The LEPR Gene Is Associated with Reproductive Seasonality Traits in Rasa Aragonesa Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Samples
2.3. Measurement of Reproductive Seasonality Traits
2.4. LEPR Gene Characterization
2.5. LEPR Polymorphism Genotyping
2.6. Statistical Analysis
2.6.1. SNP Association Studies
2.6.2. Haplotype Association Studies
3. Results
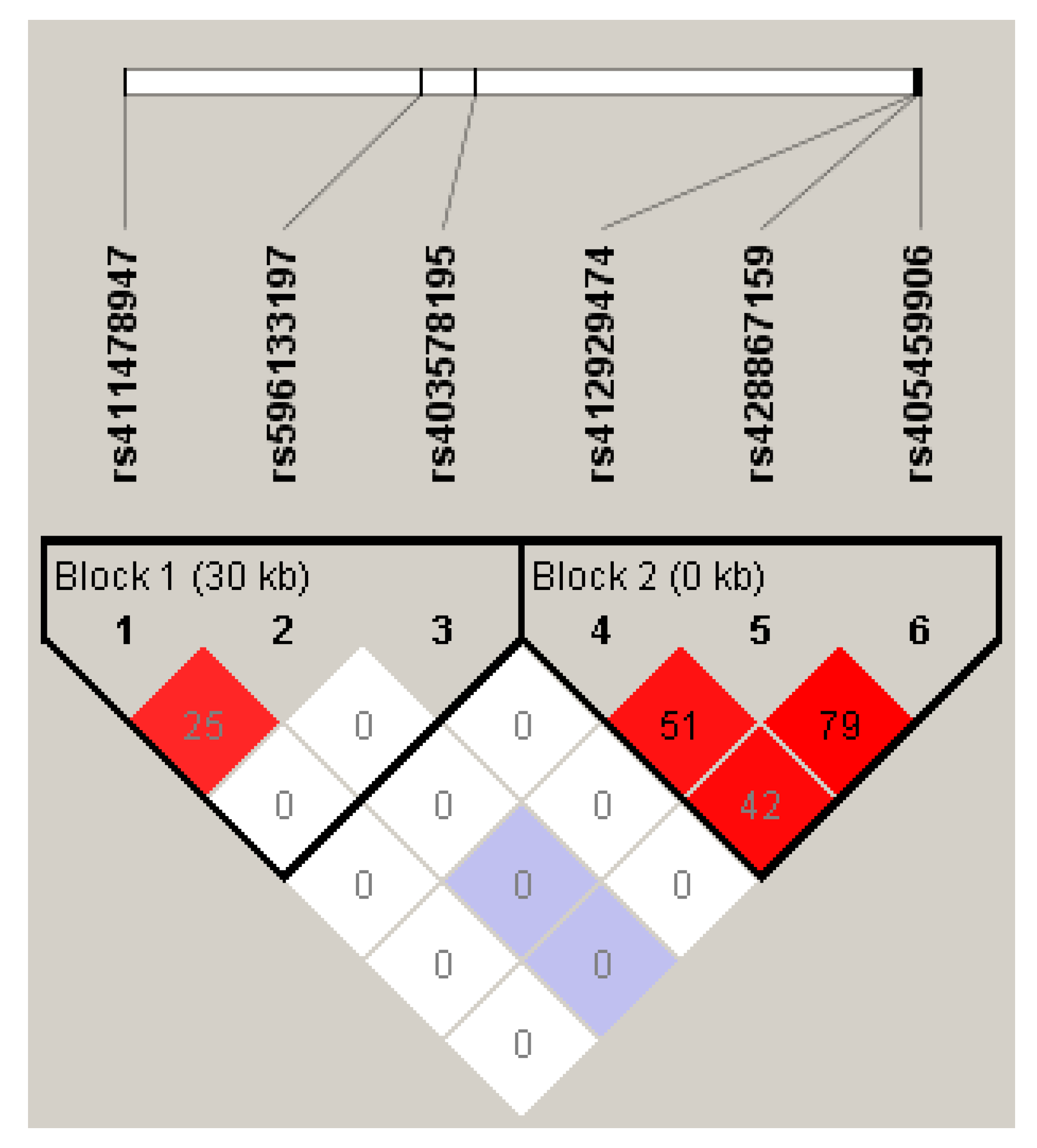
3.1. Isolation of the Partial Ovine LEPR Gene and Polymorphism Genotyping and Linkage Disequilibrium (LD)
3.2. SNP Association Studies
3.3. Haplotype Association Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malpaux, B.; Robinson, J.E.; Wayne, N.L.; Karsch, F.J. Regulation of the onset of the breeding season of the ewe: Importance of long days and of an endogenous reproductive rhythm. J. Endocrinol. 1989, 122, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Posbergh, C.J.; Thonney, M.L.; Huson, H.J. Genomic Approaches Identify Novel Gene Associations with Out of Season Lambing in Sheep. J. Hered. 2019, 110, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Alabart, J.L. Respuesta al efecto macho de ovejas rasa aragonesa según su estado cíclico tratadas o no con melatonina en primavera. ITEA 1999, 20, 651–653. [Google Scholar]
- Hanocq, E.; Bodin, L.; Thimonier, J.; Teyssier, J.; Malpaux, B.; Chemineau, P. Genetic parameters of spontaneous spring ovulatory activity in Merinos d’Arles sheep. Genet. Sel. Evol. 1999, 31, 77–90. [Google Scholar] [CrossRef]
- Notter, D.R.; Cockett, N.E.; Hadfield, T.S. Evaluation of melatonin receptor 1a as a candidate gene influencing reproduction in an autumn-lambing sheep flock. J. Anim. Sci. 2003, 81, 912–917. [Google Scholar] [CrossRef]
- Pelletier, J.; Bodin, L.; Hanocq, E.; Malpaux, B.; Teyssier, J.; Thimonier, J.; Chemineau, P. Association Between Expression of Reproductive Seasonality and Alleles of the Gene for Mel1a Receptor in the Ewe. Biol. Reprod. 2000, 62, 1096–1101. [Google Scholar] [CrossRef]
- Mura, M.C.; Luridiana, S.; Bodano, S.; Daga, C.; Cosso, G.; Diaz, M.L.; Bini, P.P.; Carcangiu, V. Influence of melatonin receptor 1A gene polymorphisms on seasonal reproduction in Sarda ewes with different body condition scores and ages. Anim. Reprod. Sci. 2014, 149, 173–177. [Google Scholar] [CrossRef]
- Calvo, J.H.; Serrano, M.; Martinez-Royo, A.; Lahoz, B.; Sarto, P.; Ibañez-Deler, A.; Folch, J.; Alabart, J.L. SNP rs403212791 in exon 2 of the MTNR1A gene is associated with reproductive seasonality in the Rasa aragonesa sheep breed. Theriogenology 2018, 113, 63–72. [Google Scholar] [CrossRef]
- Bai, D.-p.; Yu, C.-j.; Chen, Y.-l. Association between AA-NAT gene polymorphism and reproductive performance in sheep. Electron. J. Biotechnol. 2012, 15, 86–92. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Thonney, M.L. Genetic mapping of quantitative trait loci for aseasonal reproduction in sheep. Anim. Genet. 2010, 41, 454–459. [Google Scholar] [CrossRef]
