Proteomic Analysis of the Protective Effect of Early Heat Exposure against Chronic Heat Stress in Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
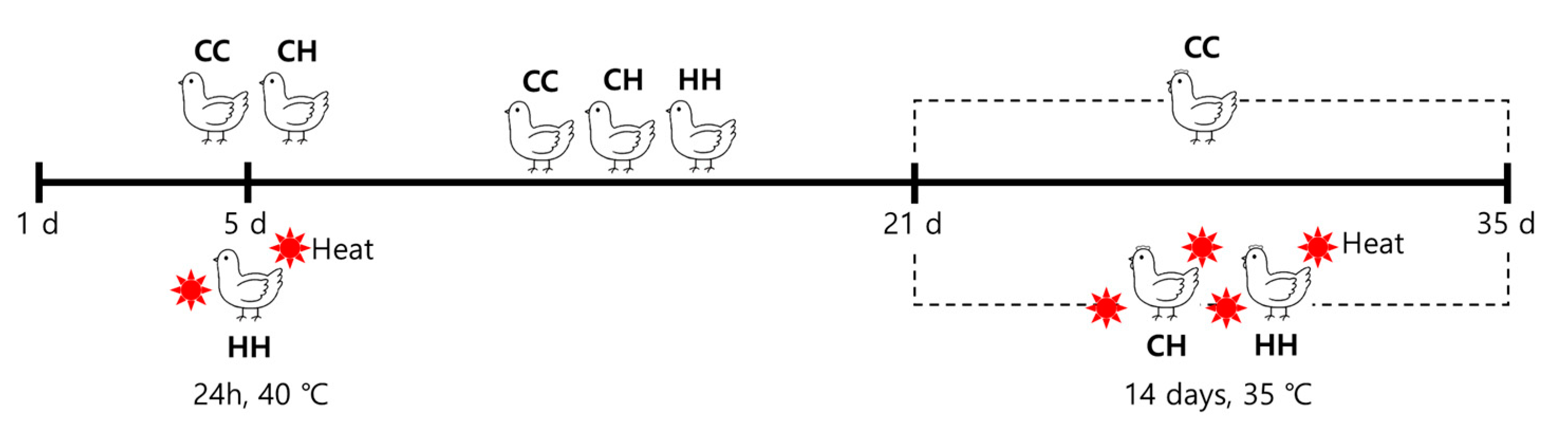
2.1. Animal Experiments
2.2. Protein Extraction and Digestion
2.3. Liquid Chromatography-Tandem Mass Spectrometry Analysis and Data Analysis
2.4. Gene Ontology Enrichment Analysis
2.5. Validation of Proteins by Their Gene Expression
2.6. Statistical Analysis
3. Results
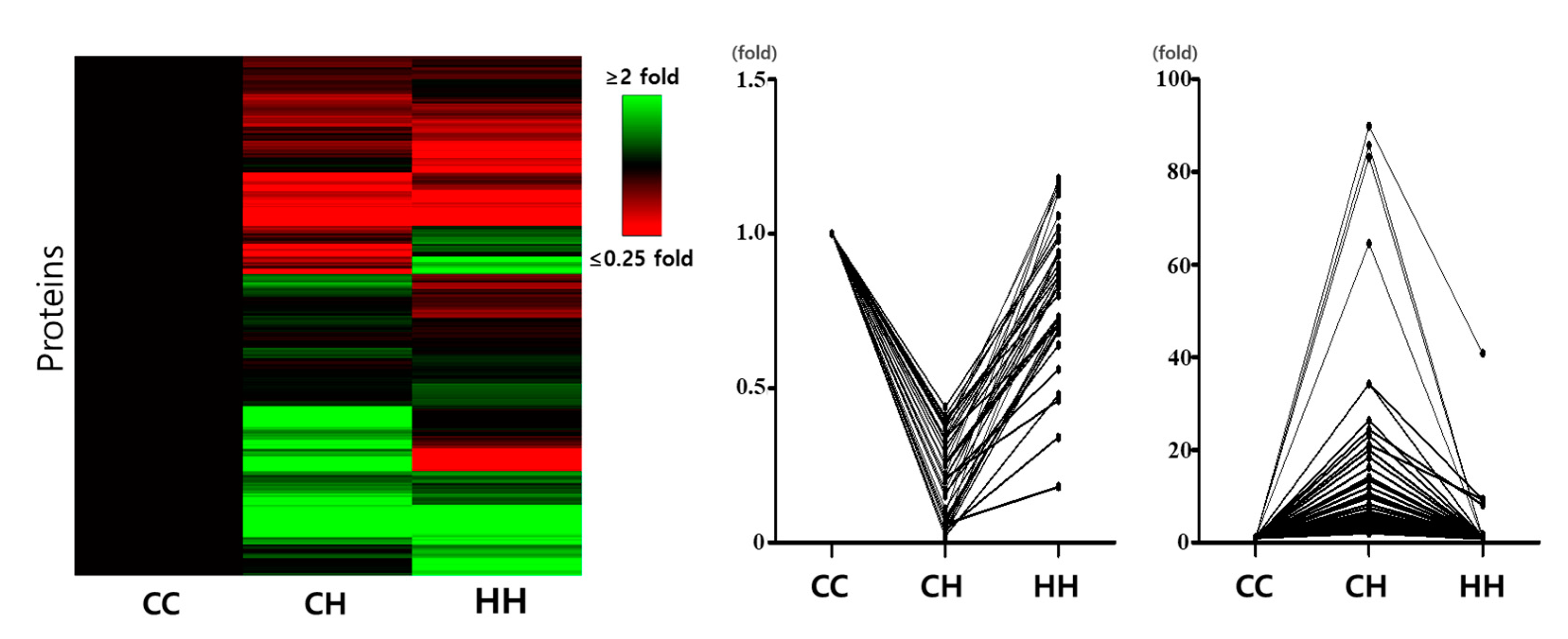
3.1. Differentially Expressed Proteins in Response to Chronic Heat Stress and Early Heat Exposure
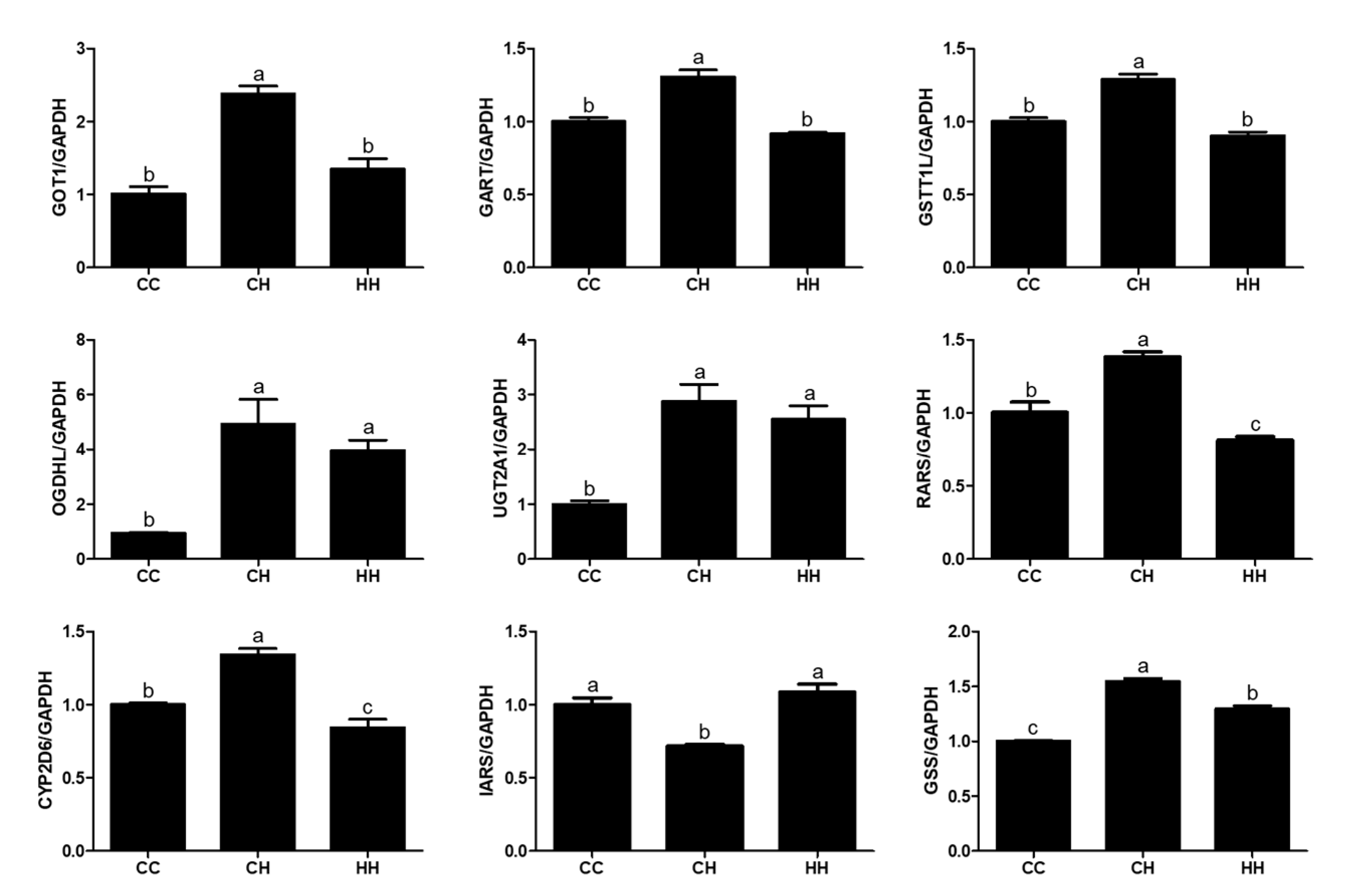
3.2. Gene Expression Analysis for Validation of the Abundant Protein
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, S.O.; Hwangbo, J.; Ryu, C.M.; Yoon, J.S.; Park, B.S.; Kang, H.K.; Seo, O.S.; Chae, H.S.; Choi, H.C.; Choi, Y.H. Effects of extreme heat stress and continuous lighting on growth performance and blood lipid in broiler chickens. J. Kor. Oil Chem. Soc. 2013, 30, 78–87. [Google Scholar] [CrossRef]
- Siegel, H. Physiological stress in birds. Bioscience 1980, 30, 529–534. [Google Scholar] [CrossRef]
- Richards, S.A. Physiology of thermal panting in birds. In Annales de Biologie Animale Biochimie Biophysique; EDP Sciences: Les Ulis, France, 1970; Volume 10, pp. 151–168. [Google Scholar]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.; Sakai, M.; Sá, L.R.M.d.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Reddy, P.G. Early age thermal conditioning improves broiler chick’s response to acute heat stress at marketing age. Am. J. Anim. Vet. Sci. 2012, 7, 1–6. [Google Scholar]
