Effects of Dietary Biological or Chemical-Synthesized Nano-Selenium Supplementation on Growing Rabbits Exposed to Thermal Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Source of SeleniumU in this Study
2.2. Antibacterial Activity of Nano-Se
2.3. Animals and Experimental Groups
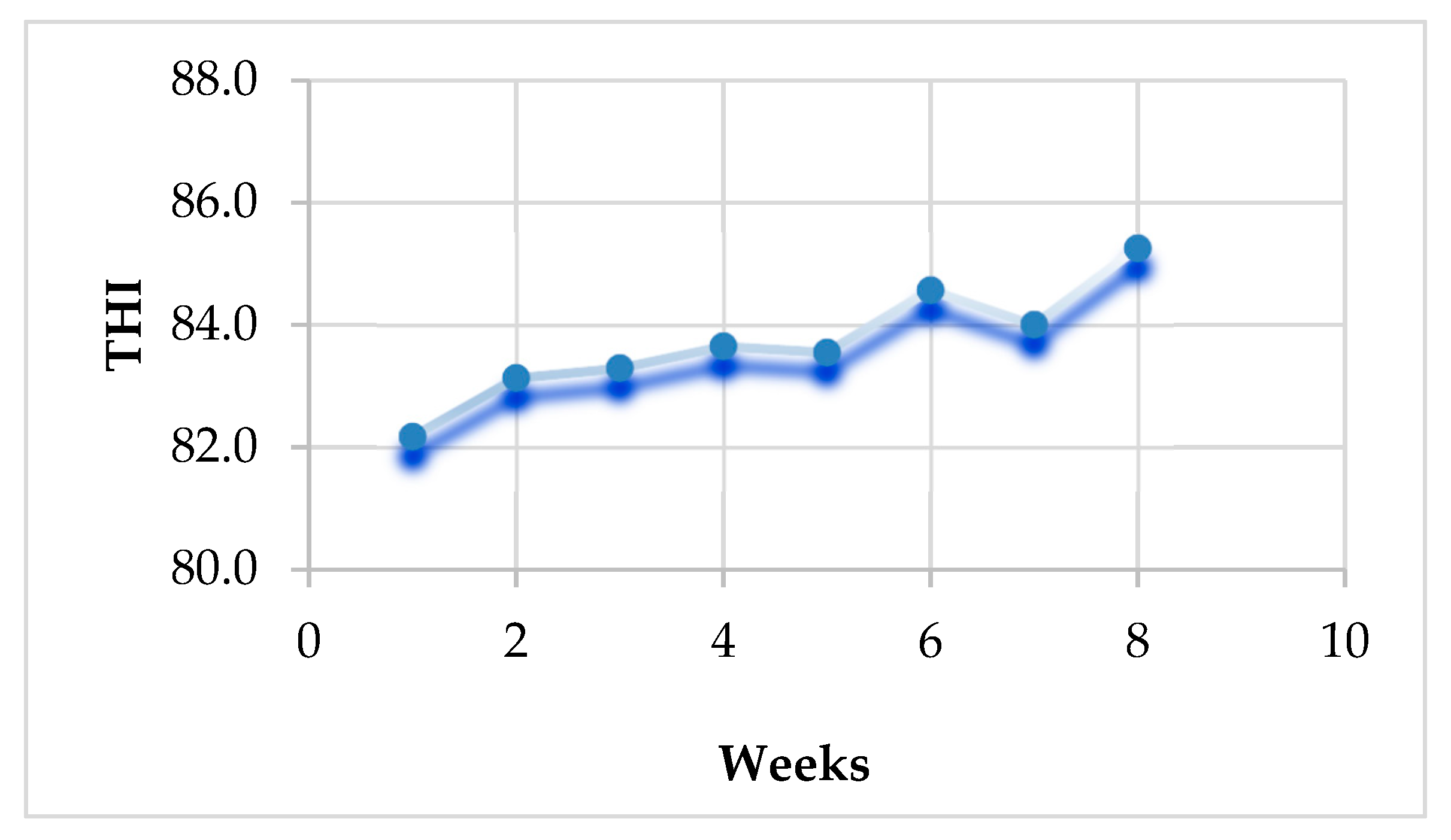
2.4. Temperature Humidity Index (THI) Calculation
2.5. Growth Performance
2.6. Carcass Measurements
2.7. Serum Biochemical, Inflammatory and Antioxidants Indices
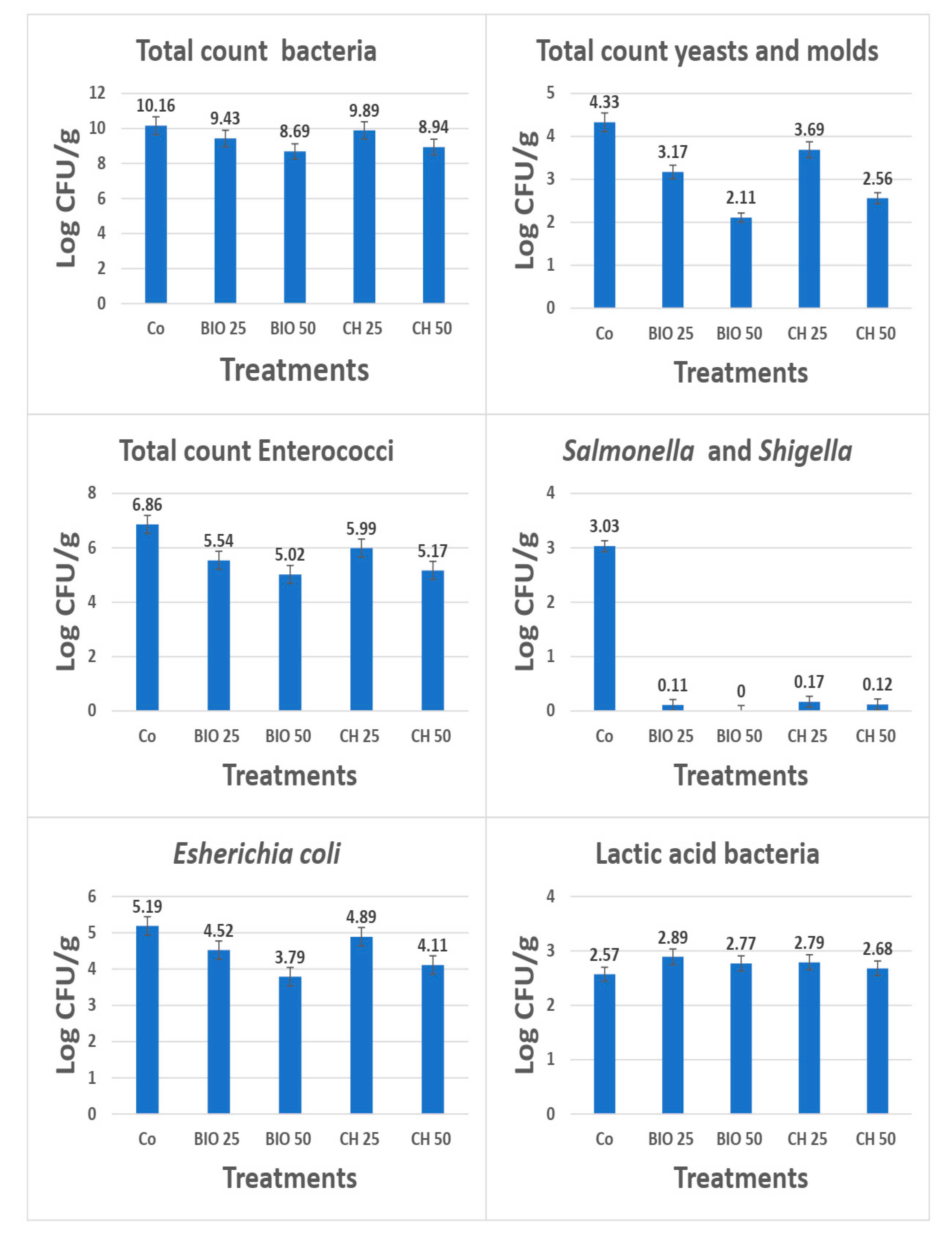
2.8. Microbiological Analyses
2.9. Statistical analysis
3. Results
3.1. Antibacterial Activity of Nano-Se
3.2. Temperature Humidity Index (THI) Values
3.3. Growth Performance
3.4. Carcass Traits
3.5. Blood Serum Metabolites
3.6. Inflammatory Responses
3.7. Antioxidant Indicators
3.8. Impact of Nano-Se (BIO or CH) on Cecal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Abdelnour, S.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Ayasan, T.; Swelum, A.A.; Abukhalil, M.H.; Alkahtani, S.; Aleya, L.; et al. Herbs as thermoregulatory agents in poultry: An overview. Sci. Total Environ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Farghly, M.F.A.; Alagawany, M.; Abd El-Hack, M.E. Feeding time can alleviate negative effects of heat stress on performance, meat quality and health status of turkey. Brit. Poult. Sci. 2018, 59, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.M.; Haeeb, A.A.M.; Gad, A.E. Biological functions in young pregnant rabbit does as affected by heat stress and lighting regime under subtropical conditions of Egypt. Trop. Subtrop. Agroecosyst. 2007, 7, 165–176. [Google Scholar]
- Abdelnour, S.; Alagawany, M.; Abd El-Hack, M.; Sheiha, A.; Swelum, A.; Saadeldin, I. Growth, carcass traits, blood hematology, serum metabolites, immunity, and oxidative indices of growing rabbits fed diets supplemented with red or black pepper oils. Animals 2018, 8, 168. [Google Scholar] [CrossRef]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Daader, A.H.; Gabr, H.A.; AbdEl-Moniem, E.A. Palliative effects of extra virgin olive oil, gallic acid, and lemongrass oil dietary supplementation on growth performance, digestibility, carcass traits, and antioxidant status of heat-stressed growing New Zealand White rabbits. Environ. Sci. Pollut. Res. 2017, 24, 6807–6818. [Google Scholar] [CrossRef]
