Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress
Abstract
Simple Summary
Abstract
1. Introduction
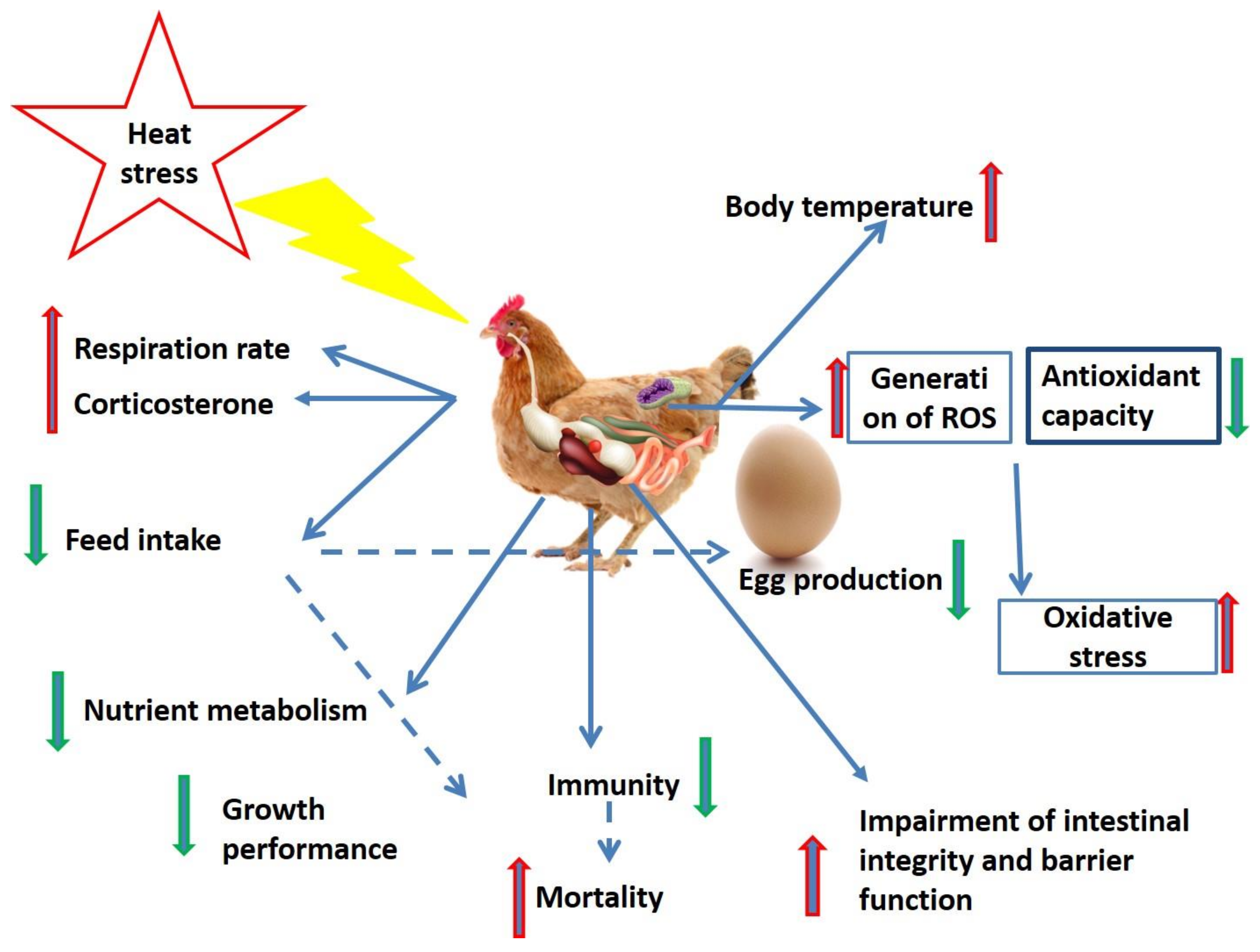
2. Heat Stress
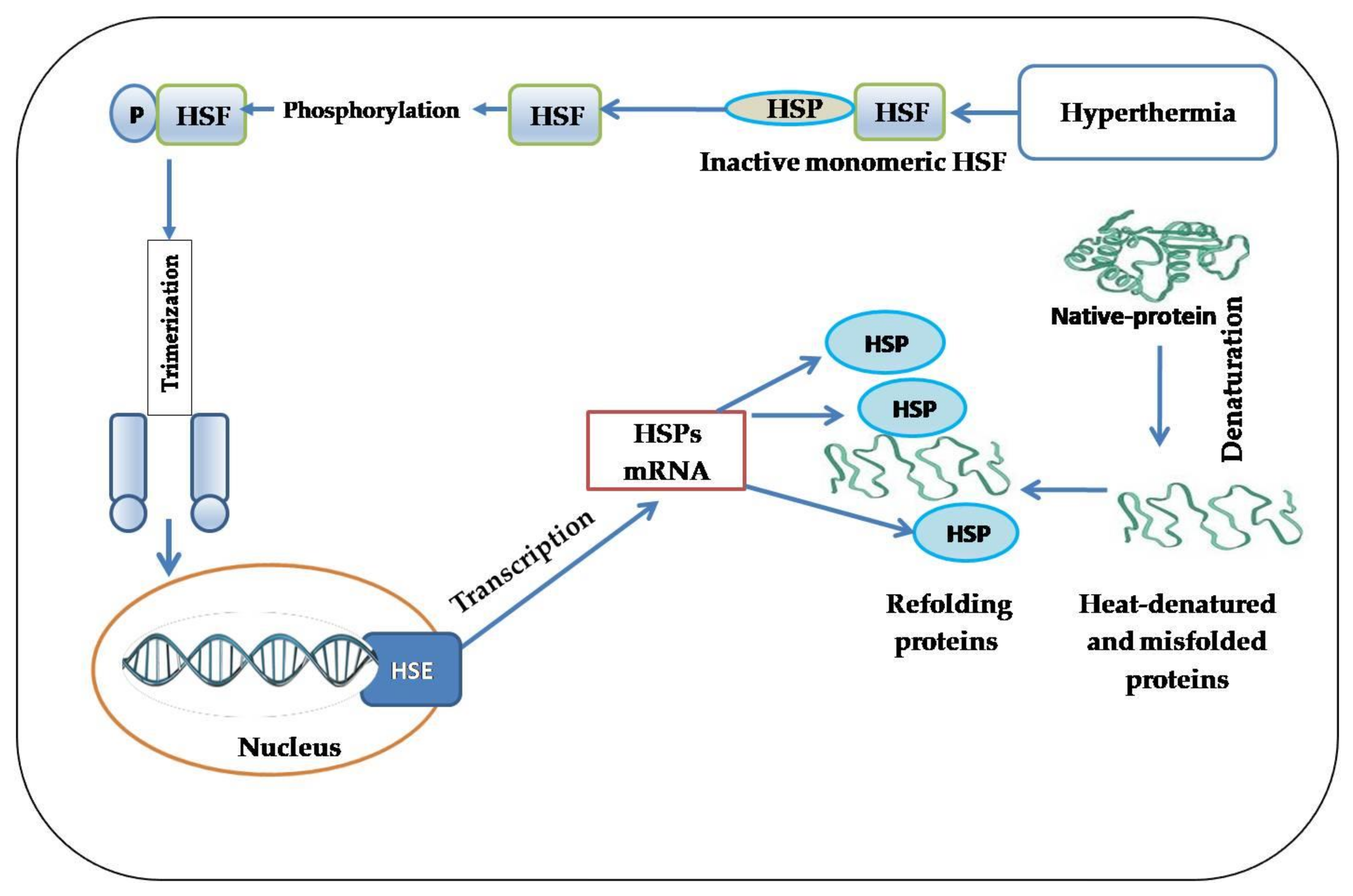
3. A brief History of HSPs
4. HSPs Regulate Apoptotic and Antiapoptotic Signaling Pathways
5. HSPs Mediate Thermotolerance
6. HSPs Modulate Cellular Redox Homeostasis under Heat-Stress Conditions
7. Nutritional and Pharmacological Tuning of HSPs in Chicken
8. Cytoprotective Role of HSPs against Heat-Stress-Induced Impairment of Intestinal Integrity
9. Cytoprotective Role of HSPs against Heat-Stress-Induced Impairment of Immune Function
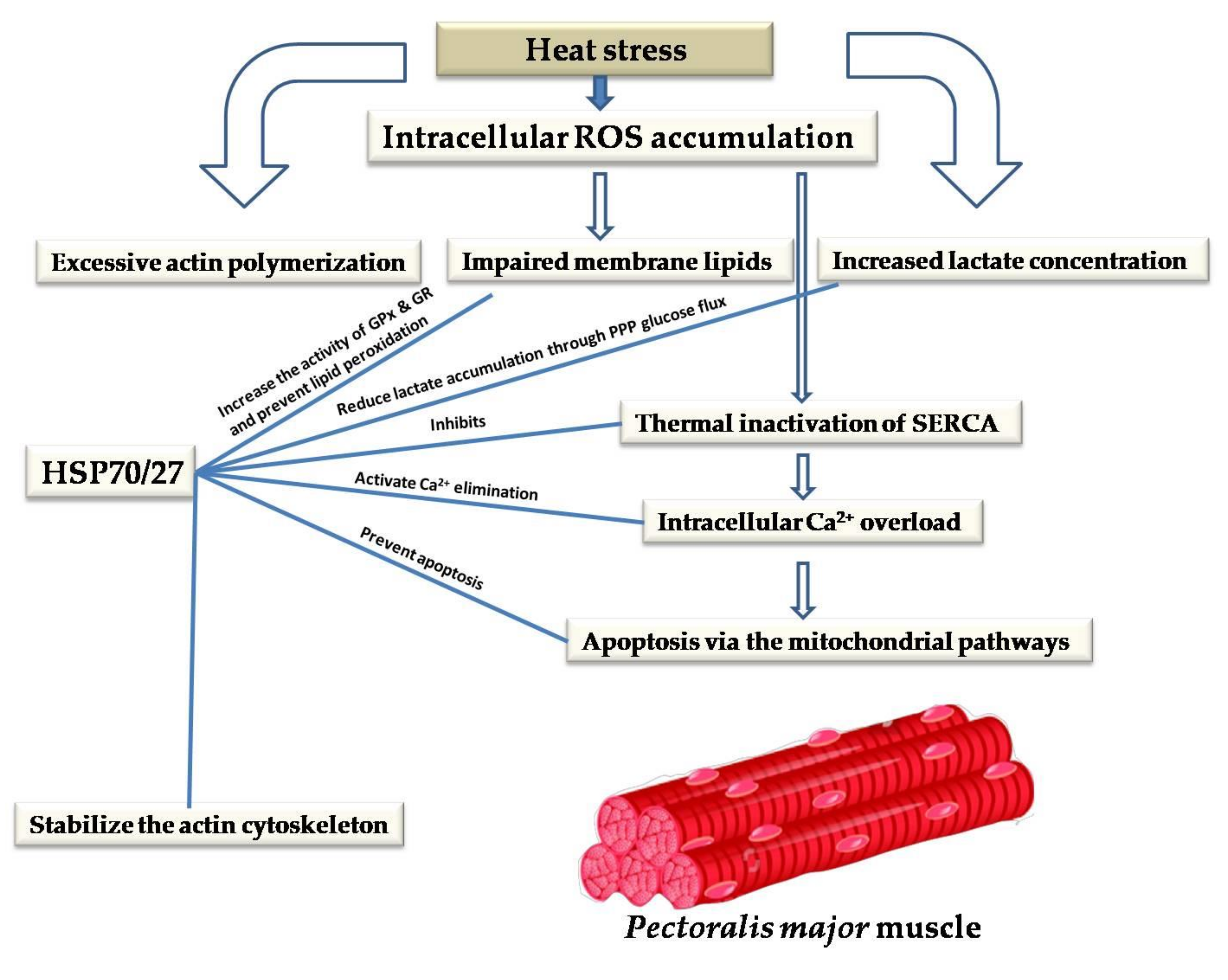
10. Cytoprotective Role of HSPs against Heat-Stress-Induced Impairment of Skeletal and Cardiac Muscles
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roushdy, E.M.; Zaglool, A.W.; El-Tarabany, M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018, 74, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.-U.; Hlodan, R.; Langer, T. Molecular chaperones in protein folding: The art of avoiding sticky situations. Trends Biochem. Sci. 1994, 19, 20–25. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Budagova, K.R.; Bryantsev, A.L.; Latchman, D.S. Heat shock protein 70 or heat shock protein 27 overexpressed in human endothelial cells during posthypoxic reoxygenation can protect from delayed apoptosis. Cell Stress Chaperones 2003, 8, 335–347. [Google Scholar] [CrossRef]
- Wegele, H.; Müller, L.; Buchner, J. Hsp70 and Hsp90—a relay team for protein folding. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–44. [Google Scholar]
- Rafiee, P.; Theriot, M.E.; Nelson, V.M.; Heidemann, J.; Kanaa, Y.; Horowitz, S.A.; Rogaczewski, A.; Johnson, C.P.; Ali, I.; Shaker, R. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am. J. Physiol. Physiol. 2006, 291, C931–C945. [Google Scholar] [CrossRef]
- Voellmy, R.; Boellmann, F. Chaperone regulation of the heat shock protein response. In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks; Springer: Berlin/Heidelberg, Germany, 2007; pp. 89–99. [Google Scholar]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef]
- Samali, A.; Orrenius, S. Heat shock proteins: Regulators of stress response and apoptosis. Cell Stress Chaperones 1998, 3, 228–236. [Google Scholar] [CrossRef]
- Tang, S.; Yin, B.; Song, E.; Chen, H.; Cheng, Y.; Zhang, X.; Bao, E.; Hartung, J. Aspirin upregulates αB-Crystallin to protect the myocardium against heat stress in broiler chickens. Sci. Rep. 2016, 6, 37273. [Google Scholar] [CrossRef]
- Yin, B.; Di, L.; Tang, S.; Bao, E. Vitamin CNa enhances the antioxidant ability of chicken myocardium cells and induces heat shock proteins to relieve heat stress injury. Res. Vet. Sci. 2020, 133, 124–130. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, J.; Song, E.; Lv, Y.; Tang, S.; Zhang, X.; Kemper, N.; Hartung, J.; Bao, E. In vitro evaluation of aspirin-induced HspB1 against heat stress damage in chicken myocardial cells. Cell Stress Chaperones 2016, 21, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kamboh, A.A.; Hang, S.Q.; Bakhetgul, M.; Zhu, W.-Y. Effects of genistein and hesperidin on biomarkers of heat stress in broilers under persistent summer stress. Poult. Sci. 2013, 92, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.E.; Shehata, A.M.; Negm, S.S.; Abd El-Hack, M.E.; Amer, M.S.; Khafaga, A.F.; Bin-Jumah, M.; Allam, A.A. The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: A review. Rev. Aquac. 2020, 12, 250–2267. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.Z.; Edens, F.W.; Eisen, E.J.; Havenstein, G.B. Effect of ascorbic acid and acute heat exposure on heat shock protein 70 expression by young white Leghorn chickens. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 329–335. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Chen, L.; Fang, F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019, 85, 102420. [Google Scholar] [CrossRef]
- Zaglool, A.W.; Roushdy, E.M.; El-Tarabany, M.S. Impact of strain and duration of thermal stress on carcass yield, metabolic hormones, immunological indices and the expression of HSP90 and Myogenin genes in broilers. Res. Vet. Sci. 2019, 122, 193–199. [Google Scholar] [CrossRef]
- Tan, G.-Y.; Yang, L.; Fu, Y.-Q.; Feng, J.-H.; Zhang, M.-H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010, 89, 115–122. [Google Scholar] [CrossRef]
