Edible Environmental Enrichments in Littered Housing Systems: Do Their Effects on Integument Condition Differ Between Commercial Laying Hen Strains?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
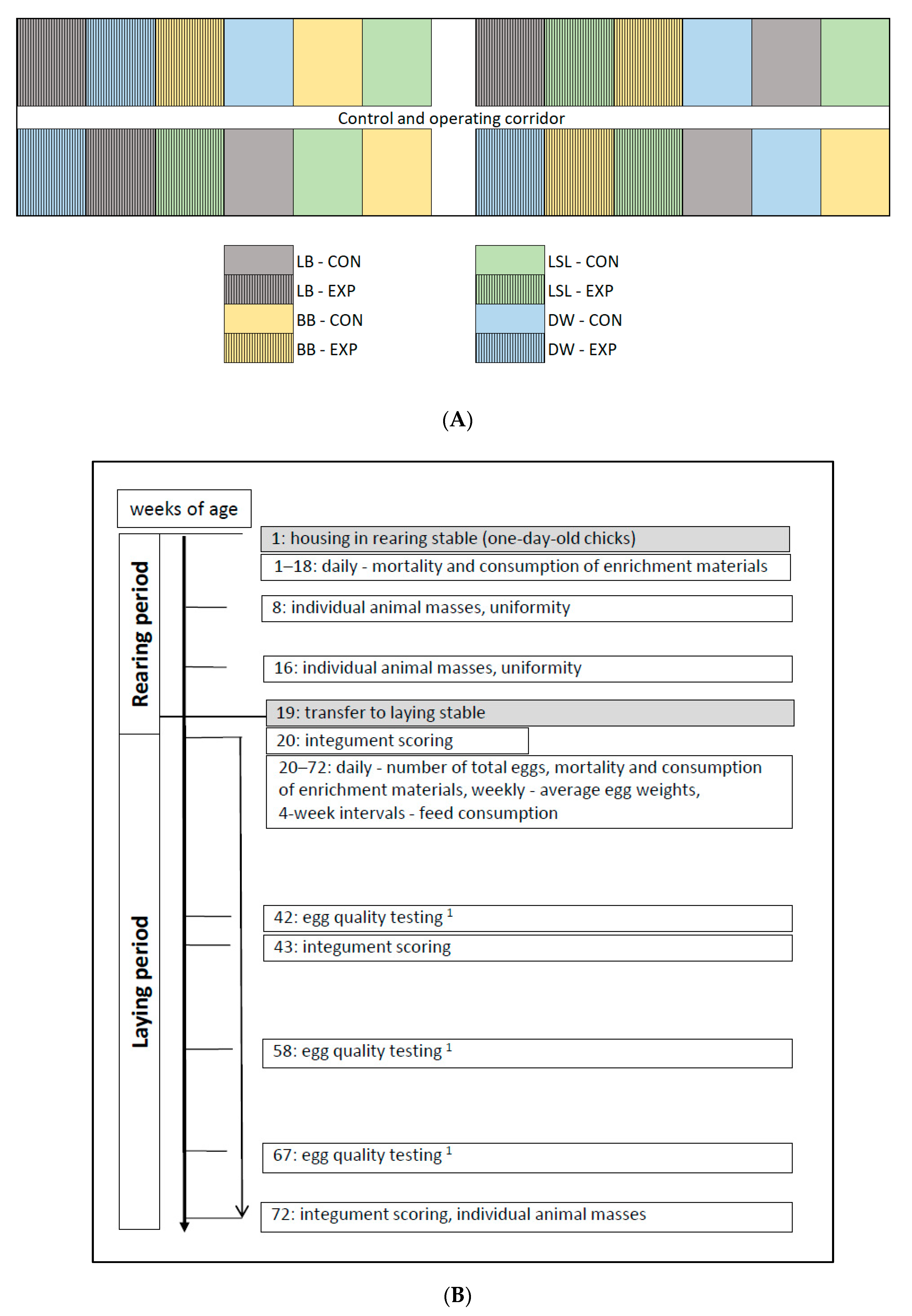
2.1. Housing and Management
2.2. Animals, Study Design, and Data Collection
2.3. Statistical Analyses
3. Results
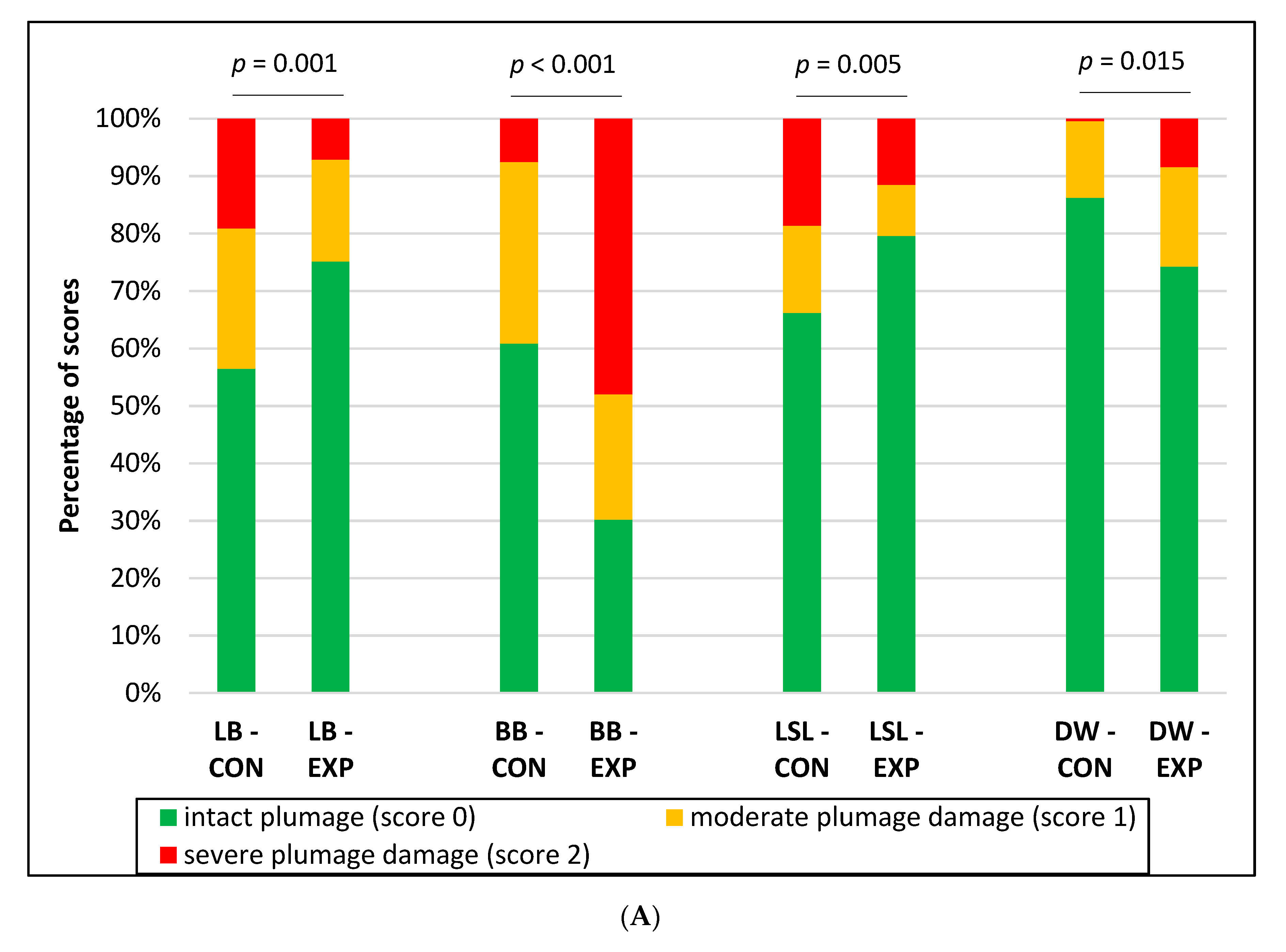
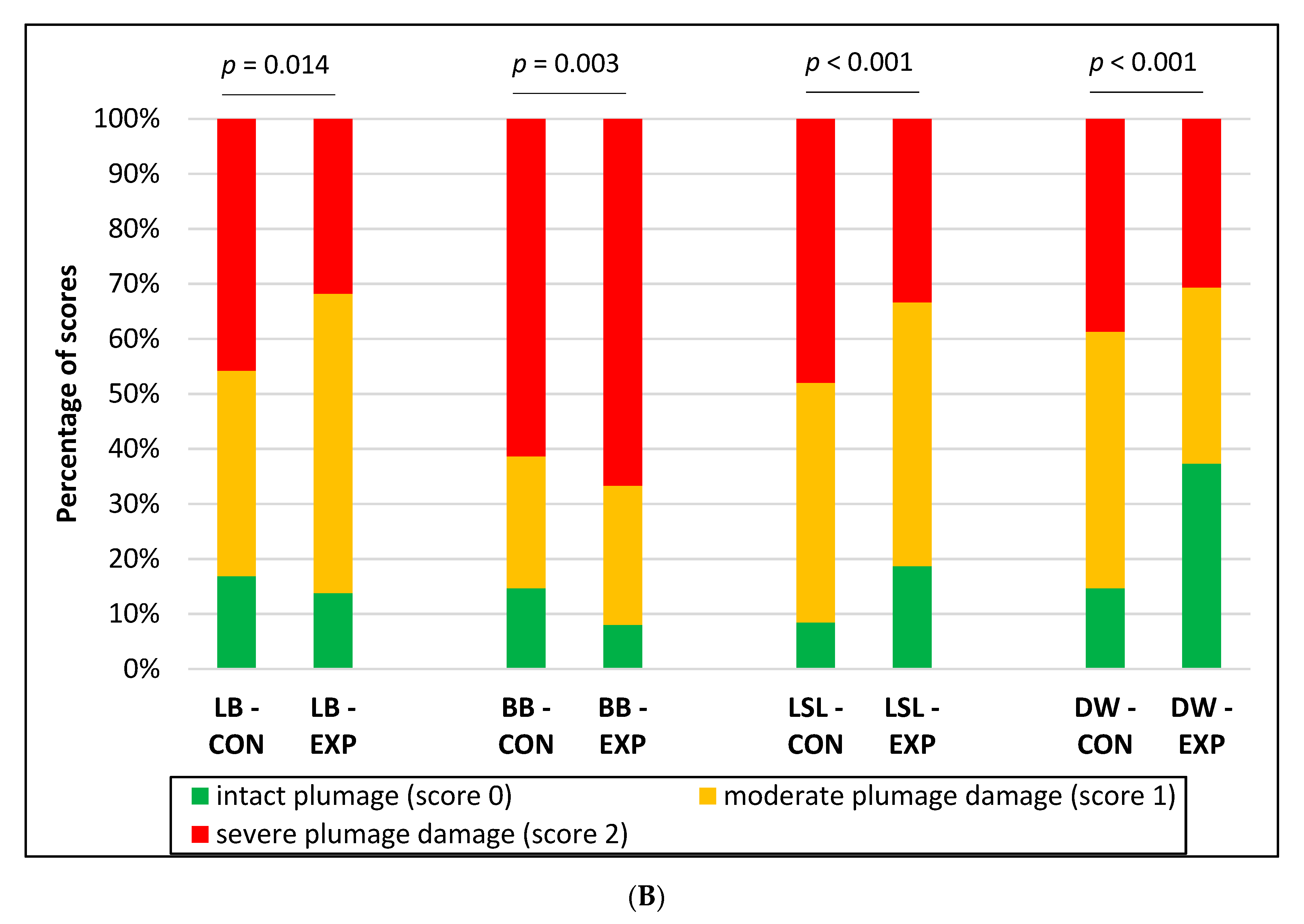
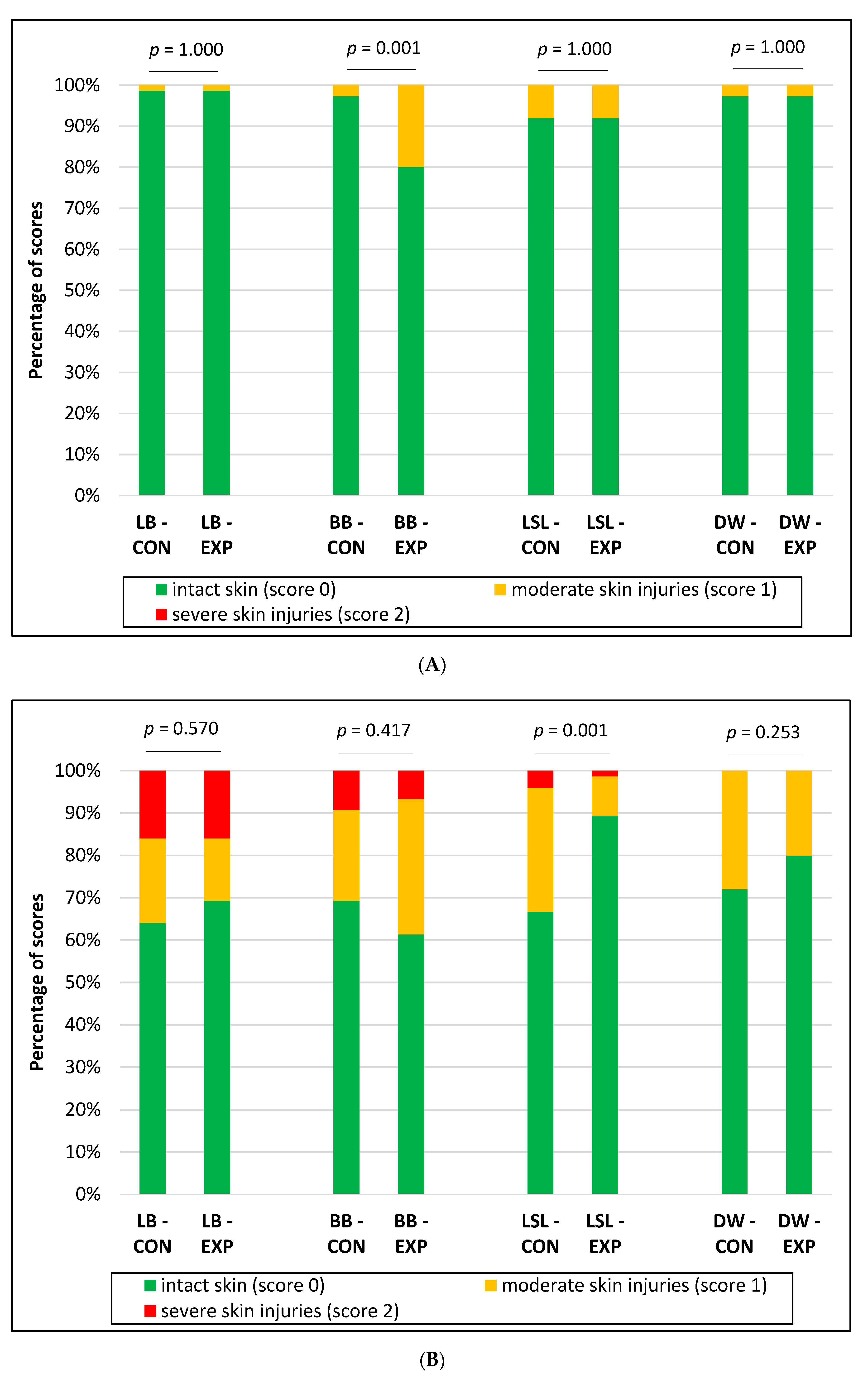
3.1. Integument Condition
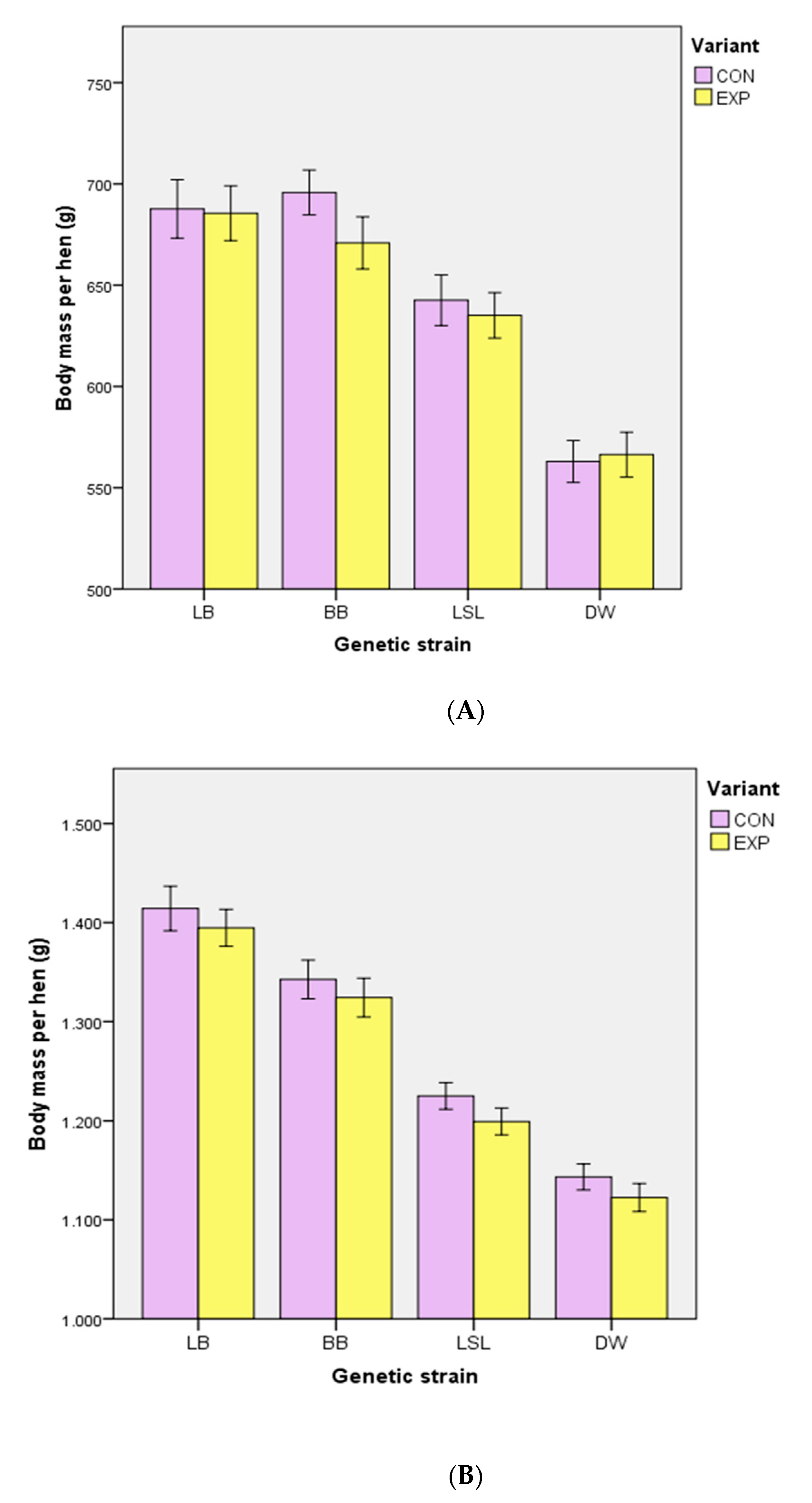
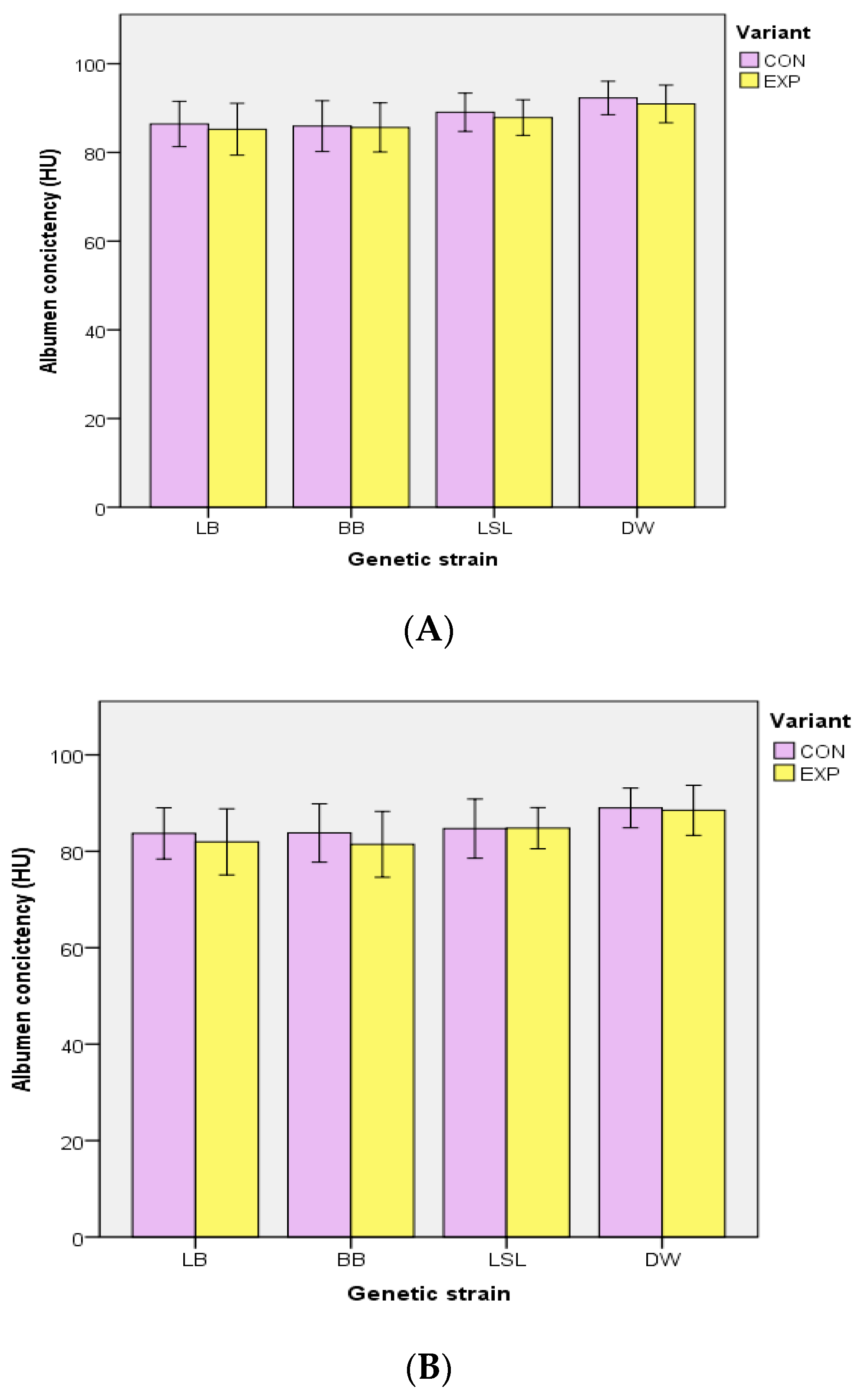
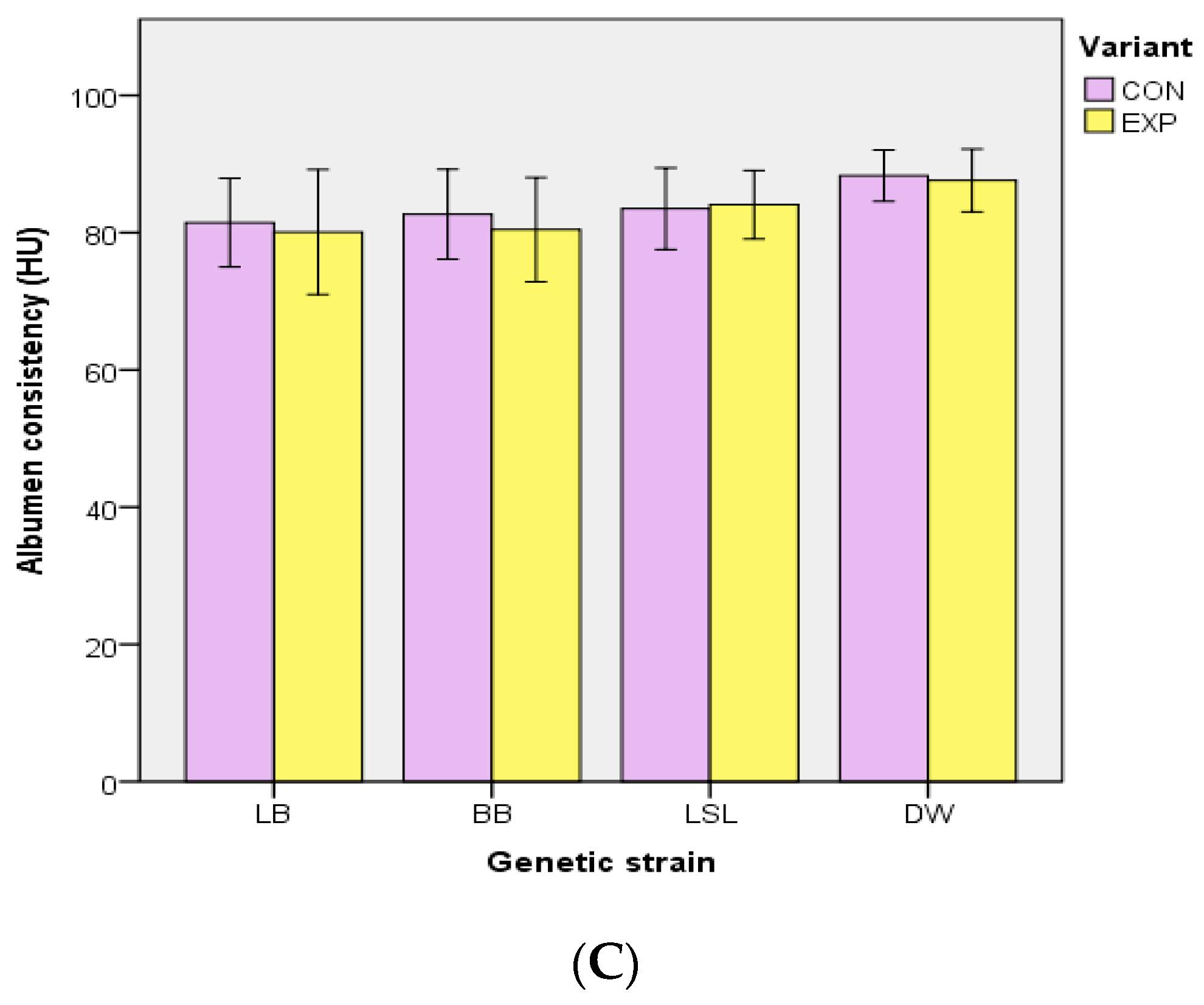
3.2. Body Mass, Performance Traits, EM Consumption and Egg Quality
4. Discussions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sherwin, C.M.; Richards, G.J.; Nicol, C.J. Comparison of the welfare of layer hens in 4 housing systems in the UK. Br. Poult. Sci. 2010, 51, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Damme, K.; Pirchner, F. Genetische Unterschiede in der Befiederung von Legehennen und Beziehungen zu Produktionsmerkmalen. Archiv. Geflügelkunde 1984, 48, 215–222. (In German) [Google Scholar]
- Appleby, M.C.; Hughes, B.O. Welfare of laying hens in cages and alternative systems, environmental, physical and behavioural aspects. World’s Poult. Sci. J. 1991, 47, 109–128. [Google Scholar] [CrossRef]
- Niebuhr, K.; Zaludik, K.; Gruber, B.; Thenmaier, I.; Lugmair, A.; Troxler, J. Epidemiologische Untersuchungen zum Auftreten von Kannibalismus und Federpicken in Alternativen Legehennenhaltungen in Österreich; Endbericht Forschungsprojekt Nr. 1313 ITT 2006; University Vienna: Vienna, Austria, 2006. (In German) [Google Scholar]
- Rodenburg, T.B.; van Krimpen, M.M.; de Jong, I.C.; de Haas, E.N.; Kops, M.S.; Riedstra, B.J.; Nordquist, R.E.; Wagenaar, J.P.; Bestman, M.; Nicol, C.J. The prevention and control of feather pecking in laying hens: Identifying the underlying principles. World’s Poult. Sci. J. 2013, 69, 361–373. [Google Scholar] [CrossRef]