- Martinez-Royo, A.; Alabart, J.L.; Sarto, P.; Serrano, M.; Lahoz, B.; Folch, J.; Calvo, J.H. Genome-wide association studies for reproductive seasonality traits in Rasa Aragonesa sheep breed. Theriogenology 2017, 99, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.X.; Ji, C.L.; Chen, G.H. Association between PCR-RFLP of melatonin receptor 1a gene and high prolificacy in Small Tail Han sheep. Asian Australas. J. Anim. Sci. 2003, 16, 1701–1704. [Google Scholar] [CrossRef]
- Faigl, V.; Kerestes, M.; Kulcsar, M.; Reiczigel, J.; Cseh, S.; Huszenicza, G.; Arnyasi, M.; Javor, A. Seasonality of reproduction and MT1 receptor gene polymorphism in Awassi sheep. Reprod. Domest. Anim. 2009, 43, 11. [Google Scholar]
- Mura, M.C.; Luridiana, S.; Vacca, G.M.; Bini, P.P.; Carcangiu, V. Effect of genotype at the MTNR1A locus and melatonin treatment on first conception in Sarda ewe lambs. Theriogenology 2010, 74, 1579–1586. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Lunsford, A.K.; Thonney, M.L. Association between melatonin receptor 1A gene polymorphism and reproductive performance in Dorset ewes. J. Anim. Sci. 2009, 87, 2485–2488. [Google Scholar] [CrossRef]
- Teyssier, J.; Migaud, M.; Debus, N.; Maton, C.; Tillard, E.; Malpaux, B.; Chemineau, P.; Bodin, L. Expression of seasonality in Merinos d’Arles ewes of different genotypes at the MT1 melatonin receptor gene. Animal 2011, 5, 329–336. [Google Scholar] [CrossRef]
- Carcangiu, V.; Mura, M.C.; Vacca, G.M.; Pazzola, M.; Dettori, M.L.; Luridiana, S.; Bini, P.P. Polymorphism of the melatonin receptor MT1 gene and its relationship with seasonal reproductive activity in the Sarda sheep breed. Anim. Reprod. Sci. 2009, 116, 65–72. [Google Scholar] [CrossRef]
- Carcangiu, V.; Luridiana, S.; Vacca, G.M.; Daga, C.; Mura, M.C. A polymorphism at the melatonin receptor 1A (MTNR1A) gene in Sarda ewes affects fertility after AI in the spring. Reprod. Fertil. Dev. 2011, 23, 376–380. [Google Scholar] [CrossRef]
- Martínez-Royo, A.; Lahoz, B.; Alabart, J.L.; Folch, J.; Calvo, J.H. Characterisation of the Melatonin Receptor 1A (MTNR1A) gene in the Rasa Aragonesa sheep breed: Association with reproductive seasonality. Anim. Reprod. Sci. 2012, 133, 169–175. [Google Scholar] [CrossRef]
- van der Lende, T.; te Pas, M.F.W.; Veerkamp, R.F.; Liefers, S.C. Leptin Gene Polymorphisms and Their Phenotypic Associations. Vitam. Horm. 2005, 71, 373–404. [Google Scholar] [CrossRef]