- Boostani, A.; Sadeghi, A.; Mousavi, S.; Chamani, M.; Kashan, N. Effects of organic, inorganic, and nano-se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest. Sci. 2015, 178, 330–336. [Google Scholar] [CrossRef]
- Cai, S.J.; Wu, C.X.; Gong, L.M.; Song, T.; Wu, H.; Zhang, L.Y. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012, 91, 2532–2539. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, J.S.; Yu, H.Q. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef]
- Zhang, J.S.; Wang, X.F.; Xu, T.W. Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Mahrose, K.M.; Arif, M.; Chaudhry, M.T.; Saadeldin, I.M.; Saeed, M.; Soomro, R.N.; Abbasi, I.H.R.; Rehman, Z.U. Alleviating the environmental heat burden on laying hens by feeding on diets enriched with certain antioxidants (vitamin E and selenium) individually or combined. Environ. Sci. Pollut. Res. 2017, 24, 10708–10717. [Google Scholar] [CrossRef] [PubMed]
- Samak, D.H.; El-Sayed, Y.S.; Shaheen, H.; El-Far, A.; Swelum, A.A.; Noreldin, A.; El-Naggar, K.; Abdelnour, S.; Saied, E.M.; El-Seedi, H.R.; et al. Developmental toxicity of carbon nanoparticles during embryogenesis in chicken. Environ. Sci. Pollut. Res. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, K.; Khan, A.Z.; Parveen, F.; Nizamani, Z.A.; Siyal, F.A.; Abd El-Hack, M.E.; Gan, F.; Liu, Y.; Hamid, M.; Sonia, A.; et al. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express 2018, 8, 112. [Google Scholar]
- Abdul, H.; Sivaraj, R.; Venckatesh, R. Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var purpurascens Benth.-lamiaceae leaf extract. Mater. Lett. 2014, 131, 16–18. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernández, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Xiangqian, L.; Huizhong, X.; Zhe-Sheng, C.; Guofang, C. Biosynthesis of nanoparticles by microorgan-isms and their applications. J. Nanomater. 2011, 270974, 1–16. [Google Scholar]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Bernardi, P.; Butler, C.S.; Vallini, G. Delayed formation of zero-valent selenium nanoparticles by Bacillus mycoides SeITE01 as a consequence of selenite reduction underaerobic conditions. Microb. Cell Fact. 2014, 13, 1–14. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilmactivity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef]