- Liebers, C.J.; Schwarzer, A.; Erhard, E.; Schmidt, P.; Louton, H. The influence of environmental enrichment and stocking density on the plumage and health conditions of laying hen pullets. Poult. Sci. 2019, 98, 2474–2488. [Google Scholar] [CrossRef] [PubMed]
- Savory, C. Feather pecking and cannibalism. World’s Poult. Sci. J. 1995, 51, 215–219. [Google Scholar]
- Bilcik, B.; Keeling, L.J. Changes in feather condition in relation to feather pecking and aggressive behaviour in laying hens. Br. Poult. Sci. 1999, 40, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Spindler, B.; Giersberg, M.F.; Andersson, R.; Kemper, N. Keeping laying hens with untrimmed beaks—A Review of the status quo in practice and science. Züchtungskunde 2016, 88, 475–493. [Google Scholar]
- Gentle, M.J.; Hunter, L.N. Physiological and behavioural responses associated with feather removal in Gallus gallus var domesticus. Res. Vet. Sci. 1991, 50, 95–101. [Google Scholar] [CrossRef]
- Buitenhuis, A.J.; Rodenburg, T.B.; Siwek, M.; Cornelissen, S.J.B.; Niewland, M.G.B.; Crooijmans, R.P.M.A.; Groenen, M.A.M.; Koene, P.; Bovenhuis, H.; van der Poel, J.J. Identification of quantitative trait loci for receiving pecks in young and adult laying hens. Poult. Sci. 2003, 82, 1661–1667. [Google Scholar] [CrossRef]
- Krause, E.T.; Petow, S.; Kjaer, J.B. A note on the physiological and behavioural consequences of cannibalistic toe pecking in laying hens (Gallus gallus domesticus). Archiv. Geflügelkunde 2011, 75, 140–143. [Google Scholar]
- Gebhardt, S. Untersuchung des ZTHZ zum Auftreten von Zehenpicken bei weissen Legehybriden. Schweiz. Geflügelzeitung 2020, 5, 14–15. (In German) [Google Scholar]
- Damme, K. Effect of beak-trimming and strain on performance, feather loss and nesting behaviour of different commercial white layer hybrids in floor pens. Archiv. Geflügelkunde 1999, 63, 93–99. [Google Scholar]