- Tona, K.; Onagbesan, O.; Bruggeman, V.; Collin, A.; Berri, C.; Duclos, M.; Tesseraud, S.; Buyse, J.; Decuypere, E.; Yahav, S. Effects of heat conditioning at d 16 to 18 of incubation or during early broiler rearing on embryo physiology, posthatch growth performance and heat tolerance. Arch. Geflügelkunde 2008, 72, 75–83. [Google Scholar]
- Yahav, S.; Hurwitz, S. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult. Sci. 1996, 75, 402–406. [Google Scholar] [CrossRef]
- He, J.; Xia, C.; He, Y.; Pan, D.; Cao, J.; Sun, Y.; Zeng, X. Proteomic responses to oxidative damage in meat from ducks exposed to heat stress. Food Chem. 2019, 295, 129–137. [Google Scholar] [CrossRef]
- Lopez, V.; van der Heijden, E.; Villar, M.; Michel, A.; Alberdi, P.; Gortázar, C.; Rutten, V.; De La Fuente, J. Comparative proteomics identified immune response proteins involved in response to vaccination with heat-inactivated Mycobacterium bovis and mycobacterial challenge in cattle. Vet. Immunol. Immunophathol. 2018, 206, 54–64. [Google Scholar] [CrossRef]
- Saelao, P.; Wang, Y.; Chanthavixay, G.; Yu, V.; Gallardo, R.A.; Dekkers, J.; Lamont, S.J.; Kelly, T.; Zhou, H. Integrated proteomic and transcriptomic analysis of differential expression of chicken lung tissue in response to NDV infection during heat stress. Genes 2018, 9, 579. [Google Scholar] [CrossRef]
- Victoria Sanz Fernandez, M.; Johnson, J.S.; Abuajamieh, M.; Stoakes, S.K.; Seibert, J.T.; Cox, L.; Kahl, S.; Elsasser, T.H.; Ross, J.W.; Clay Isom, S. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 2015, 3, e12315. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, Y.; Li, J.; Bao, W.; Li, G.; Gao, Y.; Gu, X. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: A proteomic approach. Int. J. Mol. Sci. 2016, 17, 393. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Park, J.; Shim, K. Heat Treatment at an Early Age Has Effects on the Resistance to Chronic Heat Stress on Broilers. Animals 2019, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Oehlmann, M.; Schönheit, P. Novel type of glucose-6-phosphate isomerase in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 2001, 183, 3428–3435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, H.; Takehana, K.; Date, M.; Shinozaki, T.; Raz, A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996, 56, 2960–2963. [Google Scholar]
- Ohtsuka, Y.; Yabunaka, N.; Watanabe, I.; Noro, H.; Fujisawa, H.; Agishi, Y. Thermal stress and diabetic complications. Int. J. Biometeorol. 1995, 38, 57–59. [Google Scholar] [CrossRef]
- Obrosova, I.G. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid. Redox Signal. 2005, 7, 1543–1552. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Marco, R.; Pestaña, A.; Sebastian, J.; Sols, A. Oxaloacetate metabolic crossroads in liver. Enzyme compartmentation and regulation of gluconeogenesis. Mol. Cell. Biochem. 1974, 3, 53–70. [Google Scholar] [CrossRef]
- Zhou, W.; Fujita, M.; Yamamoto, S. Thermoregulatory responses and blood viscosity in dehydrated heat-exposed broilers (Gallus domesticus). J. Therm. Biol. 1999, 24, 185–192. [Google Scholar] [CrossRef]