- Li, Q.; Chen, T.; Yang, F.; Liu, J.; Zheng, W. Facile and controllable one-step fabrication of selenium nanoparticles assisted by l-cysteine. Mater. Lett. 2010, 64, 614–617. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analyical Chemists Official Methods of Analysis; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- LPHSI. Livestock and Poultry Heat Stress Indices: Agriculture Engineering Guide; Clemson University: Clemson, SC, USA, 1990. [Google Scholar]
- Marai, I.F.M.; Bahgat, L.B.; Shalaby, T.H.; Abdel-Hafez, M.A. Fattening performance, some behavioral traits and physiological reactions of male lambs fed concentrates mixture alone with or without natural clay under hot summer of Egypt. Ann. Arid Zone 2000, 39, 449–460. [Google Scholar]
- Blasco, A.; Ouhayoun, J.; Masoero, G. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1993, 1, 3–10. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Harrigen, W.F.; Mccance-Margart, E. Laboratory Methods in Food and Dairy Microbiology; Academic Press: London, UK, 1976; pp. 1–115. [Google Scholar]
- Kurtzman, C.; Fell, J.W. The Yeasts, a Taxonomic Study3; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Suchý, P.; Straková, E.; Herzig, I. Selenium in poultry nutrition: A review. Czech J. Anim. Sci. 2014, 59, 495–503. [Google Scholar] [CrossRef]
- Wang, Y. Differential Effects of Sodium Selenite and Nano-Se on Growth Performance, Tissue Se Distribution, and Glutathione Peroxidase Activity of Avian Broiler. Biol. Trace Elem. Res. 2009, 128, 184–190. [Google Scholar] [CrossRef]
- Shi, L.G.; Xun, W.J.; Yue, W.B.; Zhang, C.X.; Ren, Y.S.; Liu, Q.; Wang, Q.; Shi, L. Effect of elemental nanoselenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Anim. Feed Sci. Technol. 2011, 163, 136–142. [Google Scholar] [CrossRef]
- Shi, L.G.; Xun, W.J.; Yue, W.B.; Zhang, C.X.; Ren, Y.S.; Shi, L.; Wang, Q.; Yang, R.J.; Lei, F.L. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Mahrose, K.; Sonbol, S.M.; Abd El-Hack, M.E. Response of laying hens to dietary vitamins A, E and selenium supplementation during summer months. Egypt. J. Anim. Prod. 2011, 47, 167–181. [Google Scholar]