- Adomako, K.; Habashy, W.S.; Milfort, M.; Fuller, A.; Rekaya, R.; Aggrey, S.E. Transcriptome analysis of genes in the protein biosynthesis and ubiquitin-proteosome pathways in meat-type chickens under heat stress. In Proceedings of the 25th World’s Poultry Congress, Beijing, China, 5–9 September 2016. [Google Scholar]
- Gu, Z.T.; Wang, H.; Li, L.; Liu, Y.S.; Deng, X.B.; Huo, S.F.; Yuan, F.F.; Liu, Z.F.; Tong, H.S.; Su, L. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci. Rep. 2014, 4, 4469. [Google Scholar] [CrossRef]
- Li, L.; Tan, H.; Gu, Z.; Liu, Z.; Geng, Y.; Liu, Y.; Tong, H.; Tang, Y.; Qiu, J.; Su, L. Heat stress induces apoptosis through a Ca2+-mediated mitochondrial apoptotic pathway in human umbilical vein endothelial cells. PLoS ONE 2014, 9, e111083. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Yoshiki, Y.; Akiba, Y.; Toyomizu, M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005, 84, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, M.; Zheng, S.; Xie, P.; Ma, A. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poult. Sci. 2008, 87, 2133–2139. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. Int. J. Biometeorol. 2019, 63, 1569–1584. [Google Scholar] [CrossRef]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.-R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Endong, B.; Yuanying, G.; Hartung, J.; Xubin, F.; Xiaojun, W.; Haitao, Z.; Zhiliang, W. Relation Between Pathologic Damages and HSP70 Changes in Acute Heat Stressed Broilers. Zhongguo Nongye Kexue 2004, 37, 301–305. [Google Scholar]
- Yu, J.; Bao, E.; Yan, J.; Lei, L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones 2008, 13, 327–335. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Kiang, J.; Smallridge, R.; Galloway, R.; Shea-Donohue, T. Induction of heat-shock protein 72 protects against ischemia/reperfusion in rat small intestine. Gastroenterology 1995, 109, 505–515. [Google Scholar] [CrossRef]
- Beck, S.C.; Paidas, C.N.; Mooney, M.L.; Deitch, E.A.; De Maio, A. Presence of the stress-inducible form of hsp-70 (hsp-72) in normal rat colon. Shock 1995, 3, 398–402. [Google Scholar] [PubMed]
- Tsuruma, T.; Yagihashi, A.; Watanabe, N.; Yajima, T.; Kameshima, H.; Araya, J.; Hirata, K. Heat-shock protein-73 protects against small intestinal warm ischemiareperfusion injury in the rat. Surgery 1999, 125, 385–395. [Google Scholar] [CrossRef]
- Tsukimi, Y.; Okabe, S. Recent advances in gastrointestinal pathophysiology: Role of heat shock proteins in mucosal defense and ulcer healing. Biol. Pharm. Bull. 2001, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.M.; Rossi, J.M.; Golic, K.; McGarry, T.; Lindquist, S. Changes in hsp70 alter thermotolerance and heat-shock regulation in Drosophila. New Biol. 1991, 3, 1106–1120. [Google Scholar] [PubMed]
- Habashy, W.S.; Milfort, M.C.; Fuller, A.L.; Attia, Y.A.; Rekaya, R.; Aggrey, S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017, 61, 2111–2118. [Google Scholar] [CrossRef]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef]
- Zhangyong, N.; Sidang, L.; Deming, Z.; Xun, T.; Shumin, Y. The influence of heat stress on morphological and ultrastructure change of respiratory, digestive and endocrine tissues in broilers. Acta Vet. Zootech. Sin. 2003, 34, 558–561. [Google Scholar]
- Hao, Y.; Gu, X.H.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012, 91, 781–789. [Google Scholar] [CrossRef]
- Yi, G.; Li, L.; Luo, M.; He, X.; Zou, Z.; Gu, Z.; Su, L. Heat stress induces intestinal injury through lysosome-and mitochondria-dependent pathway in vivo and in vitro. Oncotarget 2017, 8, 40741. [Google Scholar] [CrossRef]
- Gu, X.H.; Hao, Y.; Wang, X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012, 91, 790–799. [Google Scholar] [CrossRef]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Ullengala, R.; Amirthalingam, V. Heat Shock Protein and thermal stress in chicken. In Heat Shock Proteins in Veterinary Medicine and Sciences; Springer: Berlin/Heidelberg, Germany, 2017; pp. 179–193. [Google Scholar]
- Baird, N.A.; Douglas, P.M.; Simic, M.S.; Grant, A.R.; Moresco, J.J.; Wolff, S.C.; Yates, J.R.; Manning, G.; Dillin, A. HSF-1–mediated cytoskeletal integrity determines thermotolerance and life span. Science 2014, 346, 360–363. [Google Scholar] [CrossRef]
