The Influence of Diet Containing Wheat Gluten Supplemented with Dipeptides or Amino Acids on the Morphology of White Muscle of Yellow Perch (Perca flavescens)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
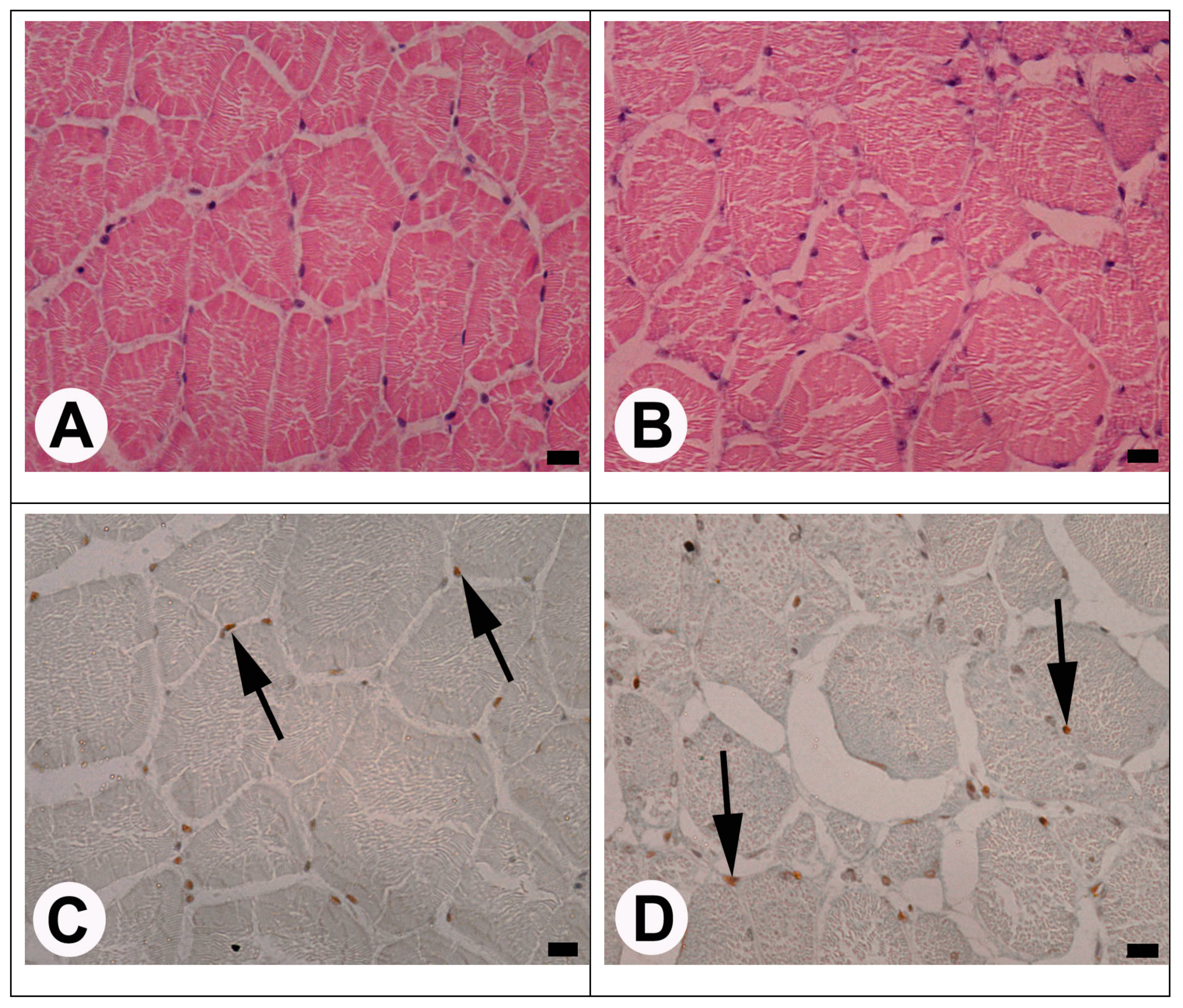
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Cultured Aquatic Species Information Programme, American Yellow Perch; Food and Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Guo, L.; Yao, H.; Shepherd, B.; Sepulveda-Villet, O.J.; Zhang, D.-C.; Wang, H.-P. Development of a genomic resource and identification of nucleotide diversity of yellow perch by rad sequencing. Front. Genet. 2019, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Apper-Bossard, E.; Feneuil, A.; Wagner, A.; Respondek, F. Use of vital wheat gluten in aquaculture feeds. Aquat. Biosyst. 2013, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Helland, S.J.; Grisdale-Helland, B. Replacement of fish meal with wheat gluten in diets for Atlantic halibut (Hippoglossus hippoglossus): Effect on whole-body amino acid concentrations. Aquaculture 2006, 261, 1363–1370. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Kamaszewski, M.; Grochowski, P.; Dabrowski, K.; Verri, T.; Aksakal, E.; Szatkowska, I.; Nowak, Z.; Dobosz, S. The effect of peptide absorption on PepT1 gene expression and digestive system hormones in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 107–114. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwashita, Y.; Matsunari, H.; Sugita, T.; Furuita, H.; Akimoto, A.; Okamatsu, K.; Suzuki, N. Influence of fermentation conditions for soybean meal in a non-fish meal diet on the growth performance and physiological condition of rainbow trout Oncorhynchus Mykiss. Aquaculture 2010, 309, 173–180. [Google Scholar] [CrossRef]
- Hansen, A.-C.; Rosenlund, G.; Karlsen, O.; Olsvik, P.A.; Hemre, G.-I. The inclusion of plant protein in cod diets, its effects on macronutrient digestibility, gut and liver histology and heat shock protein transcription. Aquac. Res. 2006, 37, 773–784. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Kamaszewski, M.; Grochowski, P.; Verri, T.; Rzepkowska, M.; Wolnicki, J. The effect of plant protein-based diet supplemented with dipeptide or free amino acids on digestive tract morphology and PepT1 and PepT2 expressions in common carp (Cyprinus carpio L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 158–169. [Google Scholar] [CrossRef]
- Kamaszewski, M.; Ostaszewska, T.; Napora-Rutkowski, Ł.; Wójcik, M.; Dabrowski, K. The role of dipeptide on fish growth and digestive enzyme activity modulation in common carp (Cyprinus carpio L.). Anim. Sci. Pap. Rep. 2019, 37, 75–86. [Google Scholar]