- El-Deep, M.H.; Shabaan, M.; Assar, M.H.; Attia, K.M.; Sayed, M.A.M. Comparative effects of different dietary selenium sources on productive performance, antioxidative properties and immunity in local laying hens exposed to high ambient temperature. J. Anim. Poult. Prod. Mansoura Univ. 2017, 8, 335–343. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Ahmed, A.E.; Hassan, H.A.; Abd El-Sadek, M.S.; Ghazalah, A.A.; Lohakare, J. Nutritional impact of nano-selenium, garlic oil, and their combination on growth and reproductive performance of male Californian rabbits. Anim. Feed Sci. Technol. 2019, 249, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, S.; Wang, X.; Wang, C.; Li, F. The effect of dietary selenium levels on growth performance, antioxidant capacity and glutathione peroxidase 1 (GSHPx1) mRNA expression in growing meat rabbits. Anim. Feed Sci. Technol. 2011, 196, 259–264. [Google Scholar] [CrossRef]
- Zeweil, H.S.; Zahran, S.M.; Ahmed, M.H.; El-Gindy, Y.M.; Khaer, A.S. Effect of organic selenium and ginger supplementation of a diet enriched with linseed oil on performance, carcass, blood lipid profile, with its traits in the meat and antioxidant property of growing rabbits. Egypt. Poult. Sci. J. 2016, 36, 1147–1161. [Google Scholar]
- Saleh, A.A. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim. Sci. Pap. Rep. 2014, 32, 65–79. [Google Scholar]
- Khafaga, A.F.; Noreldin, A.E.; Taha, A.E. The adaptogenic anti-ageing potential of resveratrol against heat stress-mediated liver injury in aged rats: Role of HSP70 and NF-kB signalling. J. Therm. Biol. 2019, 83, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Noreldin, A.E.; Arif, M.; Chaudhry, M.T.; Losacco, C.; Abdeen, A.; Abdel-Daim, M.M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lanthanum. Sci. Total Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abd El-Hack, M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y. Influence of dietary nano elemental Se on growth performance, tissue Se distribution, meat quality, and glutathione peroxidase activity in Guangxi yellow chicken. Poult. Sci. 2011, 90, 680–686. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Abdelnour, S.A. Responses of growing rabbits to supplementing diet with a mixture of black and red pepper oils as a natural growth promoter. J. Anim. Physiol. Anim. Nutr. 2019, 103, 509–517. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli and probiotics in swine: What the bleep do we know? Biosci. Microbiota Food Health 2017, 36, 75–90. [Google Scholar] [CrossRef]
