Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Hair Sampling and Steroids Extraction
2.3. Corticosterone Quantification by ELISA
2.4. Testosterone and DHEA Analysis by Radioimmunoassay (RIA)
2.5. Statistical Analyses
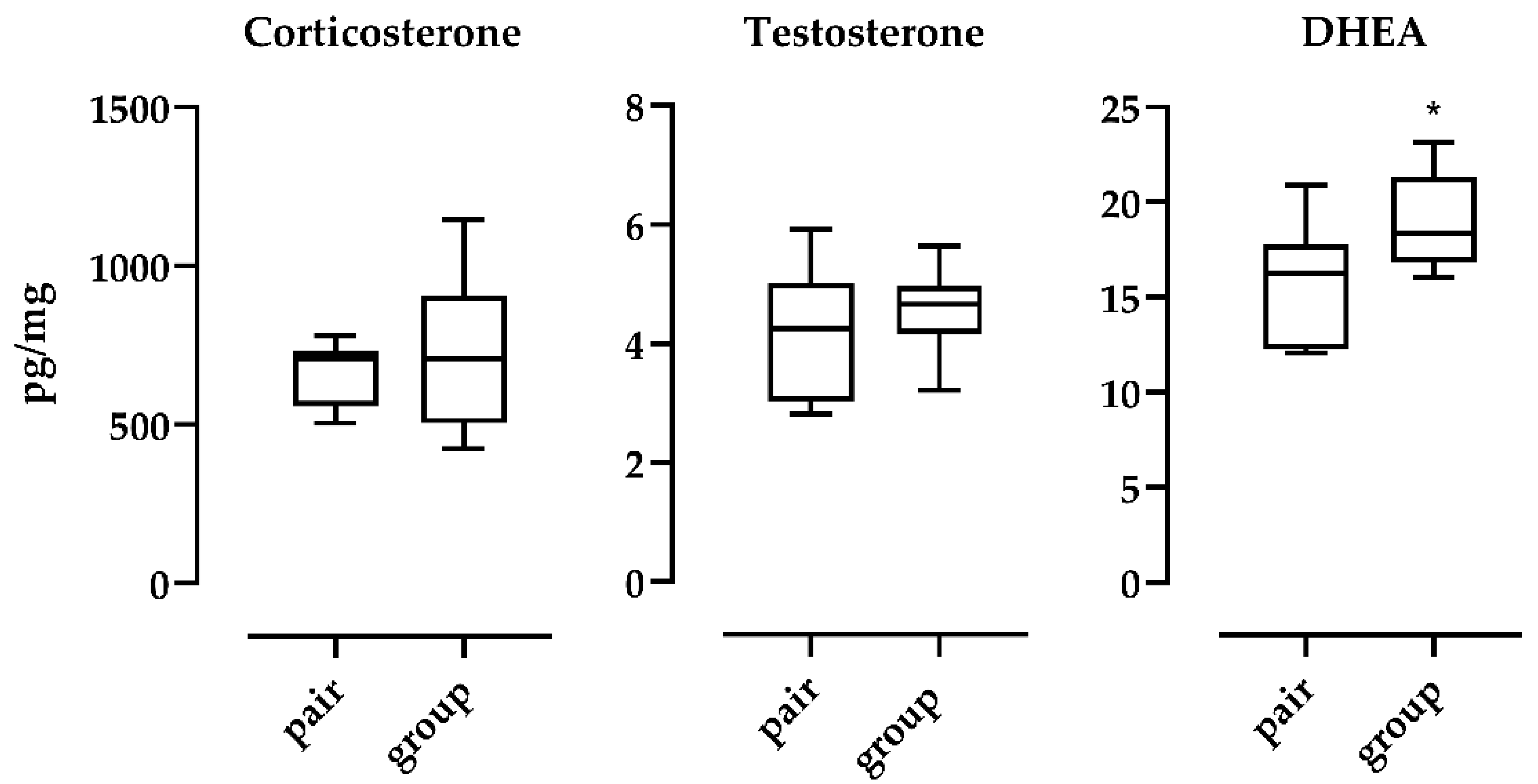
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailoo, J.D.; Murphy, E.; Varholick, J.; Novak, J.; Palme, R.; Würbel, H. Evaluation of the effects of space allowance on measures of animal welfare in laboratory mice. Sci. Rep. 2018, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Latham, N.; Mason, G. From house mouse to mouse house: The behavioural biology of free-living Mus musculus and its implications in the laboratory. Appl. Anim. Behav. Sci. 2004, 86, 261–289. [Google Scholar] [CrossRef]
- Lidster, K.; Owen, K.; Browne, W.J.; Prescott, M.J. Cage aggression in group-housed laboratory male mice: An international data crowdsourcing project. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kappel, S.; Hawkins, P.; Mendl, M. To Group or Not to Group? Good Practice for Housing Male Laboratory Mice. Animals 2017, 7, 88. [Google Scholar] [CrossRef]
- Whary, M.T.; Baumgarth, N.; Fox, J.G.; Barthold, S.W. Chapter 3—Biology and Diseases of Mice. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; American College of Laboratory Animal Medicine, Academic Press: Boston, MA, USA, 2015; pp. 43–149. ISBN 978-0-12-409527-4. [Google Scholar]
- Bell, M.R. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 2018, 159, 2596–2613. [Google Scholar] [CrossRef]
- Boleij, H.; Salomons, A.R.; Van Sprundel, M.; Arndt, S.S.; Ohl, F. Not All Mice Are Equal: Welfare Implications of Behavioural Habituation Profiles in Four 129 Mouse Substrains. PLoS ONE 2012, 7, e42544. [Google Scholar] [CrossRef]
- Ralph, C.; Tilbrook, A.J. INVITED REVIEW: The usefulness of measuring glucocorticoids for assessing animal welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef]
- Meffre, D.; Pianos, A.; Liere, P.; Eychenne, B.; Cambourg, A.; Schumacher, M.; Stein, D.G.; Guennoun, R. Steroid Profiling in Brain and Plasma of Male and Pseudopregnant Female Rats after Traumatic Brain Injury: Analysis by Gas Chromatography/Mass Spectrometry. Endocrinology 2007, 148, 2505–2517. [Google Scholar] [CrossRef]