- Paczoska-Eliasiewicz, H.E.; Proszkowiec-Weglarz, M.; Proudman, J.; Jacek, T.; Mika, M.; Sechman, A.; Rzasa, J.; Gertler, A. Exogenous leptin advances puberty in domestic hen. Domest. Anim. Endocrinol. 2006, 31, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Liefers, S.C.; Veerkamp, R.F.; Te Pas, M.F.W.; Delavaud, C.; Chilliard, Y.; Van Der Lende, T. A missense mutation in the bovine leptin receptor gene is associated with leptin concentrations during late pregnancy. Anim. Genet. 2004, 35, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.; Chua, S. Leptin receptor modulation of adiposity and fertility. Trends Endocrinol. Metab. 2010, 21, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Moschos, S.; Chan, J.L.; Mantzoros, C.S. Leptin and reproduction: A review. Science 2002, 77, 433–444. [Google Scholar] [CrossRef]
- Taheri, S.J.; Parham, A. Sheep oocyte expresses leptin and functional leptin receptor mRNA. Asian Pac. J. Reprod. 2016, 5, 395–399. [Google Scholar] [CrossRef]
- Ehrhardt, R.A.; Bell, A.W.; Boisclair, Y.R. Spatial and developmental regulation of leptin in fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282. [Google Scholar] [CrossRef]
- Agarwal, R.; Rout, P.K.; Singh, S.K. Leptin: A biomolecule for enhancing livestock productivity. Indian J. Biotechnol. 2009, 8, 169–176. [Google Scholar]
- Saleem, A.; Hussain, T.; Tahir, M.; Ali, A.; Khan, W.; Dawood, M.; Ali, R.; Rehman, Z. Role of Leptin in Growth, Reproduction and Milk Production in Farm Animals: A Review. Adv. Anim. Vet. Sci. 2015, 3, 302–307. [Google Scholar] [CrossRef]
- Zieba, D.A.; Amstalden, M.; Williams, G.L. Regulatory roles of leptin in reproduction and metabolism: A comparative review. Domest. Anim. Endocrinol. 2005, 29, 166–185. [Google Scholar] [CrossRef]
- Barb, C.R.; Kraeling, R.R. Role of leptin in the regulation of gonadotropin secretion in farm animals. Anim. Reprod. Sci. 2004, 82–83, 155–167. [Google Scholar] [CrossRef]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythms 2001, 16, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.K.; Glimm, D.R.; Kennelly, J.J. Short communication: Tissue distribution of leptin and leptin receptor mRNA in the bovine. J. Dairy Sci. 2003, 86, 2369–2372. [Google Scholar] [CrossRef]
- Dyer, C.J.; Simmons, J.M.; Matteri, R.L.; Keisler, D.H. Leptin receptor mRNA is expressed in ewe anterior pituitary and adipose tissues and is differentially expressed in hypothalamic regions of well-fed and feed-restricted ewes. Domest. Anim. Endocrinol. 1997, 14, 119–128. [Google Scholar] [CrossRef]
- Miller, D.W.; Findlay, P.A.; Morrison, M.A.; Raver, N.; Adam, C.L. Seasonal and dose-dependent effects of intracerebroventricular leptin on LH secretion and appetite in sheep. J. Endocrinol. 2002, 175, 395–404. [Google Scholar] [CrossRef]
- De Matteis, G.; Scatà, M.C.; Catillo, G.; Terzano, G.M.; Grandoni, F.; Napolitano, F. Characterization of leptin receptor gene in Bubalus bubalis and association analysis with body measurement traits. Mol. Biol. Rep. 2015, 42, 1049–1057. [Google Scholar] [CrossRef]