- Sun, Y.; Ellen, E.D.; van der Poel, J.J.; Parmentier, H.K.; Bijma, P. Modelling of feather pecking behavior in beaktrimmed and non-beak-trimmed crossbred laying hens: Variance component and trait-based approach. Poult. Sci. 2014, 93, 773–783. [Google Scholar] [CrossRef]
- Hartcher, K.M.; Tran, K.T.N.; Wilkinson, S.J.; Hemsworth, P.H.; Thomson, P.C.; Cronin, G.M. The effects of environmental enrichment and beak-trimming during the rearing period on subsequent feather damage due to feather-pecking in laying hens. Poult. Sci. 2015, 94, 852–859. [Google Scholar] [CrossRef]
- Sepeur, S.; Spindler, B.; Schulze-Bisping, M.; Habig, C.; Andersson, R.; Beyerbach, M.; Kemper, N. Comparison of plumage condition of laying hens with intact and trimmed beaks kept on commercial farms. Eur. Poult. Sci. 2015, 79. [Google Scholar] [CrossRef]
- Weeks, C.A.; Lambton, S.; Williams, A.G. Implications for Welfare, Productivity and Sustainability of the Variation in Reported Levels of Mortality for Laying Hen Flocks Kept in Different Housing Systems: A Meta-Analysis of Ten Studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef]
- Van Krimpen, M.; Kwakkel, R.; Reuvekamp, B.; van der Peet-Schwering, C.; den Hartog, L.; Verstegen, M. Impact of feeding management on feather pecking in laying hens. World’s Poult. Sci. J. 2005, 61, 663–686. [Google Scholar]
- Kjaer, J.B.; Bessei, W. The interrelationships of nutrition and feather pecking in the domestic fowl—A review. Archiv. Geflügelkunde 2013, 77, 1–9. [Google Scholar]
- Janczak, A.M.; Riber, A.B. Review of rearing-related factors affecting the welfare of laying hens. Poult. Sci. 2015, 94, 1454–1469. [Google Scholar] [CrossRef]
- Bessei, W.; Lutz, V.; Kjaer, J.B.; Grashorn, M.; Bennewitz, J. Relationships between foraging and open-field activity in young chicks and feather pecking in adult birds: Results of analyses using quantitative genetics and structural equation models. Eur. Poult. Sci. 2018, 82. [Google Scholar] [CrossRef]