- Gong, S.; Miao, Y.-L.; Jiao, G.-Z.; Sun, M.-J.; Li, H.; Lin, J.; Luo, M.-J.; Tan, J.-H. Dynamics and Correlation of Serum Cortisol and Corticosterone under Different Physiological or Stressful Conditions in Mice. PLoS ONE 2015, 10, e0117503. [Google Scholar] [CrossRef]
- Klomberg, K.F.; Garland, T.; Swallow, J.G.; A Carter, P. Dominance, plasma testosterone levels, and testis size in house mice artificially selected for high activity levels. Physiol. Behav. 2002, 77, 27–38. [Google Scholar] [CrossRef]
- Machida, T.; Yonezawa, Y.; Noumura, T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm. Behav. 1981, 15, 238–245. [Google Scholar] [CrossRef]
- Mucignat-Caretta, C.; Cavaggioni, A.; Redaelli, M.; Da Dalt, L.; Zagotto, G.; Gabai, G. Age and isolation influence steroids release and chemical signaling in male mice. Steroids 2014, 83, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.P.P.; Edwards, W.R.; Collins, W.P. The Measurement of Steroid Glucuronides in Urine from Mice to Monitor Gonadal Function. I. Pregnanediol-3α-Glucuronide as an Index of Progestogen Output. Endocrinology 1978, 103, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Decatanzaro, D.; Muir, C.; Beaton, E.A.; Jetha, M. Non-invasive repeated measurement of urinary progesterone, 17β-estradiol, and testosterone in developing, cycling, pregnant, and postpartum female mice. Steroids 2004, 69, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.; Rajabi, N.; Decatanzaro, D. Circadian Rhythm and Response to an Acute Stressor of Urinary Corticosterone, Testosterone, and Creatinine in Adult Male Mice. Horm. Metab. Res. 2012, 44, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, T.; Palme, R.; Tichy, A.; Rülicke, T. Lifetime Dependent Variation of Stress Hormone Metabolites in Feces of Two Laboratory Mouse Strains. PLoS ONE 2015, 10, e0136112. [Google Scholar] [CrossRef]
- Auer, K.E.; Kußmaul, M.; Möstl, E.; Hohlbaum, K.; Rülicke, T.; Palme, R. Measurement of Fecal Testosterone Metabolites in Mice: Replacement of Invasive Techniques. Animals 2020, 10, 165. [Google Scholar] [CrossRef]
- Nohara, M.; Tohei, A.; Sato, T.; Amao, H. Evaluation of response to restraint stress by salivary corticosterone levels in adult male mice. J. Veter. Med. Sci. 2016, 78, 775–780. [Google Scholar] [CrossRef]
- Lavitrano, M.; Nannoni, E.; Govoni, N.; Scorrano, F.; Zannoni, A.; Forni, M.; Martelli, G.; Sardi, L. Hair cortisol determination in sows in two consecutive reproductive cycles. Reprod. Biol. 2014, 14, 218–223. [Google Scholar] [CrossRef]