| Items | Average Diameter of the Inhibition Zone (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 µL | 50 µL | 75 µL | 100 µL | Synergism with Antibiotic | ||||||||
| Nano-Se CH | Nano-Se BIO | Nano-Se CH | Nano-Se BIO | Nano-Se CH | Nano-Se BIO | Nano-Se CH | Nano-Se BIO | A | B | C | D | |
| B. cereus | 23 | 25 | 27 | 28 | 29 | 31 | 30 | 34 | 27 | 30 | 31 | 35 |
| L. monocytogenes | 24 | 26 | 26 | 29 | 27 | 32 | 29 | 33 | 28 | 31 | 32 | 36 |
| S. aureus | 22 | 26 | 25 | 27 | 26 | 30 | 28 | 32 | 26 | 28 | 30 | 35 |
| E. coli | 19 | 21 | 20 | 22 | 21 | 24 | 23 | 25 | 24 | 25 | 26 | 29 |
| S. Typhimurium | 21 | 25 | 24 | 27 | 26 | 29 | 28 | 30 | 28 | 31 | 27 | 31 |
| K. pneumoniae | 20 | 21 | 22 | 24 | 23 | 26 | 26 | 29 | 25 | 27 | 25 | 30 |
| Test Pathogenic Bacteria | 20 µg/mL | 40 µg/mL | 80 µg/mL | 160 µg/mL | 320 µg/mL | 640 µg/mL |
|---|---|---|---|---|---|---|
| B. cereus | + | + | ـــ | ـــ | ـــ | ـــ |
| L. monocytogenes | + | + | ـــ | ـــ | ـــ | ـــ |
| S. aureus | + | + | + | ـــ | ـــ | ـــ |
| E. coli | + | + | + | ـــ | ـــ | ـــ |
| S. Typhimurium | + | + | ـــ | ـــ | ـــ | ـــ |
| K. pneumoniae | + | + | + | ـــ | ـــ | ـــ |
| Item | Treatments (12 Rabbits/Treatments) | Pooled SEM | p Value | ||||
|---|---|---|---|---|---|---|---|
| CO | BIO25 | BIO50 | CH25 | CH50 | |||
| Live body weight (LBW; g) at (weeks of age) | |||||||
| 5 | 661 | 689 | 663 | 676 | 666 | 11.65 | 0.513 |
| 6 | 773 b | 870 a | 843 a | 824 ab | 817 ab | 13.52 | 0.234 |
| 7 | 964 | 1079 | 1049 | 995 | 987 | 18.27 | 0.253 |
| 8 | 1169 | 1293 | 1230 | 1168 | 1170 | 19.82 | 0.177 |
| 9 | 1357 | 1457 | 1423 | 1334 | 1333 | 20.53 | 0.198 |
| 10 | 1524 | 1603 | 1612 | 1506 | 1484 | 21.72 | 0.221 |
| 11 | 1692 b | 1756 a | 1795 a | 1700a b | 1609 b | 25.50 | 0.185 |
| 12 | 1840 b | 1904 a | 1965 a | 1833 b | 1764 b | 28.45 | 0.219 |
| 13 | 1959 ab | 2077 a | 2126 a | 1954 ab | 1861 b | 30.34 | 0.028 |
| Daily body weight gain (DBWG; g/day) at (weeks of age) | |||||||
| 6 | 20.28 | 24.58 | 25.7 | 21.14 | 21.58 | 0.82 | 0.150 |
| 7 | 27.28 | 29.86 | 29.44 | 24.4 | 24.28 | 1.21 | 0.440 |
| 8 | 29.28 | 30.58 | 25.86 | 24.7 | 26.12 | 0.91 | 0.200 |
| 9 | 26.86 | 23.4 | 27.58 | 23.72 | 23.26 | 0.79 | 0.240 |