- Wennrich, G. Studie zum Verhalten verschiedener Hybrid-Herkünfte von Haushühnern (Gallus domesticus) in Boden-Intensivhaltung mit besonderer Berücksichtigung aggressiven Verhaltens sowie des Federpickens und des Kannibalismus. Archiv. Geflügelkunde 1975, 39, 37–44. (In German) [Google Scholar]
- Blokhuis, H.J. Feather pecking in poultry: Its relation with ground pecking. Appl. Anim. Behav. Sci. 1986, 16, 63–67. [Google Scholar] [CrossRef]
- Schreiter, R.; Damme, K.; von Borell, E.; Vogt, I.; Klunker, M.; Freick, M. Effects of litter and additional enrichment elements on the occurrence of feather pecking in pullets and laying hens—A focused review. Vet. Med. Sci. 2019, 5, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, H.J.; van der Haar, J.W. Effects of pecking incentives during rearing on feather pecking of laying hens. Br. Poult. Sci. 1992, 33, 17–24. [Google Scholar] [CrossRef]
- Norgaard-Nielsen, G.; Vestergaard, K.; Simonsen, H.B. Effects of rearing experience and stimulus enrichment on feather damage in laying hens. Appl. Anim. Behav. Sci. 1993, 38, 345–352. [Google Scholar] [CrossRef]
- Bari, M.S.; Cohen-Barnhouse, A.M.; Camphell, D.L.M. Early rearing enrichments influenced nest use and egg quality in free-range laying hens. Animal 2020, 14, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- McAdie, T.M.; Keeling, L.J.; Blokhuis, H.J.; Jones, R.B. Reduction in feather pecking and improvement of feather condition with the presentation of a string device to chickens. Appl. Anim. Behav. Sci. 2005, 93, 67–80. [Google Scholar] [CrossRef]
- Steenfeldt, S.; Kjaer, J.B.; Engberg, R.M. Effect of feeding silages or carrots as supplements to laying hens on production performance, nutrient digestibility, gut structure, gut microflora and feather pecking behaviour. Br. Poult. Sci. 2007, 48, 454–468. [Google Scholar] [CrossRef]
- Schreiter, R.; Damme, K.; Klunker, M.; Raoult, C.; von Borell, E.; Freick, M. Effects of edible environmental enrichments during the rearing and laying periods in a littered aviary—Part 1: Integument condition in pullets and laying hens. Poult. Sci. 2020, 99, 5184–5196. [Google Scholar] [CrossRef]