- Samali, A.; Holmberg, C.I.; Sistonen, L.; Orrenius, S. Thermotolerance and cell death are distinct cellular responses to stress: Dependence on heat shock proteins. FEBS Lett. 1999, 461, 306–310. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Garcia, A.A.P.; Farias Filho, R.V.; Souza, T.M.; de Oliveira, E.R.; de Oliveira, E.B.; do Nascimento, C.S.; Meneghetti, C.; Wenceslau, A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS ONE 2017, 12, e0186083. [Google Scholar] [CrossRef] [PubMed]
- Bijur, G.N.; Jope, R.S. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3β in the regulation of HSF-1 activity. J. Neurochem. 2000, 75, 2401–2408. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Xi, L.; Liu, H.-C.; Odle, J.; Luo, X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PLoS ONE 2014, 9, e102204. [Google Scholar] [CrossRef]
- Meng, X.; Jerome, V.; Devin, J.; Baulieu, E.E.; Catelli, M.G. Cloning of Chicken hsp90β: The Only Vertebrate hsp90 Insensitive to Heat Shock. Biochem. Biophys. Res. Commun. 1993, 190, 630–636. [Google Scholar] [CrossRef]
- Latchman, D.S. Heat shock proteins and cardiac protection. Cardiovasc. Res. 2001, 51, 637–646. [Google Scholar] [CrossRef]
- Li, S.; Chien, S.; Brånemark, P. Heat shock-induced necrosis and apoptosis in osteoblasts. J. Orthop. Res. 1999, 17, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Rupinder, S.K.; Gurpreet, A.K.; Manjeet, S. Cell suicide and caspases. Vascul. Pharmacol. 2007, 46, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018, 45, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S. Ordering the cytochrome c–initiated caspase cascade: Hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9–dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Lin, K.M.; Lin, B.; Lian, I.Y.; Mestril, R.; Scheffler, I.E.; Dillmann, W.H. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation 2001, 103, 1787–1792. [Google Scholar] [CrossRef]
- Quadrilatero, J.; Alway, S.E.; Dupont-Versteegden, E.E. Skeletal muscle apoptotic response to physical activity: Potential mechanisms for protection. Appl. Physiol. Nutr. Metab. 2011, 36, 608–617. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Beere, H.M. Death versus survival: Functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J. Clin. Investig. 2005, 115, 2633–2639. [Google Scholar] [CrossRef]
- Pandey, P.; Saleh, A.; Nakazawa, A.; Kumar, S.; Srinivasula, S.M.; Kumar, V.; Weichselbaum, R.; Nalin, C.; Alnemri, E.S.; Kufe, D. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000, 19, 4310–4322. [Google Scholar] [CrossRef]
- Bruey, J.-M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.-P.; Kroemer, G.; Solary, E. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Smaili, S.S.; Hsu, Y.T.; Sanders, K.M.; Russell, J.T.; Youle, R.J. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ. 2001, 8, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Chen, S.-T.; Tafani, M.; Snyder, J.W.; Farber, J.L. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J. Biol. Chem. 1998, 273, 7770–7775. [Google Scholar] [CrossRef] [PubMed]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Zhou, Y.; Wang, Y.; Wang, S.; Zhang, W. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. 2012, 321, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Terada, K.; Oyadomari, S.; Mori, M. hsp70-DnaJ chaperone pair prevents nitric oxide-and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004, 11, 390–402. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Xing, D.; Chen, W.R. Inhibition of the JNK/Bim pathway by Hsp70 prevents Bax activation in UV-induced apoptosis. FEBS Lett. 2010, 584, 4672–4678. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Rai, S.; Dadhania, A.; Jonnakuti, S.; Concepcion, W.; Thakor, A.S. Reversing Acute Kidney Injury Using Pulsed Focused Ultrasound and MSC Therapy: A Role for HSP-Mediated PI3K/AKT Signaling. Mol. Ther. Methods Clin. Dev. 2020, 17, 683–694. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Saving, K.L.; Gujrati, M.; Fassett, D.; Klopfenstein, J.D.; Dinh, D.H.; Rao, J.S. Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiol. Dis. 2008, 32, 486–498. [Google Scholar] [CrossRef]