- Juengel, J.L.; French, M.C.; O’Connell, A.R.; Edwards, S.J.; Haldar, A.; Brauning, R.; Farquhar, P.A.; Dodds, K.G.; Galloway, S.M.; Johnstone, P.D.; et al. Mutations in the leptin receptor gene associated with delayed onset of puberty are also associated with decreased ovulation and lambing rates in prolific Davisdale sheep. Reprod. Fertil. Dev. 2016, 28, 1318. [Google Scholar] [CrossRef]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Radford, H.M.; Watson, R.H.; Wood, G.F. A crayon and associated harness for the detection op mating under field conditions. Aust. Vet. J. 1960, 36, 57–66. [Google Scholar] [CrossRef]
- Hall, T.A.; Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. In Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; pp. 95–98. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Kumar, T.A. CFSSP: Chou and Fasman Secondary Structure Prediction server. Wide Spectr. 2013, 1, 15–19. [Google Scholar]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Bouchet-Valat, M.; Andronic, L.; Ash, M.; Boye, T.; Calza, S.; Chang, A.; Grosjean, P.; Heiberger, R.; Pour, K.K.; et al. Package “Rcmdr”. 2020. Available online: cran.ma.imperial.ac.uk/web/packages/Rcmdr/Rcmdr.pdf (accessed on 26 October 2020).
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.; French, M.C.; Brauning, R.; Edwards, S.J.; O’connell, A.R.; Farquhar, P.A.; Davis, G.H.; Johnstone, P.D.; Juengel, J.L. Single-Nucleotide Polymorphisms in the LEPR Gene Are Associated with Divergent Phenotypes for Age at Onset of Puberty in Davisdale Ewes 1. Biol. Reprod. 2014, 90, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Nunziata, A.; Funcke, J.-B.; Borck, G.; von Schnurbein, J.; Brandt, S.; Lennerz, B.; Moepps, B.; Gierschik, P.; Fischer-Posovszky, P.; Wabitsch, M. Functional and Phenotypic Characteristics of Human Leptin Receptor Mutations. J. Endocr. Soc. 2019, 3, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.; Campfield, L.; Clark, F.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 1997, 272, 6093–6096. [Google Scholar] [CrossRef]
- Chua, S.C.; Koutras, I.K.; Han, L.; Liu, S.M.; Kay, J.; Young, S.J.; Chung, W.K.; Leibel, R.L. Fine structure of the murine leptin receptor gene: Splice site suppression is required to form two alternatively spliced transcripts. Genomics 1997, 45, 264–270. [Google Scholar] [CrossRef]
- Löllmann, B.; Grüninger, S.; Stricker-Krongrad, A.; Chiesi, M. Detection and Quantification of the Leptin Receptor Splice Variants Ob-Ra, b, and e in Different Mouse Tissues. Biochem. Biophys. Res. Commun. 1997, 241, 803. [Google Scholar] [CrossRef]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.K.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Almeida, S.E.M.; Santos, L.B.S.; Passos, D.T.; Corbellini, Â.O.; Lopes, B.M.T.; Kirst, C.; Terra, G.; Neves, J.P.; Gonçalves, P.B.D.; Moraes, J.C.F.; et al. Genetic polymorphisms at the leptin receptor gene in three beef cattle breeds. Genet. Mol. Biol. 2008, 31, 680–685. [Google Scholar] [CrossRef]