- Freytag, S.; Kemper, N.; Spindler, B. Einfluss des Zugangs zu Beschäftigungsmaterial Auf Das Verhalten und Die Herdengesundheit von Jung- und Legehennen in Praxisbetrieben—Abschlussbericht. Institut Für Tierhygiene, Tierschutz und Nutztierethologie, Stiftung Tierärztliche Hochschule Hannover. 2016. Available online: https://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwi0sJ_XnfXhAhWKUBUIHSsNAvIQFjAAegQIAhAC&url=https%3A%2F%2Fwww.ml.niedersachsen.de%2Fdownload%2F107762%2FAbschlussbericht_TiHo_-_Ausstieg_Schnabelkuerzung_Einfluss_des_Zugangs_zu_Beschaeftigungsmaterial.pdf&usg=AOvVaw08urgjDm7zz5ID6F87tKel (accessed on 1 August 2020). (In German).
- Cronin, G.M.; Hopcroft, R.L.; Groves, P.J.; Hall, E.J.S.; Phalen, D.N.; Hemsworth, P.H. Why did severe feather pecking and cannibalism outbreaks occur? An unintended case study while investigating the effects of forage and stress on pullets during rearing. Poult. Sci. 2018, 97, 1484–1502. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Drake, K.; Swick, R.A.; Taylor, P.S.; Perez-Maldonado, R.A.; Ruhnke, I. Effect of pecking stones and age on feather cover, hen mortality, and performance in free-range laying hens. Poult. Sci. 2020, 99, 2307–2314. [Google Scholar] [PubMed]
- Lüke, F.; Schmitten, F.; Trappmann, W. Die Leistungen von Legehennen verschiedener Herkünfte bei unterschiedlichen Haltungs- und Fütterungsbedingungen. Archiv. Geflügelkunde 1975, 39, 138–142. (In German) [Google Scholar]
- Flock, D.K.; Heil, G. A long-term analysis of time trends in the performance profile of white-egg and brownegg hybrid laying strains based on results of official German random sample tests from 1974/75 to 1997/99. Archiv. Geflügelkunde 2002, 66, 1–20. [Google Scholar]