- Hartviksen, M.; Vecino, J.L.G.; Ringø, E.; Bakke, A.-M.; Wadsworth, S.; Krogdahl, Å.; Ruohonen, K.; Kettunen, A. Alternative dietary protein sources for Atlantic salmon (Salmo salar L.) effect on intestinal microbiota, intestinal and liver histology and growth. Aquac. Nutr. 2014, 20, 381–398. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Kamaszewski, M.; Kwasek, K.; Grodzik, M.; Bierla, J. The effect of dipeptide, Lys-Gly, supplemented diets on digestive tract histology in juvenile yellow perch (Perca flavescens). Aquac. Nutr. 2013, 19, 100–109. [Google Scholar] [CrossRef]
- Kamaszewski, M.; Prasek, M.; Ostaszewska, T.; Dabrowski, K. The influence of feeding diets containing wheat gluten supplemented with dipeptides or free amino acids on structure and development of the skeletal muscle of carp (Cyprinus carpio). Aquac. Int. 2014, 22, 259–271. [Google Scholar] [CrossRef]
- Chapalamadugu, K.C.; Robison, B.D.; Drew, R.E.; Powell, M.S.; Hill, R.A.; Amberg, J.J.; Rodnick, K.J.; Hardy, R.W.; Hill, M.L.; Murdoch, G.K. Dietary carbohydrate level affects transcription factor expression that regulates skeletal muscle myogenesis in rainbow trout. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 153, 66–72. [Google Scholar] [CrossRef]
- Hart, S.D.; Garling, D.L.; Malison, J.A. Yellow Perch (Perca flavescens) Culture Guide; NCRAC: Ames, IA, USA, 2006. [Google Scholar]
- Steenfeldt, S.; Fontaine, P.; Overton, J.L.; Policar, T.; Toner, D.; Falahatkar, B.; Horváth, Á.; Khemis, I.B.; Hamza, N.; Mhetli, M. Current status of eurasian percid fishes aquaculture. In Biology and Culture of Percid Fishes; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 817–841. [Google Scholar]
- Hara, T.J. Feeding behaviour in some teleosts is triggered by single amino acids primarily through olfaction. J. Fish Biol. 2006, 68, 810–825. [Google Scholar] [CrossRef]
- Hughes, S.G. Evaluation of glutamic acid and glycine as sources of nonessential amino acids for lake trout (Salvelinus namaycush) and rainbow trout (Salmo gairdnerii). Comp. Biochem. Physiol. Part A Physiol. 1985, 81, 669–671. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Kwasek, K.; Verri, T.; Kamaszewski, M.; Sliwinski, J.; Napora-Rutkowski, L. Effects of various diet formulations (experimental and commercial) on the morphology of the liver and intestine of rainbow trout (Oncorhynchus mykiss) juveniles: Effect of various diet on rainbow trout histology. Aquac. Res. 2011, 42, 1796–1806. [Google Scholar] [CrossRef]
- Bio-Oregon|Serving Global Customers. Available online: https://www.bio-oregon.com/ (accessed on 16 February 2020).