- Mure, H.; Matsuzaki, K.; Kitazato, K.T.; Mizobuchi, Y.; Kuwayama, K.; Kageji, T.; Nagahiro, S. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Oncol. 2009, 12, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, F.; Yin, P.; Wan, C.; He, S.; Liu, X.; Zhao, H.; Liu, T.; Xu, J.; Guo, S. Inhibition of heat-induced apoptosis in rat small intestine and IEC-6 cells through the AKT signaling pathway. BMC Vet. Res. 2013, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Cohen, P.; Alessi, D.R. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem. J. 1998, 336, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Fujiyoshi, T.; Fukui, Y.; Matsuzaki, H.; Yamamoto, T.; Ono, Y.; Andjelkovic, M.; Hemmings, B.A.; Kikkawa, U. Activation of protein kinase B induced by H2O2 and heat shock through distinct mechanisms dependent and independent of phosphatidylinositol 3-kinase. J. Biochem. 1999, 126, 1136–1143. [Google Scholar] [CrossRef]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef]
- Solit, D.B.; Basso, A.D.; Olshen, A.B.; Scher, H.I.; Rosen, N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003, 63, 2139–2144. [Google Scholar]
- Wu, R.; Kausar, H.; Johnson, P.; Montoya-Durango, D.E.; Merchant, M.; Rane, M.J. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J. Biol. Chem. 2007, 282, 21598–21608. [Google Scholar] [CrossRef] [PubMed]
- Maira, S.-M.; Galetic, I.; Brazil, D.P.; Kaech, S.; Ingley, E.; Thelen, M.; Hemmings, B.A. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 2001, 294, 374–380. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef]
- Mogk, A.; Ruger-Herreros, C.; Bukau, B. Cellular Functions and Mechanisms of Action of Small Heat Shock Proteins. Annu. Rev. Microbiol. 2019, 73, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lv, Y.; Chen, H.; Adam, A.; Cheng, Y.; Hartung, J.; Bao, E. Comparative analysis of αB-crystallin expression in heat-stressed myocardial cells in vivo and in vitro. PLoS ONE 2014, 9, e86937. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Tang, S.; Xu, J.; Sun, J.; Zhang, X.; Li, Y.; Bao, E. CRYAB protects cardiomyocytes against heat stress by preventing caspase-mediated apoptosis and reducing F-actin aggregation. Cell Stress Chaperones 2019, 24, 59–68. [Google Scholar] [CrossRef]
- Fan, G.-C.; Zhou, X.; Wang, X.; Song, G.; Qian, J.; Nicolaou, P.; Chen, G.; Ren, X.; Kranias, E.G. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ. Res. 2008, 103, 1270–1279. [Google Scholar] [CrossRef]
- Kim, D.S.; Ko, Y.J.; Lee, M.W.; Park, H.J.; Park, Y.J.; Kim, D.-I.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones 2016, 21, 1089–1099. [Google Scholar] [CrossRef]
- Wu, D.; Xu, J.; Song, E.; Tang, S.; Zhang, X.; Kemper, N.; Hartung, J.; Bao, E. Acetyl salicylic acid protected against heat stress damage in chicken myocardial cells and may associate with induced Hsp27 expression. Cell Stress Chaperones 2015, 20, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, S.; Song, E.; Yin, B.; Wu, D.; Bao, E. Hsp70 expression induced by Co-Enzyme Q10 protected chicken myocardial cells from damage and apoptosis under in vitro heat stress. Poult. Sci. 2017, 96, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, I.J.; McMillan, D.R. Stress (heat shock) proteins: Molecular chaperones in cardiovascular biology and disease. Circ. Res. 1998, 83, 117–132. [Google Scholar] [CrossRef]
- Tupling, A.R.; Bombardier, E.; Vigna, C.; Quadrilatero, J.; Fu, M. Interaction between Hsp70 and the SR Ca2+ pump: A potential mechanism for cytoprotection in heart and skeletal muscle. Appl. Physiol. Nutr. Metab. 2008, 33, 1023–1032. [Google Scholar] [CrossRef]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Yahav, S. Alleviating heat stress in domestic fowl: Different strategies. Worlds. Poult. Sci. J. 2009, 65, 719–732. [Google Scholar] [CrossRef]
- Somero, G.N. Proteins and temperature. Annu. Rev. Physiol. 1995, 57, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Klemenz, R.; Hultmark, D.; Gehring, W.J. Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J. 1985, 4, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef]
- Yamashita, M.; Yabu, T.; Ojima, N. Stress protein HSP70 in fish. Aqua-BioSci. Monogr. 2010, 3, 111–141. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Brunsting, J.F.; Stege, G.J.J.; Burgman, P.W.J.J.; Konings, A.W.T. Thermal Protein Denaturation and Protein Aggregation in Cells Made Thermotolerant by Various Chemicals: Role of Heat Shock Proteins. Exp. Cell Res. 1995, 219, 536–546. [Google Scholar] [CrossRef]
