Does Seasonality, Tidal Cycle, and Plumage Color Influence Feeding Behavior and Efficiency of Western Reef Heron (Egretta gularis)?
Simple Summary
Abstract
1. Introduction
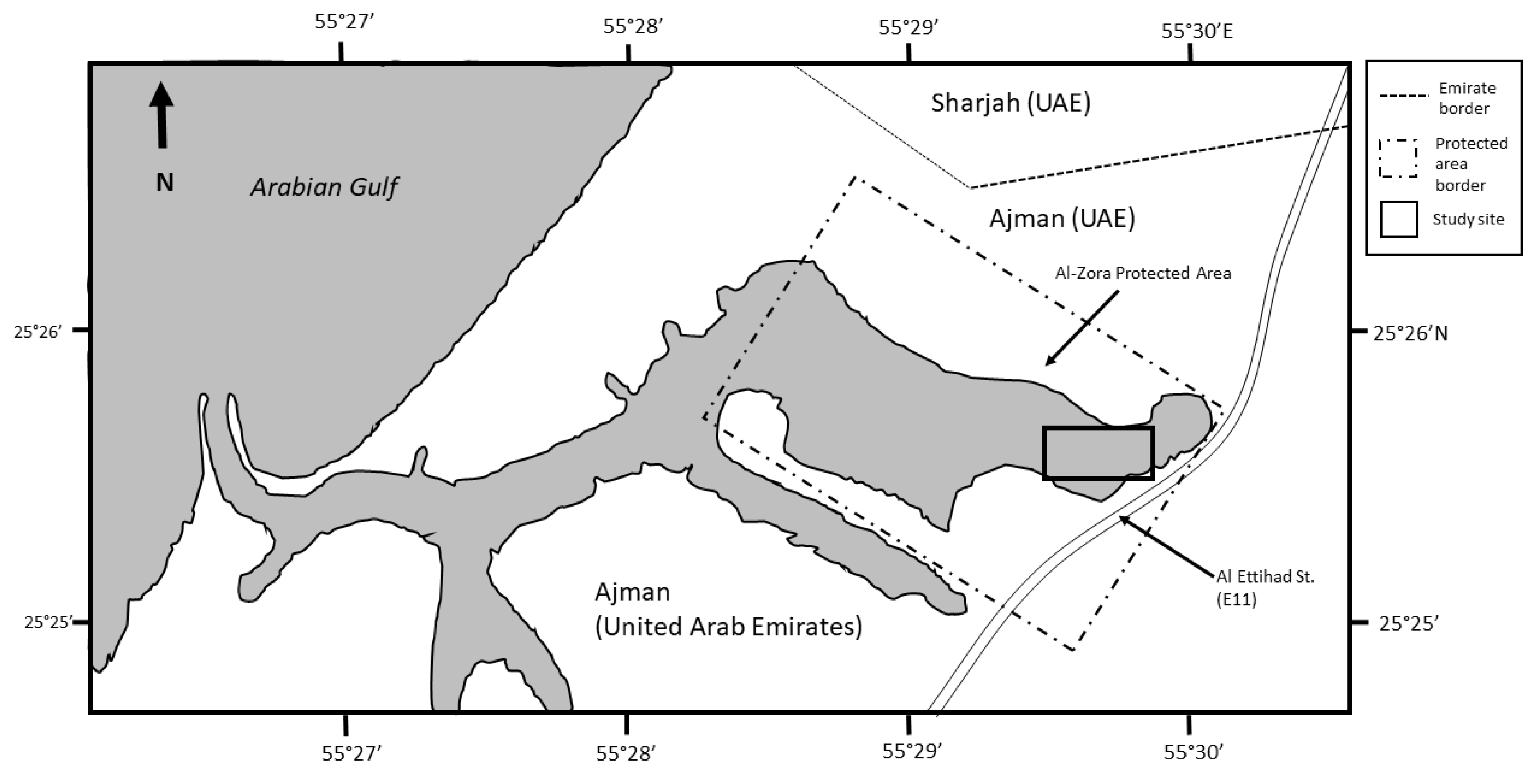
