ani10030494
Chicago/Turabian StylePetruccelli, Angela, Tiziana Zottola, Gianmarco Ferrara, Valentina Iovane, Cristina Di Russo, Ugo Pagnini, and Serena Montagnaro. 2020. "West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study" Animals 10, no. 3: 494. https://doi.org/10.3390/ani10030494
APA StylePetruccelli, A., Zottola, T., Ferrara, G., Iovane, V., Di Russo, C., Pagnini, U., & Montagnaro, S. (2020). West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study. Animals, 10(3), 494. https://doi.org/10.3390/ani10030494