- Buchner, J. Hsp90 & Co.—A holding for folding. Trends Biochem. Sci. 1999, 24, 136–141. [Google Scholar]
- Wang, S.; Edens, F.W. Stress-induced heat-shock protein synthesis in peripheral leukocytes of turkeys, Meleagris gallopavo. Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 621–628. [Google Scholar] [CrossRef]
- Wang, S.; Edens, F.W. Heat conditioning induces heat shock proteins in broiler chickens and turkey poults. Poult. Sci. 1998, 77, 1636–1645. [Google Scholar] [CrossRef]
- Li, G.C.; Werb, Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc. Natl. Acad. Sci. USA 1982, 79, 3218–3222. [Google Scholar] [CrossRef]
- Li, G.C.; Laszlo, A. Amino acid analogs while inducing heat shock proteins sensitize CHO cells to thermal damage. J. Cell. Physiol. 1985, 122, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqil, A.; Zulkifli, I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009, 88, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.-B.; Dalab, A.E.S.; Yahya, I.E.; Althnaian, T.A.; Al-ramadan, S.Y.; Ali, A.M.; Albokhadaim, I.F.; El-Bahr, S.M.; Al Busadah, K.A.; Hannon, K.M. Thermal manipulation during broiler chicken embryogenesis: Effect on mRNA expressions of Hsp108, Hsp70, Hsp47 and Hsf-3 during subsequent post-hatch thermal challenge. Res. Vet. Sci. 2015, 103, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S.; Shamay, A.; Horev, G.; Bar-Ilan, D.; Genina, O.; Friedman-Einat, M. Effect of acquisition of improved thermotolerance on the induction of heat shock proteins in broiler chickens. Poult. Sci. 1997, 76, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Loyau, T.; Bedrani, L.; Berri, C.; Métayer-Coustard, S.; Praud, C.; Coustham, V.; Mignon-Grasteau, S.; Duclos, M.J.; Tesseraud, S.; Rideau, N. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: A review. Animal 2015, 9, 76–85. [Google Scholar] [CrossRef]
- Hatayama, T.; Kano, E.; Taniguchi, Y.; Nitta, K.; Wakatsuki, T.; Kitamura, T.; Imahara, H. Role of heat-shock proteins in the induction of thermotolerance in Chinese hamster V79 cells by heat and chemical agents. Int. J. Hyperth. 1991, 7, 61–74. [Google Scholar] [CrossRef]
- Labunskay, G.; Meiri, N. R-Ras3/(M-Ras) is involved in thermal adaptation in the critical period of thermal control establishment. J. Neurobiol. 2006, 66, 56–70. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B. Thermal manipulation during broiler chicken embryogenesis increases basal mRNA levels and alters production dynamics of heat shock proteins 70 and 60 and heat shock factors 3 and 4 during thermal stress. Poult. Sci. 2018, 97, 3661–3670. [Google Scholar] [CrossRef]
- Al-Zhgoul, M.-B.; Dalab, A.E.S.; Ababneh, M.M.; Jawasreh, K.I.; Al Busadah, K.A.; Ismail, Z.B. Thermal manipulation during chicken embryogenesis results in enhanced Hsp70 gene expression and the acquisition of thermotolerance. Res. Vet. Sci. 2013, 95, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.-B.; Ismail, Z.B.; Dalab, A.E.S.; Al-Ramadan, A.; Althnaian, T.A.; Al-ramadan, S.Y.; Ali, A.M.; Albokhadaim, I.F.; Al Busadah, K.A.; Eljarah, A.; et al. Hsp90, Hsp60 and HSF-1 genes expression in muscle, heart and brain of thermally manipulated broiler chicken. Res. Vet. Sci. 2015, 99, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Leandro, N.S.M.; Gonzales, E.; Ferro, J.A.; Ferro, M.I.T.; Givisiez, P.E.N.; Macari, M. Expression of heat shock protein in broiler embryo tissues after acute cold or heat stress. Mol. Reprod. Dev. Inc. Gamete Res. 2004, 67, 172–177. [Google Scholar] [CrossRef]
- Piestun, Y.; Halevy, O.; Shinder, D.; Ruzal, M.; Druyan, S.; Yahav, S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J. Therm. Biol. 2011, 36, 469–474. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Saleh, K.M.; Ababneh, M.M.K. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult. Sci. 2019, 98, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Basaki, M.; Sahraiy, N.; Keykavusi, K.; Akbari, G.; Shahbazfar, A.A.; Kianifard, D. Differential expression of small heat shock proteins in the brain of broiler embryo; the effects of embryonic thermal manipulation. J. Therm. Biol. 2020, 93, 102719. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.N.; Lambert, H.; Hickey, E.; Weber, L.A.; Landry, J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell. Biol. 1995, 15, 505–516. [Google Scholar] [CrossRef]