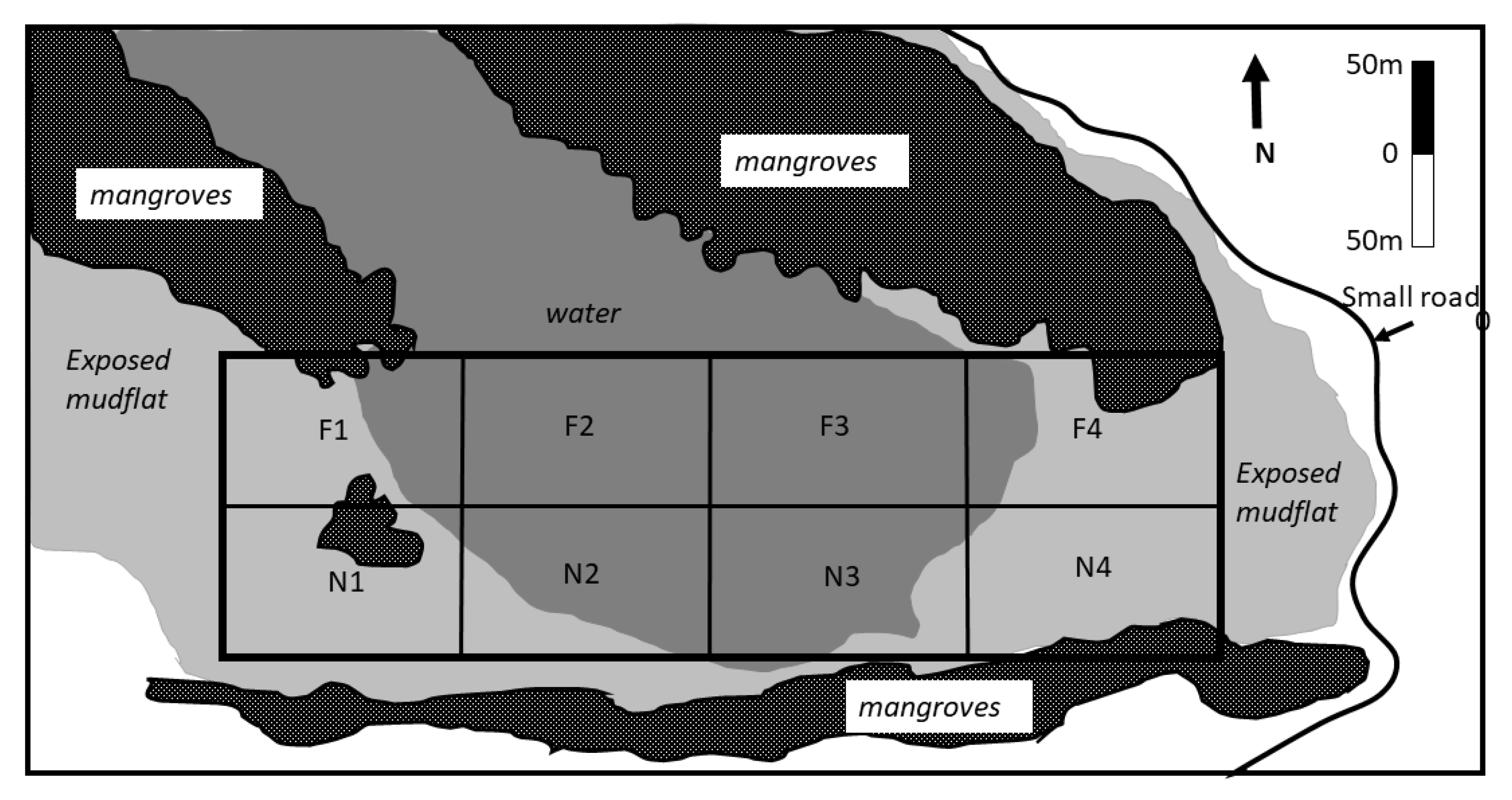
2. Materials and Methods
- S = the total number of behavior types;
- i = the number of observations of each behavior type; and
- pi = relative proportion of each behavior type.