- Ostaszewska, T.; Dabrowski, K.; Wegner, A.; Krawiec, M. The effects of feeding on muscle growth dynamics and the proliferation of myogenic progenitor cells during pike perch development (Sander lucioperca). J. World Aquac. Soc. 2008, 39, 184–195. [Google Scholar] [CrossRef]
- Mittakos, I.; Ayala, M.D.; López-Albors, O.; Grigorakis, K.; Lenas, D.; Kakali, F.; Nathanailides, C. Muscle cellularity, enzyme activities, and nucleic acid content in meagre (Argyrosomus regius). Can. J. Zool. 2012, 90, 1270–1277. [Google Scholar] [CrossRef]
- Nathanailides, C.; Lopez-Albors, O.; Abellan, E.; Vazquez, J.M.; Tyler, D.D.; Rowlerson, A.; Stickland, N.C. Muscle cellularity in relation to somatic growth in the European sea bass Dicentrarchus labrax (L.). Aquac. Res. 1996, 27, 885–889. [Google Scholar] [CrossRef]
- Rowlerson, A.; Mascarello, F.; Radaelli, G.; Veggetti, A. Differentiation and growth of muscle in the fish Sparus aurata (L): II. Hyperplastic and hypertrophic growth of lateral muscle from hatching to adult. J. Muscle Res. Cell Motil. 1995, 16, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A. Muscle development and growth: Potential implications for flesh quality in fish. Aquaculture 1999, 177, 99–115. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Moutou, K.A.; Conceição, L.E.C.; Engrola, S.; Fernandes, J.M.O.; Johnston, I.A. What determines growth potential and juvenile quality of farmed fish species? Rev. Aquac. 2013, 5, S168–S193. [Google Scholar] [CrossRef]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Andersen, Ø.; Vieira, V.; Dessen, J.; Johnston, I.A. Influence of feed ration size on somatic and muscle growth in landlocked dwarf and farmed Atlantic salmon Salmo salar. J. Fish Biol. 2019, 94, 614–620. [Google Scholar] [CrossRef]
- Herce, H.D.; Rajan, M.; Lättig-Tünnemann, G.; Fillies, M.; Cardoso, M.C. A novel cell permeable DNA replication and repair marker. Nucleus 2014, 5, 590–600. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef]
- Villasante, A.; Powell, M.S.; Moutou, K.; Murdoch, G.K.; Overturf, K.; Wacyk, J.; Hardy, R.W. Effects of anthocyanidins on myogenic differentiation and antioxidant defense in primary myogenic cells isolated from rainbow trout (Oncorhynchus mykiss). Aquaculture 2016, 454, 81–89. [Google Scholar] [CrossRef]
- Plakas, S.M.; Lee, T.-C.; Wolke, R.E.; Meade, T.L. Effect of Maillard Browning Reaction on Protein Utilization and Plasma Amino Acid Response by Rainbow Trout (Salmo gairdneri). J. Nutr. 1985, 115, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Kwasek, K.; Dabrowski, K.; Ware, K.; Reddish, J.M.; Wick, M. The effect of lysine-supplemented wheat gluten-based diet on yellow perch Perca flavescens (Mitchill) performance. Aquac. Res. 2012, 43, 1384–1391. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
| Ingredients, g/kg Feed | Experimental Feeds | ||
|---|---|---|---|
| Supplemented with Lys-Gly Dipeptide (DP) | Supplemented with Free Lys and Gly Amino Acids (FAA) | Without Lys Amino Acid Supplementation (LF) | |