- Damme, K.; Simon, I.; Flock, D.K. Adaptability of Laying Hens to Different Environments: Analysis of German Random Sample Tests 2010/11 with floor management and enriched cages. Lohmann Inf. 2012, 47, 9–14. [Google Scholar]
- Schreiter, R.; Damme, K.; Simon, I. Random Sample Test in terms of performance and economics of various laying hen hybrids in Germany 2016–2017. Eur. Poult. Sci. 2018, 82. [Google Scholar] [CrossRef]
- Lohmann Tierzucht GmbH. Management Guide Alternative Haltung. 2017. Available online: https://www.ltz.de/de/downloads/management-guides.php (accessed on 1 August 2020).
- Council Directive 1999/74/EC of 19 July 1999 Laying down Minimum Standards for the Protection of Laying Hens. Available online: http://www.legislation.gov.uk/eudr/1999/74/contents (accessed on 1 August 2020).
- TierschG. Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206, 1313), das Zuletzt Durch Artikel 4 Absatz 8 des Gesetzes vom 18. Juli 2016 (BGBl. I S. 1666) Geändert Worden ist. Available online: http://www.gesetze-im-internet.de/tierschg/TierSchG.pdf (accessed on 1 August 2020). (In German).
- TierSchNutztV. Verordnung zum Schutz Landwirtschaftlicher Nutztiere und Anderer zur Erzeugung Tierischer Produkte Gehaltener Tiere bei Ihrer Haltung.—Tierschutz-Nutztierhaltungsverordnung in der Fassung der Bekanntmachung vom 22. August 2006, BGBl.I, 2043, die Durch Artikel 3 Absatz 2 des Gesetzes vom 30. Juni 2017 (BGBL I, 2147) Geändert Worden ist. Available online: http://www.gesetze-im-internet.de/tierschnutztv/ (accessed on 1 August 2020). (In German).
- American Dairy Science Association. Guide for the Care and Use of Agricultural Animals in Research and Teaching. 2010. Available online: https://www.asas.org/docs/default-source/default-document-library/ag_guide_3rded.pdf?sfvrsn=4 (accessed on 1 August 2020).
- Council Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official, J. 2010, L 276, (October 10). 33. Available online: http://eur-lex.europa.eu/legal-content/DE/TXT/PDF/?uri=CELEX:32010L0063&from=en (accessed on 1 August 2020).