3. Results
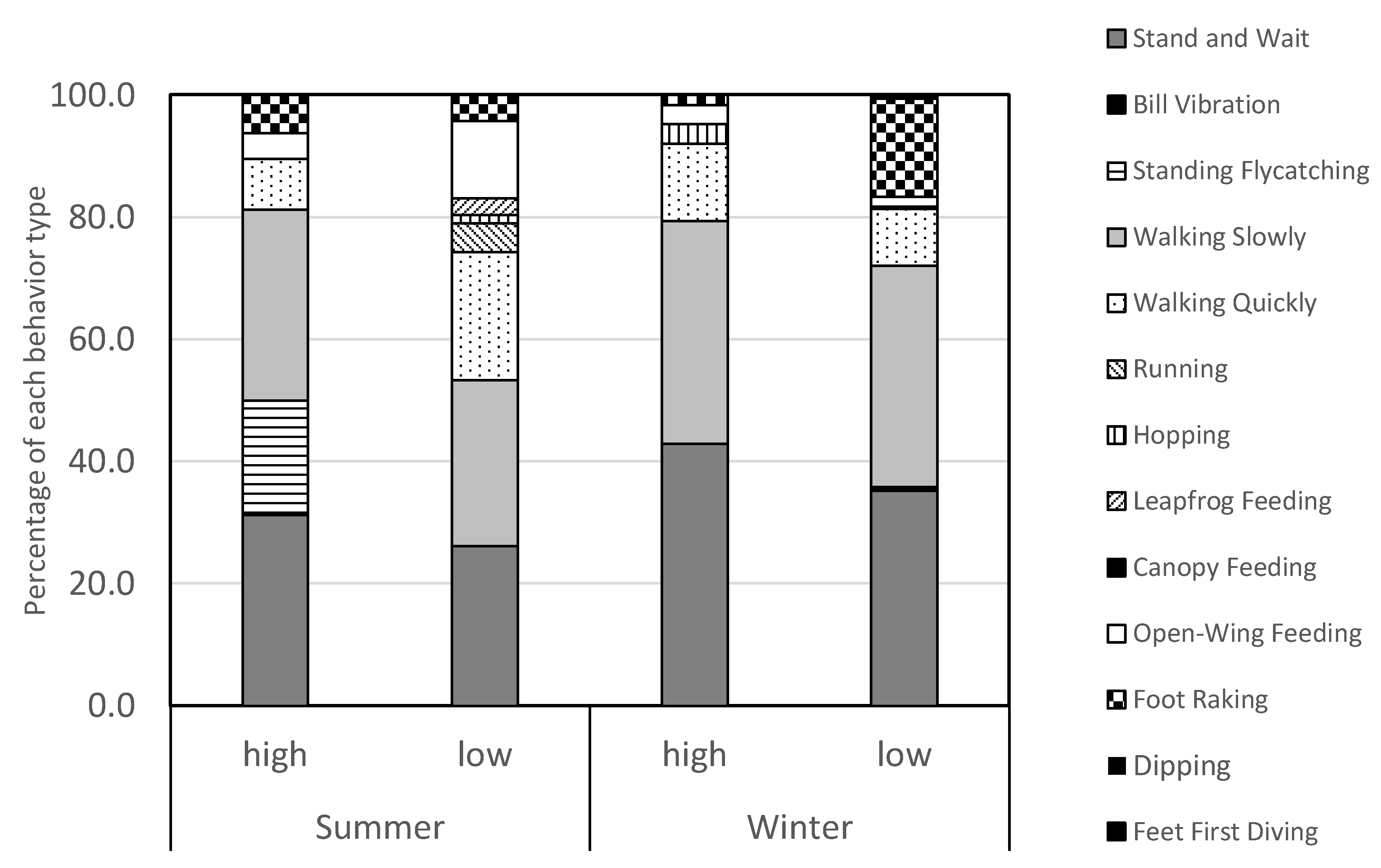
3.1. Feeding Behavior Types
3.2. Seasonal Effects, Tidal Influence, and Inter-Morph Differences
3.3. Foraging Efficiency
4. Discussion
4.1. Behavioral Diversity
4.2. Behavioral Diversity and Foraging Efficiency
4.3. Foraging Efficiency
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, T.B.; Skúlason, S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Evol. Syst. 1996, 27, 111–133. [Google Scholar] [CrossRef]
- Roulin, A. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol. Rev. 2004, 79, 815–848. [Google Scholar] [CrossRef] [PubMed]
- Green, M.C. Plumage Dimorphism in the Reddish Egret: Does Plumage Coloration Influence Foraging Habitat Use and Tactics? Waterbirds 2005, 28, 519–524. [Google Scholar] [CrossRef]
- Western Reef-egret (Egretta gularis) Handbook of the Birds of the World Alive. Available online: https://www.hbw.com/species/western-reef-egret-egretta-gularis (accessed on 1 June 2019).