- Elias, C.F. Leptin action in pubertal development: Recent advances and unanswered questions. Trends Endocrinol. Metab. 2012, 23, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J.; Smith, J.T.; Caraty, A.; Goodman, R.L.; Lehman, M.N. Kisspeptin and seasonality in sheep. Peptides 2009, 30, 154–163. [Google Scholar] [CrossRef]
- Lutz, T.A.; Woods, S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. 2012, 58, 5–61. [Google Scholar] [CrossRef] [PubMed]
- Von Schnurbein, J.; Moss, A.; Nagel, S.A.; Muehleder, H.; Debatin, K.M.; Farooqi, I.S.; Wabitsch, M. Leptin substitution results in the induction of menstrual cycles in an adolescent with leptin deficiency and hypogonadotropic hypogonadism. Horm. Res. Paediatr. 2012, 77, 127–133. [Google Scholar] [CrossRef] [PubMed]
| PCR | Primer Sequence (5′–3′) 1 | Site | 2 AT (°C) | Size (bp) |
|---|---|---|---|---|
| 1 | F: TTTTTCTGTGTCTTTTGAATGTCC R: AAGTAACAACTAATGCTTGGAACA | Exon 4 | 57 | 397 |
| 2 | F: GCTCTTTAAGCTGGGTGTGC R: TTCAGCCTGTTTGAATGACTG | Exon 6 | 55 | 386 |
| 3 | F: TGCTAAAAATTCATTTTGACTTCG R: GGAGGGCATCTCACCTTTTC | Exon 7 | 55 | 293 |
| 4 | F: CTGTCGCCAGCTAACTCCTC R: CCTCCTTTTGAGTTACCACCA | Exon 8 | 55 | 378 |
| 5 | F: TGCCTGGTGAATCCTTTTTA R: TCTCACCATATCCACAGAAAAAT | Exons 9–10 | 53 | 700 |
| 6 | F: AGAGCTGGGAATTCAGAAATG R: TCTTTTCAATCCCACTGCAA | Exon 11 | 53 | 496 |
| 7 | F: CTGCTTGGCAGGTGGATT R: CAGGAGGATGTATTTTATGCCAGT | Exon 12 | 55 | 392 |
| 8 | F: TGCCTACCAATGGGAAATGT R: ATGGGAGGGGTTTGAAAGAT | Exon 15 | 55 | 383 |
| 9 | F: CCTGCTTTCTCTTCCTTCTTCC R: TTTTTGAAGTTTTCATTAACTGTGTT | Exon 16 | 55 | 389 |
| 10 | F: CCAGTTTCAATCCATAAATCATCA R: TGGCAGCATTGTTGCTAACT | Exon 17 | 55 | 299 |
| 11 | F: TGAAGCAAAACAAAACAAAACA R: ACTCTCCTAACCAATGGTGAAA | Exon 20 | 52 | 974 |
| SNP | Alias 1 | Location | Position in OAR Version 3.1 | Nucleotide Change | Amino Acid Change | VEP (SIFT Score) | PolyPhen-2 (Score) |
|---|---|---|---|---|---|---|---|
| rs411478947 | snp_ex4 | Exon 4 | Oar1: g.40787726 | C > T | Arg62Cys | Tolerated (0.05) | Possibly damaging (0.74) |
| rs159694506 | Oar1: g.40787782 | T > C | Asn80 = 2 | - | - | ||
| rs159694508 | Oar1: g.40787821 | T > C | Ser93 = | - | - | ||
| rs596133197 | snp_ex7 | Exon 7 | Oar1: g.40813963 | C > T | Thr248Ile | Deleterious (0) | Probably damaging (0.98) |
| rs403578195 | snp_ex8 | Exon 8 | Oar1: g.40818703 | C > G | Ala284Gly | Deleterious (0) | Possibly damaging (0.77) |
| rs416296450 | Intron 9 | Oar1: g.40825576 | G > A | - | - | - | |
| rs404892216 | Intron 10 | Oar1: g.40828606 | A > G | - | - | - | |
| rs407234698 | Exon 12 | Oar1: g.40833201 | A > G | Pro561 = | - | - | |
| rs421946862 | Exon 16 | Oar1: g.40840634 | C > T | Ser791 = | - | - | |
| rs401262081 | Intron 16 | Oar1: g.40840703 | C > T | - | - | - | |
| rs403654953 | Exon 20 | Oar1: g.40857538 | C > T | Gly908 = | - | - | |