- Sands, J.; Smith, M. Effects of dietary manganese proteinate or chromium picolinate supplementation on plasma insulin, glucagon, glucose and serum lipids in broiler chickens reared under thermoneutral or heat stress conditions. Int. J. Poult. Sci. 2002, 1, 145–149. [Google Scholar]
- Schnabl, B. Linking intestinal homeostasis and liver disease. J. Curr. Opin. Gastroenterol. 2013, 29, 264. [Google Scholar] [CrossRef] [PubMed]
- Hasan Siddiqui, S.; Kang, D.; Park, J.; Choi, H.W.; Shim, K. Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration. J. Anim. 2020, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.H.; Kang, D.; Park, J.; Khan, M.; Shim, K. Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.; Wheelock, J.; Sanders, S.; Moore, C.; Green, H.; Waldron, M.; Rhoads, R. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J. Dairy Sci. 2011, 94, 5620–5633. [Google Scholar] [CrossRef] [PubMed]
- Biamonti, G.; Caceres, J.F.J.T.i.b.s. Cellular stress and RNA splicing. Trends Biochem. Sci. 2009, 34, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.J.N.r.g. The environmental contribution to gene expression profiles. Nat. Rev. Genet. 2008, 9, 575–581. [Google Scholar] [CrossRef]
- Holcik, M.; Sonenberg, N.J.N.r.M.c.b. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef]
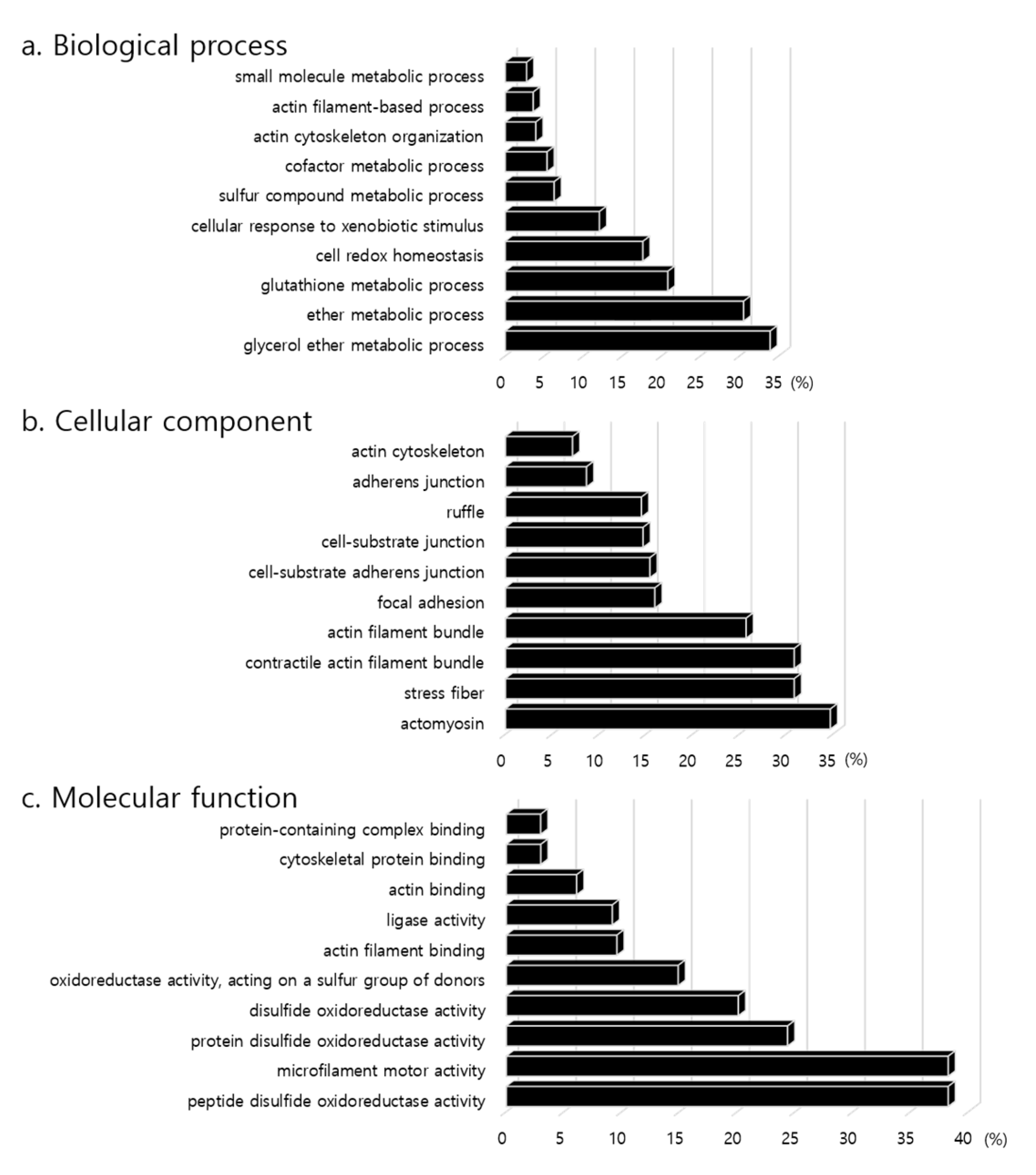

| Term ID | Term | No. | Genes | p-Value |
|---|---|---|---|---|
| Carbohydrate metabolism | ||||
| 00010 | Glycolysis/Gluconeogenesis | 3 | ACSS1L, GPI, HKDC1 | 0.02 |
| 00020 | Citrate cycle (TCA cycle) | 2 | CS, OGDHL | 0.04 |
| 00040 | Pentose and glucoronate interconversions | 2 | SORD, UGT2A1 | 0.02 |
| 00051 | Fructose and mannose metabolism | 2 | HKDC1, SORD | 0.04 |
| 00500 | Starch and sucrose metabolism | 3 | GPI, HKDC1, UGT2A1 | 0.01 |