- Kushlan, J.A.; Hancock, J.A. The Herons: Ardeidae; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Murton, R.K. Polymorphism in Ardeidae. IBIS 1971, 113, 97–99. [Google Scholar] [CrossRef]
- Caldwell, G.S. Predation as a selective force on foraging herons: Effects of plumage color and flocking. Auk 1986, 103, 494–505. [Google Scholar] [CrossRef]
- Rohwer, S. Foraging differences between white and dark morphs of the Pacific Reef Heron Egretta sacra. Ibis 1990, 132, 21–26. [Google Scholar] [CrossRef]
- Green, M.C.; Leberg, P.L. Influence of plumage colour on prey response: Does habitat alter heron crypsis to prey? Anim. Behav. 2005, 70, 1203–1208. [Google Scholar] [CrossRef]
- Nefla, A.; Nouira, S. Environmental factors affecting the foraging behavior of herons in Ichkeul National Park, Tunisia. Waterbirds 2016, 39, 99–103. [Google Scholar] [CrossRef]
- Matsunaga, K. Effects of Tidal Cycle on the Feeding Activity and Behavior of Grey Herons in a Tidal Flat in Notsuke Bay, Northern Japan Katsutoshi Matsunaga. Waterbirds 2000, 23, 226–235. [Google Scholar]
- Itoh, S. Geographical Variation of the Plumage Polymorphism in the Eastern Reef Heron (Egretta sacra). Condor 1991, 93, 383–389. [Google Scholar] [CrossRef]
- Jennings, M.C.; Krupp, F. Atlas of the Breeding Birds of Arabia; Senckenberg-Ges. für Naturforschung: Frankfurt, Germany, 2010. [Google Scholar]
- Bird Checklist-UAEbirding. EBRC Annotated Checklist of the Birds of the United Arab Emirates. Available online: https://www.uaebirding.com/bird-checklists (accessed on 1 November 2018).
- Shah, J.N.; Javed, S.; Khan, S.A.S.B.; Hammadi, A.A.; Al Hammadi, E.A.; Soorae, P.S. Distribution and Temporal Trends of Western Reef Heron (Egretta gularis) Populations along the Arabian Gulf Coast of Abu Dhabi, United Arab Emirates. Waterbirds 2018, 41, 376–383. [Google Scholar]
- Dubois, P.J.; Yésou, P. Identification of Western Reef Egret and dark Little Egret. Br. Birds 1995, 88, 307–319. [Google Scholar]
- Hamza, W.; Munawar, M. Protecting and managing the Arabian Gulf: Past, present and future. Aquat. Ecosyst. Health Manag. 2009, 12, 429–439. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Penha-Lopes, G.; Torres, P.; Cannicci, S.; Narciso, L.; Paula, J. Monitoring anthropogenic sewage pollution on mangrove creeks in southern Mozambique: A test of Palaemon concinnus Dana, 1852 (Palaemonidae) as a biological indicator. Environ. Pollut. 2011, 159, 636–645. [Google Scholar] [CrossRef]
- Al-Zora Protected Area-Ramsar Sites Information Service. Available online: https://rsis.ramsar.org/ris/2309 (accessed on 1 November 2018).
- Rayer, H.U.; Migongo-Bake, W.; Schmidt, L. Field Studies and Experiments on Distribution and Foraging of Pied and Malachite Kingfishers at Lake Nakuru (Kenya). J. Anim. Ecol. 1988, 57, 595. [Google Scholar] [CrossRef]
- Kuranchie, A.; Rosina, K.; Kyerematen, R.; Holbech, L. Foraging activities, success and efficiency of cattle egrets (Bubulcus ibis) in three habitat types in the greater accra region of ghana. J. Biol. Food Sci. Res. 2013, 2, 45–50. [Google Scholar]
- Ministry of Presidential Affairs-National Centre of Meteorology. Available online: https://www.ncm.ae/en/climate-reports-yearly.html?id=386 (accessed on 1 November 2018).