- Institute for Medical Statistics and Bioinformatics at the University of Cologne: Webtool Sample Size. Available online: https://imsiewebarchiv.uni-koeln.de/beratung/rechner/b2.html (accessed on 1 August 2020).
- Welfare Quality. Welfare Quality Assessment Protocol for Poultry (Broilers, Laying Hens); Welfare Quality Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Keppler, C. Managementtool Beurteilungskarten—Legehennen. Anleitung zur Beurteilung des Tierzustandes. University Kassel. 2017. Available online: https://www.mud-tierschutz.de/fileadmin/user_upload/2017-08-22_Beurteilungskarten_Legehennen_web.pdf (accessed on 1 August 2020).
- Jeroch, H.; Müller, R. Nutritional recommendations for laying hens including the rearing period. In Geflügeljahrbuch 2019; Damme, K., Mayer, A., Eds.; Ulmer: Stuttgart, Germany, 2018; pp. 201–227. [Google Scholar]
- Damme, K.; Schreiter, R.; Schneider, M.; Hildebrand, R.A. 13. Bayerischer Herkunftsvergleich von Legehybriden in Bodenhaltung. 2018. Available online: https://www.lfl.bayern.de/mam/cms07/lvfz/kitzingen/dateien/13._bayerischer_herkunftsvergleich.pdf (accessed on 1 August 2020). (In German).
- Haugh, R. The Haugh unit for measuring egg quality. US Egg Poult. Mag. 1937, 43, 552–555. [Google Scholar]
- Weiß, C. Basic Knowledge of Medical Statistics, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 98–256. [Google Scholar]
- Du Prel, J.-B.; Röhrig, B.; Hommel, G.; Blettner, M. Auswahl statistischer Testverfahren. Dtsch. Aerztebl. Int. 2010, 107, 343–348. (In German) [Google Scholar]
- Rasch, B.; Friese, M.; Hofmann, W.J.; Naumann, E. Quantitative Methods—Volume 2, 3th ed.; Springer: Heidelberg, Germany, 2010; pp. 55–98. [Google Scholar]
- Victor, A.; Elsäßer, A.; Hommel, G.; Blettner, M. Wie bewertet man die p-Wert-Flut? Dtsch. Aerztebl. Int. 2010, 107, 50–56. (In German) [Google Scholar]
- Baltes-Götz, B. Logistische Regressionsanalyse Mit SPSS. 2000. Available online: https://www.uni-trier.de/fileadmin/urt/doku/logist/logist.pdf (accessed on 1 August 2020). (In German).
- Menard, S. Applied Logistic Regression Analysis, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2002; pp. 41–101. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage: Thousand Oaks, CA, USA, 2013; pp. 293–356. [Google Scholar]
- Backhaus, K.; Erichson, B.; Plinke, W.; Weiber, R. Multivariate Analysemethoden, 14th ed.; Springer: Berlin, Germany, 2016; pp. 283–356. (In German) [Google Scholar]
- Huber-Eicher, B.; Wechsler, B. Feather pecking in domestic chicks: Its relation to dustbathing and foraging. Anim. Behav. 1997, 54, 757–768. [Google Scholar] [CrossRef]
- Icken, W.; Cavero, D.; Schmutz, M. Selection on beak shape to reduce feather pecking in laying hens. Lohmann Inf. 2017, 51, 22–27. [Google Scholar]
- Cooke, B.C. Cannibalism in laying hens. Vet. Rec. 1992, 131, 495. [Google Scholar] [CrossRef] [PubMed]
- Van Krimpen, M.M.; Kwakkel, R.P.; van der Peet-Schwering, C.M.; den Hartog, L.A.; Verstegen, M.W. Low dietary energy concentration, high nonstarch polysaccharide concentration, and coarse particle sizes of nonstarch polysaccharides affect the behavior of feather-pecking-prone laying hens. Poult. Sci. 2008, 87, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Muir, W.M. Relative efficiency of selection for performance of birds housed in colony cages based on performance in single bird cages. Poult. Sci. 1985, 64, 2239–2247. [Google Scholar] [CrossRef]
- Leyendecker, M.; Hamann, H.; Hartung, J.; Kamphues, J.; Ring, C.; Glünder, G.; Ahlers, C.; Sander, I.; Neumann, U.; Distl, O. Analysis of genotype-environment interactions between layer lines and housing systems for performance trails, egg quality and bone strength. 2nd communication: Egg quality traits. Züchtungskunde 2001, 73, 308–323. [Google Scholar]
- Ledvinka, Z.; Tůmová, E.; Englmaierová, M.; Podsedníček, M. Egg quality of three laying hen genotypes kept in conventional cages and on litter. Archiv. Geflügelkunde 2016, 76, 38–43. [Google Scholar]
- Yakubu, A.; Salako, A.E.; Ige, A.O. Effects of Genotype and Housing System on the Laying Performance of Chickens in Different Seasons in the Semi-Humid Tropics. Int. J. Poult. Sci. 2007, 6, 434–439. [Google Scholar] [CrossRef]
- Küçükyılmaz, K.; Bozkurt, M.; nur Herken, E.; Çınar, M.; Uğur Çatlı, A.; Bintaş, E.; Çöven, F. Effects of Rearing Systems on Performance, Egg Characteristics and Immune Response in Two Layer Hen Genotype. Asian-Aust. J. Anim. Sci. 2012, 25, 559–568. [Google Scholar]
- Wall, H.; Tauson, R.; Elwinger, K. Effects of Litter Substrate and Genotype on Layers’ Use of Litter, Exterior Appearance, and Heterophil: Lymphocyte Ratios in Furnished Cages. Poult. Sci. 2008, 87, 2458–2465. [Google Scholar] [CrossRef]
- De Haas, E.N.; Kemp, B.; Bollhuis, J.E.; Groothuis, T.; Rodenburg, T.B. Fear, stress, and feather pecking in commercial white and brown laying hen parent-stock flocks and their relationships with production parameters. Poult. Sci. 2013, 92, 2259–2269. [Google Scholar] [CrossRef]
- Purdum, S.; Eusebio, P.; Hanford, K. The effects of 2 genetic lines on spatial distribution and use and preference of perch and nest area in an aviary system. Poult. Sci. 2020, 99, 3328–3333. [Google Scholar] [CrossRef] [PubMed]
- Berk, J.; Stehle, E.; Bartels, T. Haltung schnabelunbehandelter Puten–Beschäftigung hält nicht immer vom Picken ab. DGS-Magazin 2016, 39, 35–38. (In German) [Google Scholar]
- Falker-Gieske, C.; Mott, A.; Preuß, S.; Franzenburg, S.; Bessei, W.; Bennewitz, J.; Tentens, J. Analysis of the brain transcriptome in lines of laying hens divergently selected for feather pecking. BMC Genom. 2020, 21, 595. [Google Scholar] [CrossRef] [PubMed]
- Alm, M.; Holm, L.; Tauson, R.; Wall, H. Corticosterone metabolites in laying hen droppings—Effects of fiber enrichment, genotype, and daily variations. Poult. Sci. 2014, 93, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Schreiter, R.; Damme, K. Nutrition of Laying Hens—Use of Domestic Feed and Feeding of Laying Hens with Untrimmed Beaks; Bavarian Institute for Agriculture: Freising, Germany, 2017; Available online: www.baysg.bayern.de/mam/cms16/zentren/kitzingen/dateien/legehennenfuetterung_baysg-publikation.pdf (accessed on 1 August 2020).