| 00630 | Glyoxylate and dicarboxylate metabolism | 4 | AMT, CS, PCCA, ACSS1L | 0.004 |
| 00640 | Propanoate metabolism | 2 | ACSS1L, PCCA | 0.04 |
| Metabolism of cofactors and vitamins | ||||
| 00670 | One carbon pool by folate | 2 | AMT, GART | 0.01 |
| 00860 | Porphyrin and chlorophyll metabolism | 2 | UGT2A1, UROD | 0.03 |
| Xenobiotics biodegradation and metabolism | ||||
| 00982 | Drug metabolism (cytochrome P450) | 2 | GSTAL3, GSTT1L | 0.04 |
| 00983 | Drug metabolism (other enzymes) | 3 | LOC769704, GSTAL3, GSTT1L | 0.02 |
| Translation | ||||
| 00970 | Aminoacyl-tRNA biosynthesis | 4 | IARS, QARS, RARS, TARS | 0.001 |
| Metabolism of other amino acids | ||||
| 00480 | Glutathione metabolism | 3 | GSR, GSS, GSTT1L | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.R.; Shim, K.S. Proteomic Analysis of the Protective Effect of Early Heat Exposure against Chronic Heat Stress in Broilers. Animals 2020, 10, 2365. https://doi.org/10.3390/ani10122365
Kang DR, Shim KS. Proteomic Analysis of the Protective Effect of Early Heat Exposure against Chronic Heat Stress in Broilers. Animals. 2020; 10(12):2365. https://doi.org/10.3390/ani10122365
Chicago/Turabian StyleKang, Da Rae, and Kwan Seob Shim. 2020. "Proteomic Analysis of the Protective Effect of Early Heat Exposure against Chronic Heat Stress in Broilers" Animals 10, no. 12: 2365. https://doi.org/10.3390/ani10122365
APA StyleKang, D. R., & Shim, K. S. (2020). Proteomic Analysis of the Protective Effect of Early Heat Exposure against Chronic Heat Stress in Broilers. Animals, 10(12), 2365. https://doi.org/10.3390/ani10122365