- Kushlan, J.A. Feeding Behaviour of North American Herons. Auk 1976, 93, 86–94. [Google Scholar]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Katzir, G.; Intrator, N. Striking of underwater prey by a reef heron, Egretta gularis schistacea. J. Comp. Physiol. A 1987, 160, 517–523. [Google Scholar] [CrossRef]
- Tojo, H. Habitat selection, foraging behaviour and prey of five heron species in Japan. Jpn. J. Ornithol. 1996, 45, 141–158. [Google Scholar] [CrossRef]
- Quinney, T.E.; Smith, P.C. Comparative foraging behaviour and efficiency of adult and juvenile great blue herons. Can. J. Zool. 1980, 58, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.M. Foraging efficiency of sympatric egrets. Colonial Waterbirds 1986, 9, 81–85. [Google Scholar] [CrossRef]
- Connors, P.G.; Mayers, J.P.; Connors, C.S.W.; Pitelka, F.A. Interhabitat Movement by Sanderling in Relation to Foraging Profitability and the Tidal Cycle. Auk 1981, 98, 49–64. [Google Scholar]
- Richner, H. Winter feeding strategies of individually marked herons. Anim. Behav. 1986, 34, 881–886. [Google Scholar] [CrossRef]
- Slagsvold, T.; Wiebe, K.L. Social learning in birds and its role in shaping a foraging niche. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 969–977. [Google Scholar] [CrossRef]
- Wiggins, D.A. Foraging Success and Aggression in Solitary and Group-Feeding Great Egrets (Casmerodius albus). Colonial Waterbirds 1991, 14, 176–179. [Google Scholar] [CrossRef]
| Category and Number | Behavior Type |
|---|---|
| A | Stand (Stalk) |
| 1 | Stand and Wait: the bird stands still and waits for prey to approach |
| 2 | Bill Vibration: the bird places its bill in the water and rapidly opens and closes it to attract prey |
| 3 | Standing and Flycatching: bird remains standing and catches flying insects |
| 4 | Walking slowly: the bird walks slowly in shallow water, searching for prey |
| B | Disturb and Chase |
| 5 | Walking quickly: the bird walks quickly, causing disturbance which forces prey to emerge |
| 6 | Running: the bird runs to chase specific prey, often using the wings for balance and uplift |
| 7 | Hopping: the bird flies to a feeding site a short distance away |
| 8 | Leapfrog feeding: the bird flies from the rear to the front of a forward moving feeding flock |
| 9 | Open wing feeding: the bird extends one wing and keeps it extended for a few seconds before retracting it |
| C | Aerial and Deepwater Feeding |
| 10 | Canopy Feeding: the bird extends both wings like an umbrella with wing-tips touching the water and keeps its head underneath the wings to strike prey |
| 11 | Foot Raking: the bird drags its toes across the substrate to disturb prey |
| 12 | Dipping: the bird flies along and periodically dips into the water to pick up prey |
| 13 | Feet First Diving: the bird plunges from the air into the water feet first to strike prey |
| Statistical Test | Summer | Winter | t | Df | p |
|---|---|---|---|---|---|
| Shannon’s H (all birds) | 1.79 ± 0.002 | 1.42 ± 0.002 | 5.72 | 591 | <0.00001 |
| Rising tide | Falling tide | ||||
| Shannon’s H (all birds) | 2.22 ± 0.001 | 2.08 ± 0.006 | −1.66 | 165 | 0.090 |
| Dark morph | Light morph | ||||
| Shannon’s H (morphs | 1.68 ± 0.002 | 1.53 ± 0.003 | 2.07 | 467 | 0.039 |
| Shannon’s H (summer, falling tide) | 1.78 ± 0.003 | 1.56 ± 0.008 | 2.03 | 101 | 0.044 |
| Feeding Efficiency in Relation to Different Variables | Summer | Winter | Mann-Whitney U | z-Score | p |
|---|---|---|---|---|---|
| FEseason | 17.30 ± 4.97 | 13.82 ± 3.48 | 53.0 | −0.0356 | 0.9716 |
| Rising | Falling | ||||
| FEtides | 20.08 ± 4.99 | 7.58 ± 2.19 | 33.0 | −2.2409 | 0.0250 |
| Dark | Light | ||||
| FEmorph | 12.02 ± 2.28 | 20.85 ± 6.57 | 41.5 | −0.85447 | 0.3929 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ali, A.; Bin Muzaffar, S.; Hamza, W. Does Seasonality, Tidal Cycle, and Plumage Color Influence Feeding Behavior and Efficiency of Western Reef Heron (Egretta gularis)? Animals 2020, 10, 373. https://doi.org/10.3390/ani10030373
Al-Ali A, Bin Muzaffar S, Hamza W. Does Seasonality, Tidal Cycle, and Plumage Color Influence Feeding Behavior and Efficiency of Western Reef Heron (Egretta gularis)? Animals. 2020; 10(3):373. https://doi.org/10.3390/ani10030373
Chicago/Turabian StyleAl-Ali, Ahmed, Sabir Bin Muzaffar, and Waleed Hamza. 2020. "Does Seasonality, Tidal Cycle, and Plumage Color Influence Feeding Behavior and Efficiency of Western Reef Heron (Egretta gularis)?" Animals 10, no. 3: 373. https://doi.org/10.3390/ani10030373
APA StyleAl-Ali, A., Bin Muzaffar, S., & Hamza, W. (2020). Does Seasonality, Tidal Cycle, and Plumage Color Influence Feeding Behavior and Efficiency of Western Reef Heron (Egretta gularis)? Animals, 10(3), 373. https://doi.org/10.3390/ani10030373