| Fish meal | 124 | 124 | 124 |
| Wheat gluten (WG) a | 370 | 370 | 370 |
| Wheat | 172 | 172 | 172 |
| Fish oil | 50 | 50 | 50 |
| Lecithin | 150 | 150 | 150 |
| Mineral mix | 30 | 30 | 30 |
| Vitamin mix | 40 | 40 | 40 |
| Lys-Gly Dipeptide a | 34 | - | - |
| Lysine b | - | 21 | - |
| Glycine b | - | 13 | 13 |
| Glutamate d | - | - | 21 |
| Cysteine c | 2 | 2 | 2 |
| Arginine | 6.5 | 6.5 | 6.5 |
| Methionine | 3.3 | 3.3 | 3.3 |
| Threonine | 2.9 | 2.9 | 2.9 |
| Calcium monophosphate | 15 | 15 | 15 |
| Vitamin C | 0.5 | 0.5 | 0.5 |
| Parametres (%) | Diets | |||
|---|---|---|---|---|
| DP | FAA | LF | C | |
| Crude protein | 46 | 46 | 46 | 43,5 |
| Crude lipids | 6 | 6 | 6 | 24 |
| Ash | 7 | 7 | 7 | 12 |
| Moisture | 8 | 8 | 8 | 8.5 |
| Gross Energy (MJ/kg) | 19.6 | 19.6 | 19.6 | 19.7 |
| Parameters | Feed Group | ||||
|---|---|---|---|---|---|
| DP | FAA | LF | C | p Value | |
| Body weight, g | 0.91 ± 0.02 | 0.90 ± 0.02 | 0.83 ± 0.01 | 0.79 ± 0.02 | |
| CSAw | 2557.1 ± 140.6 | 2873.5 ± 216.6 | 2032.6 ± 57.9 | 2701.2 ± 236.4 | |
| TFN | 797.7 ± 26.0 a | 768.0 ± 54.6 a | 515.3 ± 2.8 b | 679.3 ± 28.8 a,b | DP vs. LF p = 0.0160 FAA vs. LF p = 0.0328 |
| FA | 1433.1 ± 12.7 | 1462.6 ± 52.2 | 1321.4 ± 20.2 | 1158.5 ± 80 | |
| TNN | 1424.3 ± 56.9 | 1590.0 ± 109.8 | 1049.0 ± 1.7 | 1392.3 ± 95.1 | |
| PCNA—positive myonuclei | 349.7 ± 5.3 b | 416.0 ± 17.2 a | 297.0 ± 20.8 b | 324.7 ± 4.8 a,b | FAA vs. LF p = 0.0150 |
| TFN/CSAw | 3.17 × 10−4 ± 9.8 × 10−6 | 2.68 × 10−4 ± 1.6 × 10−6 | 2.56 × 10−4 ± 6.2 × 10−6 | 2.66 × 10−4 ± 1.4 × 10−5 | |
| TNN/TFN | 1.78 ± 0,02 | 2.07 ± 0.01 a | 2.036 ± 0.01 a | 2.02 ± 0.5 a | DP vs. FAA p = 0.0045 DP vs. LF p = 0.0129 DP vs. C p = 0.0187 |
| PCNA/TNN | 0.25 ± 0.01 | 0.27 ± 0.01 | 0.28 ± 0.02 | 0.24 ± 0.01 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaszewski, M.; Wójcik, M.; Krawczyńska, A.; Ostaszewska, T. The Influence of Diet Containing Wheat Gluten Supplemented with Dipeptides or Amino Acids on the Morphology of White Muscle of Yellow Perch (Perca flavescens). Animals 2020, 10, 388. https://doi.org/10.3390/ani10030388
Kamaszewski M, Wójcik M, Krawczyńska A, Ostaszewska T. The Influence of Diet Containing Wheat Gluten Supplemented with Dipeptides or Amino Acids on the Morphology of White Muscle of Yellow Perch (Perca flavescens). Animals. 2020; 10(3):388. https://doi.org/10.3390/ani10030388
Chicago/Turabian StyleKamaszewski, Maciej, Maciej Wójcik, Agata Krawczyńska, and Teresa Ostaszewska. 2020. "The Influence of Diet Containing Wheat Gluten Supplemented with Dipeptides or Amino Acids on the Morphology of White Muscle of Yellow Perch (Perca flavescens)" Animals 10, no. 3: 388. https://doi.org/10.3390/ani10030388
APA StyleKamaszewski, M., Wójcik, M., Krawczyńska, A., & Ostaszewska, T. (2020). The Influence of Diet Containing Wheat Gluten Supplemented with Dipeptides or Amino Acids on the Morphology of White Muscle of Yellow Perch (Perca flavescens). Animals, 10(3), 388. https://doi.org/10.3390/ani10030388