- Kapoor, A.; Schultz-Darken, N.; Ziegler, T.E. Radiolabel validation of cortisol in the hair of rhesus monkeys. Psychoneuroendocrinology 2018, 97, 190–195. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, D.; Elmi, A.; Bertocchi, M.; Aniballi, C.; Parmeggiani, A.; Govoni, N.; Lavitrano, M. Progesterone and Cortisol Levels in Blood and Hair of Wild Pregnant Red Deer (Cervus Elaphus) Hinds. Animals 2020, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, D.; Elmi, A.; Barone, F.; Carnevali, G.; Govoni, N.; Lavitrano, M. Hair Testosterone and Cortisol Concentrations in Pre- and Post-Rut Roe Deer Bucks: Correlations with Blood Levels and Testicular Morphometric Parameters. Animals 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Jahangard, L.; Mikoteit, T.; Bahiraei, S.; Zamanibonab, M.; Haghighi, M.; Bahmani, D.S.; Brand, S. Prenatal and Postnatal Hair Steroid Levels Predict Post-Partum Depression 12 Weeks after Delivery. J. Clin. Med. 2019, 8, 1290. [Google Scholar] [CrossRef]
- Meyer, J.S.; Novak, M.; Hamel, A.; Rosenberg, K. Extraction and analysis of cortisol from human and monkey hair. J. Vis. Exp. 2014, 83, e50882. [Google Scholar] [CrossRef]
- Scorrano, F.; Carrasco, J.; Pastor-Ciurana, J.; Belda, X.; Rami-Bastante, A.; Bacci, M.L.; Armario, A. Validation of the long-term assessment of hypothalamic-pituitary-adrenal activity in rats using hair corticosterone as a biomarker. FASEB J. 2015, 29, 859–867. [Google Scholar] [CrossRef]
- Carlitz, E.H.D.; Runge, J.-N.; König, B.; Winkler, L.; Kirschbaum, C.; Gao, W.; Lindholm, A.K. Steroid hormones in hair reveal sexual maturity and competition in wild house mice (Mus musculus domesticus). Sci. Rep. 2019, 9, 16925. [Google Scholar] [CrossRef]
- Jarcho, M.; Massner, K.; Eggert, A.; Wichelt, E. Behavioral and physiological response to onset and termination of social instability in female mice. Horm. Behav. 2016, 78, 135–140. [Google Scholar] [CrossRef]
- Erickson, R.L.; Browne, C.A.; Lucki, I. Hair corticosterone measurement in mouse models of type 1 and type 2 diabetes mellitus. Physiol. Behav. 2017, 178, 166–171. [Google Scholar] [CrossRef]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice—Assessing the degree of distress. PLoS ONE 2017, 12, e0179588. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Zhao, H.; Liang, Y.; Chao, R.; Chen, L.; Yang, S.; Yu, P. Differences in cocaine- and morphine-induced cognitive impairments and serum corticosterone between C57BL/6J and BALB/cJ mice. Pharmacol. Biochem. Behav. 2019, 182, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oyola, M.G.; Handa, R.J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Dungan, H.M.; Stoll, E.A.; Gottsch, M.L.; Braun, R.E.; Eacker, S.M.; Clifton, D.K.; Steiner, R.A. Differential Regulation of KiSS-1 mRNA Expression by Sex Steroids in the Brain of the Male Mouse. Endocrinology 2005, 146, 2976–2984. [Google Scholar] [CrossRef]
- Chichinadze, K. Stress-induced increase of testosterone: Contributions of social status and sympathetic reactivity. Physiol. Behav. 2008, 94, 595–603. [Google Scholar] [CrossRef]
- Dong, Q.; Salva, A.; Sottas, C.M.; Niu, E.; Holmes, M.; Hardy, M.P. Rapid Glucocorticoid Mediation of Suppressed Testosterone Biosynthesis in Male Mice Subjected to Immobilization Stress. J. Androl. 2004, 25, 973–981. [Google Scholar] [CrossRef]
- Labrie, F.; Luu-The, V.; Bélanger, A.; Lin, S.-X.; Simard, J.; Pelletier, G.; Labrie, C. Is dehydroepiandrosterone a hormone? J. Endocrinol. 2005, 187, 169–196. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocr. 2009, 30, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.E.M.; Pradhan, D.S.; Soma, K.K. Dehydroepiandrosterone and Corticosterone Are Regulated by Season and Acute Stress in a Wild Songbird: Jugular Versus Brachial Plasma. Endocrinology 2008, 149, 2537–2545. [Google Scholar] [CrossRef]
- Wolf, O.T.; Kirschbaum, C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: Effects on cognition and emotion in animals and humans. Brain Res. Rev. 1999, 30, 264–288. [Google Scholar] [CrossRef]
- European Union (Protection of Animals used for Scientific Purposes) Regulations 2012 (S.I. No. 543 of 2012). Available online: https://www.ecolex.org/details/legislation/european-union-protection-of-animals-used-for-scientific-purposes-regulations-2012-si-no-543-of-2012-lex-faoc121063/ (accessed on 10 November 2020).
- Directive 2010/63/EU of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 10 November 2020).
- Commission Recommendation of 18 June 2007 on Guidelines for the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes (Notified under Document Number C(2007) 2525) (Text with EEA Relevance); EEA: Brussels, Belgium, 2007.