- Jäättelä, M. Escaping cell death: Survival proteins in cancer. Exp. Cell Res. 1999, 248, 30–43. [Google Scholar] [CrossRef]
- Xu, Q.; Schett, G.; Li, C.; Hu, Y.; Wick, G. Mechanical stress–induced heat shock protein 70 expression in vascular smooth muscle cells is regulated by Rac and Ras small G proteins but not mitogen-activated protein kinases. Circ. Res. 2000, 86, 1122–1128. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Zhang, Z.W.; Qu, J.P.; Yao, H.D.; Li, M.; Li, S.; Xu, S.W. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones 2014, 19, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.M.M.; Tarkhan, A.H.; Al-Zghoul, M.B. Embryonic Thermal Manipulation Affects the Antioxidant Response to Post-Hatch Thermal Exposure in Broiler Chickens. Animals 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J. Neurochem. 1997, 68, 2061–2069. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef]
- Matsuo, K.; Hosoda, K.; Tanaka, J.; Yamamoto, Y.; Imahori, T.; Nakai, T.; Irino, Y.; Shinohara, M.; Sasayama, T.; Kohmura, E. Abstract WP342: Activate the Pentose Phosphate Pathway to Reduce the Cerebral Ischemia/Reperfusion Injury: The Impact of Heat Shock Protein 27 Phosphorylation. Stroke 2019, 50, AWP342. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Bánfi, B.; Molnár, G.; Maturana, A.; Steger, K.; Hegedûs, B.; Demaurex, N.; Krause, K.-H. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef]
- Hu, Y.; Rosen, D.G.; Zhou, Y.; Feng, L.; Yang, G.; Liu, J.; Huang, P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005, 280, 39485–39492. [Google Scholar] [CrossRef]
- Vu, H.V.; Acosta, T.J. Catalase and glutathione peroxidase expression in bovine corpus luteum during the estrous cycle and their modulation by prostaglandin F2α and H2O2. Anim. Reprod. 2018, 11, 74–84. [Google Scholar]
- Tandogan, B.; Kuruüzüm-Uz, A.; Sengezer, C.; Güvenalp, Z.; Demirezer, L.Ö.; Nuray Ulusu, N. In vitro effects of rosmarinic acid on glutathione reductase and glucose 6-phosphate dehydrogenase. Pharm. Biol. 2011, 49, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wharton, W.; Moseley, P.; Shi, H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 2007, 12, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.Z.; Edens, F.W.; Eisen, E.J.; Havenstein, G.B. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 137, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Banan, A.; Fitzpatrick, L.; Zhang, Y.; Keshavarzian, A. OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic. Biol. Med. 2001, 30, 287–298. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, Y.; Xu, J.; Qin, J.; Li, C.; Ma, X.; Zhang, Z.; Zhang, B.; Tang, J. Evaluating the protective mechanism of panax notoginseng saponins against oxidative stress damage by quantifying the biomechanical properties of single cell. Anal. Chim. Acta 2019, 1048, 186–193. [Google Scholar] [CrossRef]
- Huot, J.; Houle, F.; Spitz, D.R.; Landry, J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996, 56, 273–279. [Google Scholar]
- Venkatakrishnan, C.D.; Tewari, A.K.; Moldovan, L.; Cardounel, A.J.; Zweier, J.L.; Kuppusamy, P.; Ilangovan, G. Heat shock protects cardiac cells from doxorubicin-induced toxicity by activating p38 MAPK and phosphorylation of small heat shock protein 27. Am. J. Physiol. Circ. Physiol. 2006, 291, H2680–H2691. [Google Scholar] [CrossRef]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.; He, J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Ahmad, H.; Zhang, L.; Wang, Z.; Wang, T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agric. 2018, 98, 3715–3721. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Kumbhar, S.; Hamid, M.; Afzal, S.; Parveen, F.; Liu, Y.; Shu, H.; Mengistu, B.M.; Huang, K. Effects of Selenium-Enriched Probiotics on Heart Lesions by Influencing the mRNA Expressions of Selenoproteins and Heat Shock Proteins in Heat Stressed Broiler Chickens. Pak. Vet. J. 2016, 36, 460–464. [Google Scholar]
- Tang, S.; Yin, B.; Xu, J.; Bao, E. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens. Oxid. Med. Cell. Longev. 2018, 2018, 7014126. [Google Scholar] [CrossRef]
- Kumbhar, S.; Khan, A.Z.; Parveen, F.; Nizamani, Z.A.; Siyal, F.A.; El-Hack, M.E.A.; Gan, F.; Liu, Y.; Hamid, M.; Nido, S.A.; et al. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express 2018, 8, 112. [Google Scholar] [CrossRef]