| rs412929474 | snp_ex20_1 | Oar1: g.40857581 | G > A | Val923Ile | Tolerated (0.5) | Benign (0.04) | |
| rs426037269 | Oar1: g.40857583 | C > T | Val923 = | - | - | ||
| rs415715948 | Oar1: g.40857634 | C > T | Ala940 = | - | - | ||
| rs428867159 | snp_ex20_2 | Oar1: g.40857869 | C > T | Pro1019Ser | Tolerated (0.77) | Benign (0.06) | |
| rs405459906 | snp_ex20_3 | Oar1: g.40858019 | A > G | Lys1069Glu | Tolerated (1) | Benign (0) | |
| rs414501727 | Oar1: g.40858045 | C > T | Val1077 = | - | - | ||
| rs427778198 | Oar1: g.40858219 | G > A | Gln1135 = | - | - |
| SNP | Trait | p Value SNP | A | SNP LSMs | ||
| CC | GC | GG | ||||
| snp_ex8 | OCM | 0.003 | All | 0.54 ± 0.01 a | 0.42 ± 0.03 b | - |
| SNP | Trait | p Value SNP × A | A | SNP × A LSMs | ||
| AA | AG | GG | ||||
| snp_ex20_1 | TDA | 0.0004 | Young | 80.6 ± 31.09 a,b | 45.6 ± 11.98 a | 83.3 ± 6.41 b |
| Haplotype Block 1 | Trait | Haplotype | Frequency | p Value Haplotype | A | Haplotype LSMs 2 | ||
| 0 Copies | 1 Copy | 2 Copies | ||||||
| Block 0 | OCM | h2 (GCCGTG) | 0.07 | 0.002 | All | 0.53 ± 0.01 a | 0.44 ± 0.03 b | - |
| OCM | h8 (GCGATG) | 0.01 | 0.004 | All | 0.52 ± 0.01 a | 0.26 ± 0.10 b | - | |
| Block 1 | OCM | h1(GCG) | 0.05 | 0.003 | All | 0.55 ± 0.01 a | 0.42 ± 0.03 b | - |
| Block 2 | OCM | h2 (GTG) | 0.07 | 0.002 | All | 0.54 ± 0.01 a | 0.44 ± 0.03 b | - |
| Haplotype Block 1 | Trait | Haplotype | Frequency | p Value Haplotype × A | A | Haplotype ×A LSMs 2 | ||
| 0 Copies | 1 Copy | 2 Copies | ||||||
| Block 0 | OCM | h1(GCCATG) | 0.09 | 0.004 | Young | 0.46 ± 0.02 a | 0.66 ± 0.05 b | 0.55 ± 0.14 a,b |
| Block 2 | TDA | h1 (ATG) | 0.10 | 0.0003 | Young | 83.9 ± 6.38 a | 46.3 ± 11.97 b | 81.4 ± 31.09 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakhssassi, K.; Serrano, M.; Lahoz, B.; Sarto, M.P.; Iguácel, L.P.; Folch, J.; Alabart, J.L.; Calvo, J.H. The LEPR Gene Is Associated with Reproductive Seasonality Traits in Rasa Aragonesa Sheep. Animals 2020, 10, 2448. https://doi.org/10.3390/ani10122448
Lakhssassi K, Serrano M, Lahoz B, Sarto MP, Iguácel LP, Folch J, Alabart JL, Calvo JH. The LEPR Gene Is Associated with Reproductive Seasonality Traits in Rasa Aragonesa Sheep. Animals. 2020; 10(12):2448. https://doi.org/10.3390/ani10122448
Chicago/Turabian StyleLakhssassi, Kenza, Malena Serrano, Belén Lahoz, María Pilar Sarto, Laura Pilar Iguácel, José Folch, José Luis Alabart, and Jorge Hugo Calvo. 2020. "The LEPR Gene Is Associated with Reproductive Seasonality Traits in Rasa Aragonesa Sheep" Animals 10, no. 12: 2448. https://doi.org/10.3390/ani10122448
APA StyleLakhssassi, K., Serrano, M., Lahoz, B., Sarto, M. P., Iguácel, L. P., Folch, J., Alabart, J. L., & Calvo, J. H. (2020). The LEPR Gene Is Associated with Reproductive Seasonality Traits in Rasa Aragonesa Sheep. Animals, 10(12), 2448. https://doi.org/10.3390/ani10122448