- FELASA Working Group on Revision of Guidelines for Health Monitoring of Rodents and Rabbits. Erratum to “FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units”. Lab. Anim. 2014, 49, 88. [Google Scholar] [CrossRef]
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, K.; Hurst, J.L. Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, K.; Hurst, J.L. Reducing Mouse Anxiety during Handling: Effect of Experience with Handling Tunnels. PLoS ONE 2013, 8, e66401. [Google Scholar] [CrossRef]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar]
- Smith, A.J.; Lilley, E. The Role of the Three Rs in Improving the Planning and Reproducibility of Animal Experiments. Animals 2019, 9, 975. [Google Scholar] [CrossRef]
- Chu, L.; Li, N.; Deng, J.; Wu, Y.; Yang, H.; Wang, W.; Zhou, D.; Deng, H. LC-APCI+-MS/MS method for the analysis of ten hormones and two endocannabinoids in plasma and hair from the mice with different gut microbiota. J. Pharm. Biomed. Anal. 2020, 185, 113223. [Google Scholar] [CrossRef]
- Canastar, A.; Maxson, S.C. Sexual Aggression in Mice: Effects of Male Strain and of Female Estrous State. Behav. Genet. 2003, 33, 521–528. [Google Scholar] [CrossRef]
- Chen, F.; Knecht, K.; Birzin, E.; Fisher, J.; Wilkinson, H.; Mojena, M.; Moreno, C.T.; Schmidt, A.; Harada, S.-I.; Freedman, L.P.; et al. Direct Agonist/Antagonist Functions of Dehydroepiandrosterone. Endocrinology 2005, 146, 4568–4576. [Google Scholar] [CrossRef]
- Ma, J.; Yue, J.; Huang, R.; Liao, Y.; Li, S.; Liu, W. Reversion of aging-related DHEAS decline in mouse plasma alleviates aging-related glucose tolerance impairment by potentiation of glucose-stimulated insulin secretion of acute phase. Biochem. Biophys. Res. Commun. 2018, 500, 671–675. [Google Scholar] [CrossRef]
- Hohlbaum, K.; Frahm, S.; Rex, A.; Palme, R.; Thöne-Reineke, C.; Ullmann, K. Social enrichment by separated pair housing of male C57BL/6JRj mice. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Varholick, J.A.; Pontiggia, A.; Murphy, E.; Daniele, V.; Palme, R.; Voelkl, B.; Würbel, H.; Bailoo, J.D. Social dominance hierarchy type and rank contribute to phenotypic variation within cages of laboratory mice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Corticosterone | Testosterone | DHEA | Age | |
|---|---|---|---|---|
| Corticosterone | – | −0.587 p < 0.0001 | −0.479 p = 0.0003 | −0.604 p < 0.0001 |
| Testosterone | – | – | 0.855 p < 0.0001 | 0.451 p = 0.0006 |
| DHEA | – | – | – | 0.354 p = 0.0086 |
| Age | – | – | – | – |
| Models | R2 | Angular Coefficient (β) | 95% C.I. | |
|---|---|---|---|---|
| (log)Corticosterone | 0.41 | Intercept | 2.9472 | 2.8491; 3.0452 |
| Age | −0.0018 | −0.0014; −0.0007 | ||
| Testosterone | 0.24 | Intercept | 3.8278 | 2.4543; 5.2013 |
| Age | 0.0103 | 0.0052; 0.0155 | ||
| (log)DHEA | 0.14 | Intercept | 1.2245 | 1.1330; 1.3160 |
| Age | 0.0005 | 0.0001; 0.0008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmi, A.; Galligioni, V.; Govoni, N.; Bertocchi, M.; Aniballi, C.; Bacci, M.L.; Sánchez-Morgado, J.M.; Ventrella, D. Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice. Animals 2020, 10, 2408. https://doi.org/10.3390/ani10122408
Elmi A, Galligioni V, Govoni N, Bertocchi M, Aniballi C, Bacci ML, Sánchez-Morgado JM, Ventrella D. Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice. Animals. 2020; 10(12):2408. https://doi.org/10.3390/ani10122408
Chicago/Turabian StyleElmi, Alberto, Viola Galligioni, Nadia Govoni, Martina Bertocchi, Camilla Aniballi, Maria Laura Bacci, José M. Sánchez-Morgado, and Domenico Ventrella. 2020. "Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice" Animals 10, no. 12: 2408. https://doi.org/10.3390/ani10122408
APA StyleElmi, A., Galligioni, V., Govoni, N., Bertocchi, M., Aniballi, C., Bacci, M. L., Sánchez-Morgado, J. M., & Ventrella, D. (2020). Quantification of Hair Corticosterone, DHEA and Testosterone as a Potential Tool for Welfare Assessment in Male Laboratory Mice. Animals, 10(12), 2408. https://doi.org/10.3390/ani10122408