- Ramiah, S.K.; Atta Awad, E.; Hemly, N.I.M.; Ebrahimi, M.; Joshua, O.; Jamshed, M.; Saminathan, M.; Soleimani, A.F.; Idrus, Z. Effects of zinc oxide nanoparticles on regulatory appetite and heat stress protein genes in broiler chickens subjected to heat stress. J. Anim. Sci. 2020, 98, skaa300. [Google Scholar] [CrossRef]
- Hu, H.; Chen, L.; Dai, S.; Li, J.; Bai, X. Effect of Glutamine on Antioxidant Capacity and Lipid Peroxidation in the Breast Muscle of Heat-stressed Broilers via Antioxidant Genes and HSP70 Pathway. Animals 2020, 10, 404. [Google Scholar] [CrossRef]
- Belal, S.A.; Kang, D.R.; Cho, E.S.R.; Park, G.H.; Shim, K.S. Taurine Reduces Heat Stress by Regulating the Expression of Heat Shock Proteins in Broilers Exposed to Chronic Heat. Brazilian J. Poult. Sci. 2018, 20, 479–486. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Lu, C.; Bai, K.; Zhang, L.; Wang, T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 2015, 63, 3880–3886. [Google Scholar] [CrossRef]
- Hajati, H.; Hassanabadi, A.; Golian, A.; Nassiri-Moghaddam, H.; Nassiri, M.R. The effect of grape seed extract and vitamin C feed supplementation on some blood parameters and HSP70 gene expression of broiler chickens suffering from chronic heat stress. Ital. J. Anim. Sci. 2015, 14, 3273. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Golian, A.; Buyse, J.; Wang, Y.; De Smet, S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fedOriganum compactum andCurcuma xanthorrhiza essential oils. Poult. Sci. 2014, 93, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, S.J.; Baker, M.S.; Buffinton, G.D.; Doe, W.F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Investig. 1996, 98, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Sedghi, S.; Kanofsky, J.; List, T.; Robinson, C.; Ibrahim, C.; Winship, D. Excessive production of reactive oxygen metabolites by inflamed colon: Analysis by chemiluminescence probe. Gastroenterology 1992, 103, 177–185. [Google Scholar] [CrossRef]
- Ruff, J.; Barros, T.L.; Tellez, G., Jr.; Blankenship, J.; Lester, H.; Graham, B.D.; Selby, C.A.M.; Vuong, C.N.; Dridi, S.; Greene, E.S. Research Note: Evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poult. Sci. 2020, 99, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Sander, G.R.; Cummins, A.G.; Powell, B.C. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005, 579, 4851–4855. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci. 1997, 110, 1113–1121. [Google Scholar]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Molecular Adaptations to Exercise, Heat Acclimation, and Thermotolerance: Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Paraskeuas, V.V.; Fegeros, K. Priming of intestinal cytoprotective genes and antioxidant capacity by dietary phytogenic inclusion in broilers. Anim. Nutr. 2020, 6, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Jeon, S.M.; Ha, S.H.; Jang, A.; Son, J.S.; Kim, G.Y.; Kang, H.K.; Kim, J.S. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants 2020, 9, 1032. [Google Scholar] [CrossRef]
- Xu, D.; Li, W.; Huang, Y.; He, J.; Tian, Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz.(PAMK) on immune response in chicken spleen under heat stress. Biol. Trace Elem. Res. 2014, 160, 232–237. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Smith, M.O. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef]
- Xu, D.; Li, B.; Cao, N.; Li, W.; Tian, Y.; Huang, Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget 2017, 8, 70394–70405. [Google Scholar] [CrossRef]
- Pritts, T.A.; Wang, Q.; Sun, X.; Moon, M.R.; Fischer, D.R.; Fischer, J.E.; Wong, H.R.; Hasselgren, P.-O. Induction of the Stress Response In Vivo Decreases Nuclear Factor–Kappa B Activity in Jejunal Mucosa of Endotoxemic Mice. Arch. Surg. 2000, 135, 860–866. [Google Scholar] [CrossRef]
- Dangi, S.S.; Bharati, J.; Samad, H.A.; Bhure, S.K.; Singh, G.; Maurya, V.P.; Sarkar, M.; Kumar, P. Expression Dynamics of Heat Shock Proteins (HSP) in Livestock under Thermal Stress. In Heat Shock Proteins in Veterinary Medicine and Sciences; Springer: Berlin/Heidelberg, Germany, 2017; pp. 37–79. [Google Scholar]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat shock proteins as immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Ramalingam, T.; Srivastava, P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001, 14, 303–313. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Klinkerfuss, G.H.; Terjung, R.L.; Mole, P.A.; Holloszy, J.O. Respiratory capacity of white, red, and intermediate muscle: Adaptative response to exercise. Am. J. Physiol. Content 1972, 222, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Hepple, R.T.; Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: Tailoring the organelle for optimal function. Am. J. Physiol. Physiol. 2012, 302, C629–C641. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Toyomizu, M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS ONE 2013, 8, e64412. [Google Scholar] [CrossRef]