| 10 | 23.86 b | 20.86 b | 27 a | 24.58 b | 21.56 b | 0.82 | 0.110 |
| 11 | 24.02 b | 21.86 b | 26.14 a | 27.72 a | 17.86 b | 1.19 | 0.060 |
| 12 | 21.14 | 21.12 | 24.28 | 19 | 22.16 | 0.76 | 0.290 |
| 13 | 17 bc | 23a | 24.72 a | 17.26 bc | 13 c | 1.21 | 0.010 |
| Feed intake (FI; g) at (weeks of age) | |||||||
| 6 | 70 | 80 | 79 | 73 | 70 | 1.9 | 0.280 |
| 7 | 109 | 93 | 94 | 83 | 84 | 3.96 | 0.236 |
| 8 | 102 | 114 | 98 | 103 | 110 | 3.41 | 0.615 |
| 9 | 93 | 88 | 102 | 87 | 93 | 2.49 | 0.351 |
| 10 | 103 a | 82 b | 107a | 104 a | 93 a | 3.34 | 0.094 |
| 11 | 82 | 84 | 107 | 95 | 83 | 6.02 | 0.670 |
| 12 | 87 | 91 | 106 | 87 | 103 | 3.19 | 0.162 |
| 13 | 80 ab | 99 a | 93 ab | 74 ab | 66 c | 4.57 | 0.124 |
| Feed conversion ratio (FCR; g feed/g gain) at (weeks of age) | |||||||
| 6 | 3.48 | 3.27 | 3.11 | 3.47 | 3.26 | 0.059 | 0.217 |
| 7 | 4.08 a | 3.11 b | 3.20 b | 3.40 b | 3.48 b | 0.087 | 0.001 |
| 8 | 3.48 c | 3.72 b | 3.82 b | 4.18 a | 4.22 a | 0.065 | 0.001 |
| 9 | 3.56 | 3.76 | 3.72 | 3.66 | 4.04 | 0.076 | 0.363 |
| 10 | 4.35 a | 3.95 b | 3.96 b | 4.23 ab | 4.32 a | 0.059 | 0.058 |
| 11 | 3.44 | 3.85 | 4.09 | 3.58 | 4.69 | 0.185 | 0.234 |
| 12 | 4.20 | 4.31 | 4.38 | 4.59 | 4.66 | 0.086 | 0.419 |
| 13 | 4.69 a | 4.01 b | 4.04 b | 4.43 ab | 4.77 a | 0.087 | 0.002 |
| Item * | Treatments (12 Rabbits/Treatments) | Pooled SEM | p Value | ||||
|---|---|---|---|---|---|---|---|
| % | CO | BIO25 | BIO50 | CH25 | CH50 | ||
| Carcass | 59.14 c | 64.38 a | 61.80 b | 60.98 b | 60.71 b | 0.49 | 0.001 |
| Liver | 2.80 a | 2.37b c | 2.70 a | 2.45 b | 2.30 c | 0.05 | 0.001 |
| Lungs | 0.73 a | 0.57 c | 0.60 bc | 0.68 ab | 0.66 abc | 0.018 | 0.016 |
| Heart | 0.24 a | 0.23 ab | 0.24 a | 0.23 b | 0.24 ab | 0.002 | 0.044 |
| Kidney | 0.73 | 0.72 | 0.70 | 0.73 | 0.72 | 0.013 | 0.927 |
| Spleen | 0.052 | 0.051 | 0.053 | 0.055 | 0.055 | 0.001 | 0.662 |
| Items * | Treatments (12 Rabbits/Treatments) | Pooled SEM | p Value | ||||
|---|---|---|---|---|---|---|---|
| CO | BIO25 | BIO50 | CH25 | CH50 | |||
| Creatinine (mg/dL) | 1.46 | 4.15 | 1.12 | 1.26 | 1.35 | 0.60 | 0.500 |
| Urea (mg/dL) | 52.81 a | 51.11 ab | 45.85 c | 49.42 b | 51.34 ab | 0.74 | 0.010 |
| GGT (U/L) | 5.36 ab | 5.02 bc | 4.74 c | 4.95 c | 5.48 a | 0.08 | 0.001 |
| TG (mg/dL) | 144.78 a | 75.44 c | 73.09 c | 73.74 c | 107.33 b | 7.51 | 0.050 |