- Kjaer, J.B.; Sorensen, P. Feather pecking and cannibalism in free-range laying hens as affected by genotype, dietary level of methionine + cystine, light intensity during rearing and age at first access to the range area. Appl. Anim. Behav. Sci. 2002, 76, 21–39. [Google Scholar] [CrossRef]
- Schreiter, R.; Damme, K.; Hartmann, J.; Klunker, M.; Freick, M.; Wolff, N.; von Borell, E. Effect of a specially to reduce feather pecking designed feed on the performance and the occurrence of behavioural disorders in laying hens. Eur. Poult. Sci. 2019, 83. [Google Scholar] [CrossRef]
- Craig, J.V.; Lee, H.Y. Beak Trimming and Genetic Stock Effects on Behavior and Mortality from Cannibalism in White Leghorn-Type Pullets. Appl. Anim. Behav. Sci. 1990, 25, 107–123. [Google Scholar] [CrossRef]
- Schreiter, R.; Damme, K.; Klunker, M.; Raoult, C.; von Borell, E.; Freick, M. Effects of edible environmental enrichment during the rearing and laying period in a littered aviary—Part 2: Physical development of pullets and performance, egg quality and carcase composition in laying hens. Poult. Sci. 2020, 99, 6685–6696. [Google Scholar] [CrossRef]
- Elsherif, H.; Foaud, A.; Nassar, S.; Wahba, F.; Elsabagh, M.; ElIraqi, K. Effect of dietary copper sulphate on laying hen performance, egg quality, and oxidative stress in hot climate conditions. Eur. Poult. Sci. 2019, 83. [Google Scholar] [CrossRef]
| Trait | Score 1 (%) | Coefficients (SE) | Odds Ratio (95% CI) | Individual p-Value | Overall p-Value |
|---|---|---|---|---|---|
| Total Plumage Score | |||||
| LB | 45.6 | reference | baseline | <0.001 | |
| BB | 52.7 | −0.74 (0.31) | 0.48 (0.26–0.87) | 0.016 | |
| LSL | 46.4 | 0.14 (0.31) | 1.14 (0.63–2.09) | 0.662 | |
| DW | 35.6 | −1.59 (0.32) | 0.20 (0.11–0.38) | <0.001 | |
| week 20 | 1.3 | reference | baseline | <0.001 | |
| week 43 | 40.7 | 4.35 (0.38) | 77.43 (36.47–164.36) | <0.001 | |
| week 72 | 93.2 | 7.69 (0.42) | 2199.89 (963.13–5024.78) | <0.001 | |
| CON | 45.2 | reference | baseline | 0.001 | |
| EXP | 44.9 | −1.09 (0.32) | 0.34 (0.18–0.63) | ||
| compartment | - | 0.04 (0.01) | 1.04 (1.01–1.06) | 0.003 | |
| CON × LB | 49.8 | reference | baseline | <0.001 | |
| EXP × BB | 61.3 | 3.24 (0.49) | 25.49 (9.79–66.20) | <0.001 | |
| EXP × LSL | 40.4 | −0.09 (0.44) | 0.91 (0.38–2.17) | 0.838 | |
| EXP × DW | 36.4 | 1.22 (0.44) | 3.40 (1.43–8.09) | 0.006 | |
| constant | −4.66 (0.45) | ||||
| Skin Injuries | |||||
| LB | 11.6 | reference | baseline | 0.632 | |
| BB | 15.3 | −0.19 (0.32) | 0.83 (0.44–1.56) | 0.560 | |
| LSL | 10.0 | 0.11 (0.31) | 1.11 (0.61–2.06) | 0.728 | |
| DW | 8.9 | −0.26 (0.32) | 0.77 (0.41–1.45) | 0.416 | |
| week 20 | 0.0 | reference | baseline | <0.001 | |
| week 43 | 5.8 | 18.41 (1620.70) | 50.71 (0.74–77.51) | 0.991 | |
| week 72 | 28.5 | 20.32 (1620.70) | 1008 (0.89–2171.81) | 0.990 | |
| CON | 11.9 | reference | baseline | 0.436 | |
| EXP | 11.0 | −0.26 (0.33) | 0.77 (0.40–1.48) | ||
| compartment | - | 0.01 (0.01) | 1.01 (0.98–1.04) | 0.527 | |
| CON × LB | 12.4 | reference | baseline | 0.001 | |
| EXP × BB | 19.6 | 1.14 (0.47) | 3.14 (1.24–7.91) | 0.016 | |
| EXP × LSL | 6.2 | −0.74 (0.49) | 0.48 (0.18–1.26) | 0.134 | |
| EXP × DW | 7.6 | −0.13 (0.48) | 0.87 (0.34–2.26) | 0.784 | |
| constant | −21.20 (1620.44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiter, R.; Damme, K.; Freick, M. Edible Environmental Enrichments in Littered Housing Systems: Do Their Effects on Integument Condition Differ Between Commercial Laying Hen Strains? Animals 2020, 10, 2434. https://doi.org/10.3390/ani10122434
Schreiter R, Damme K, Freick M. Edible Environmental Enrichments in Littered Housing Systems: Do Their Effects on Integument Condition Differ Between Commercial Laying Hen Strains? Animals. 2020; 10(12):2434. https://doi.org/10.3390/ani10122434
Chicago/Turabian StyleSchreiter, Ruben, Klaus Damme, and Markus Freick. 2020. "Edible Environmental Enrichments in Littered Housing Systems: Do Their Effects on Integument Condition Differ Between Commercial Laying Hen Strains?" Animals 10, no. 12: 2434. https://doi.org/10.3390/ani10122434
APA StyleSchreiter, R., Damme, K., & Freick, M. (2020). Edible Environmental Enrichments in Littered Housing Systems: Do Their Effects on Integument Condition Differ Between Commercial Laying Hen Strains? Animals, 10(12), 2434. https://doi.org/10.3390/ani10122434