- Koh, T.J.; Escobedo, J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am. J. Physiol. Physiol. 2004, 286, C713–C722. [Google Scholar] [CrossRef]
- Arrigo, A.-P. Human small heat shock proteins: Protein interactomes of homo-and hetero-oligomeric complexes: An update. FEBS Lett. 2013, 587, 1959–1969. [Google Scholar] [CrossRef]
- Morrow, G.; Hightower, L.E.; Tanguay, R.M. Small heat shock proteins: Big folding machines. Cell Stress Chaperones 2015, 20, 207–212. [Google Scholar] [CrossRef]
- Selsby, J.T.; Dodd, S.L. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R134–R139. [Google Scholar] [CrossRef]
- Liu, Y.; Gampert, L.; Nething, K.; Steinacker, J.M. Response and function of skeletal muscle heat shock protein 70. Front Biosci 2006, 11, 2802–2827. [Google Scholar] [CrossRef]
- Gehrig, S.M.; van der Poel, C.; Sayer, T.A.; Schertzer, J.D.; Henstridge, D.C.; Church, J.E.; Lamon, S.; Russell, A.P.; Davies, K.E.; Febbraio, M.A.; et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 2012, 484, 394–398. [Google Scholar] [CrossRef]
- Dremina, E.S.; Sharov, V.S.; Schöneich, C. Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: Possible implications for the ER Ca2+-mediated apoptosis. Biochem. J. 2012, 444, 127–139. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol. 2019, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Kumar, H.; Park, W.; Byun, M.; Lim, D.; Kemp, S.; te Pas, M.F.W.; Kim, J.-M.; Park, J.-E. Cardiac and Skeletal Muscle Transcriptome Response to Heat Stress in Kenyan Chicken Ecotypes Adapted to Low and High Altitudes Reveal Differences in Thermal Tolerance and Stress Response. Front. Genet. 2019, 10, 993. [Google Scholar] [CrossRef] [PubMed]

| Bioactive Compounds and Medicinal Herbs * | Regulation of HSP Expression | Tissue | Reference |
|---|---|---|---|
| Flavonoids genistein and hesperidin | Decreased HSP70 | Breast muscle | [14] |
| Ascorbic acid | Decreased HSP70 | Heart | [17] |
| Resveratrol | Decreased HSP27, HSP70, and HSP90 by attenuating heat-stress-induced overexpression | Bursa of Fabricius and spleen | [145] |
| Increased HSP27 and HSP90 by increasing heat-stress-induced low expression | Thymus | [145] | |
| Decreased HSP70 and HSP90 after 15 days of heat stress by attenuating heat-stress-induced overexpression | Jejunal villi | [146] | |
| Artemisia annua L. (A. annua) | Decreased HSP70 and HSP90 | Breast muscles | [147] |
| Rosemary | Increased HSP70 for 5–10 h; increased HSP27 at 3 h; increased CRYAB at 0 and 3–10 h | Heart | [149] |
| Vitamin E (VE) and selenium (Se) | Increased HSP90, HSP70, and HSP60 with Se and Se + VE supplementation. Increased HSP90 and HSP70 with VE supplementation. | Breast muscle | [150] |
| Zinc oxide nanoparticles | Increased HSP70 &HSP90 | Jejunum, Duodenum, and ileum | [151] |
| Glutamine | Increased HSP70 | Breast muscle | [152] |
| Taurine | Decreased HSP90 | Breast muscle | [153] |
| Curcumin | Decreased HSP70 and HSP90 | Breast muscles | [154] |
| Grape-seed extract (GSE) and vitamin C | Decreased HSP70 | Heart | [155] |
| Origanum compactum and Curcuma xanthorrhiza | Decreased HSP70 | Heart | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. https://doi.org/10.3390/ani10122407
Shehata AM, Saadeldin IM, Tukur HA, Habashy WS. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals. 2020; 10(12):2407. https://doi.org/10.3390/ani10122407
Chicago/Turabian StyleShehata, Abdelrazeq M., Islam M. Saadeldin, Hammed A. Tukur, and Walid S. Habashy. 2020. "Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress" Animals 10, no. 12: 2407. https://doi.org/10.3390/ani10122407
APA StyleShehata, A. M., Saadeldin, I. M., Tukur, H. A., & Habashy, W. S. (2020). Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals, 10(12), 2407. https://doi.org/10.3390/ani10122407