| Total bilirubin (mg/dL) | 0.88 | 0.89 | 0.82 | 0.83 | 0.89 | 6.01 | 0.310 |
| Direct bilirubin (mg/dL) | 0.21 | 0.19 | 0.20 | 0.20 | 0.19 | 0.00 | 0.270 |
| Indirect bilirubin (mg/dL) | 0.76 | 0.76 | 0.74 | 0.74 | 0.71 | 0.01 | 0.001 |
| IL-4 (pg/mL) | 0.31 a | 0.27 b | 0.28 b | 0.26 b | 0.31 a | 0.01 | 0.010 |
| IFNγ (pg/mL) | 0.18 b | 0.16 b | 0.17 b | 0.17 b | 0.20 a | 0.00 | 0.001 |
| LZM (ng/mL) | 0.22 b | 0.24 b | 0.27 a | 0.29 a | 0.22 b | 0.01 | 0.001 |
| NO (µmol/L) | 0.26 a | 0.20 c | 0.20 d | 0.22 c | 0.24 b | 0.01 | 0.001 |
| MDA (nmol/mL) | 0.31 a | 0.26 c | 0.24 d | 0.246 cd | 0.29 b | 0.01 | 0.001 |
| SOD (U/mL) | 0.29 a | 0.24 cd | 0.22 d | 0.25 c | 0.27 b | 0.01 | 0.001 |
| GSH (ng/mL) | 0.13 c | 0.16 b | 0.17 b | 0.19 a | 0.12 d | 0.01 | 0.001 |
| CAT (ng/mL) | 0.21 c | 0.25 b | 0.25 b | 0.29 a | 0.18 d | 0.01 | 0.050 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheiha, A.M.; Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Metwally, K.A.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; El-Saadony, M.T. Effects of Dietary Biological or Chemical-Synthesized Nano-Selenium Supplementation on Growing Rabbits Exposed to Thermal Stress. Animals 2020, 10, 430. https://doi.org/10.3390/ani10030430
Sheiha AM, Abdelnour SA, Abd El-Hack ME, Khafaga AF, Metwally KA, Ajarem JS, Maodaa SN, Allam AA, El-Saadony MT. Effects of Dietary Biological or Chemical-Synthesized Nano-Selenium Supplementation on Growing Rabbits Exposed to Thermal Stress. Animals. 2020; 10(3):430. https://doi.org/10.3390/ani10030430
Chicago/Turabian StyleSheiha, Asmaa M., Sameh A. Abdelnour, Mohamed E. Abd El-Hack, Asmaa F. Khafaga, Khaled A. Metwally, Jamaan S. Ajarem, Saleh N. Maodaa, Ahmed A. Allam, and Mohamed T. El-Saadony. 2020. "Effects of Dietary Biological or Chemical-Synthesized Nano-Selenium Supplementation on Growing Rabbits Exposed to Thermal Stress" Animals 10, no. 3: 430. https://doi.org/10.3390/ani10030430
APA StyleSheiha, A. M., Abdelnour, S. A., Abd El-Hack, M. E., Khafaga, A. F., Metwally, K. A., Ajarem, J. S., Maodaa, S. N., Allam, A. A., & El-Saadony, M. T. (2020). Effects of Dietary Biological or Chemical-Synthesized Nano-Selenium Supplementation on Growing Rabbits Exposed to Thermal Stress. Animals, 10(3), 430. https://doi.org/10.3390/ani10030430










