Sexual Maturity Promotes Yolk Precursor Synthesis and Follicle Development in Hens via Liver-Blood-Ovary Signal Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection for Blood and Tissues
2.3. Histological Staining
2.4. Serum Biochemical Parameters Assay
2.5. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.6. Protein Extraction and Western Blot Analysis
2.7. Statistical Analysis
3. Results
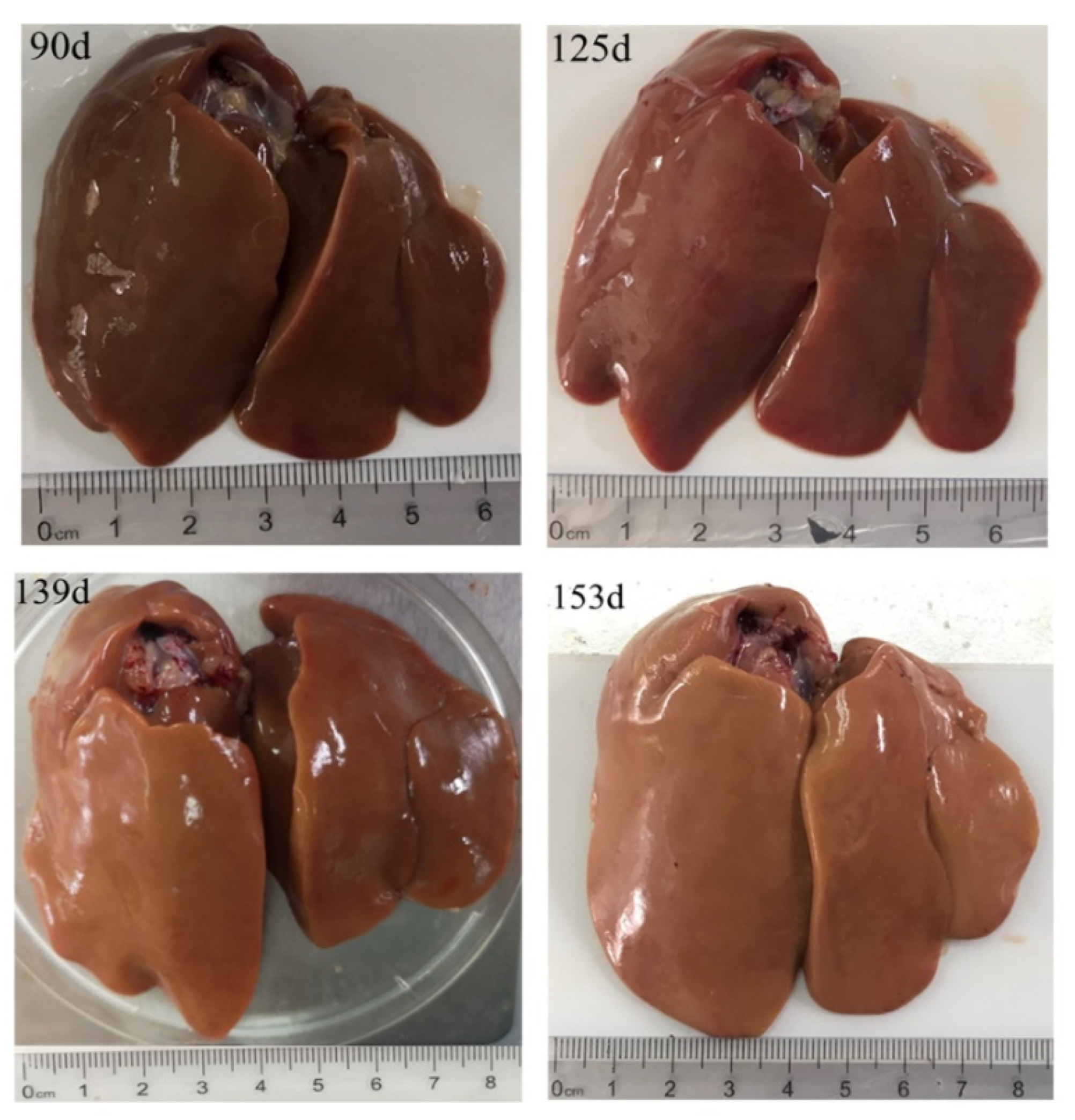
3.1. Weight Measurements for Body, Liver, and Ovary Tissues
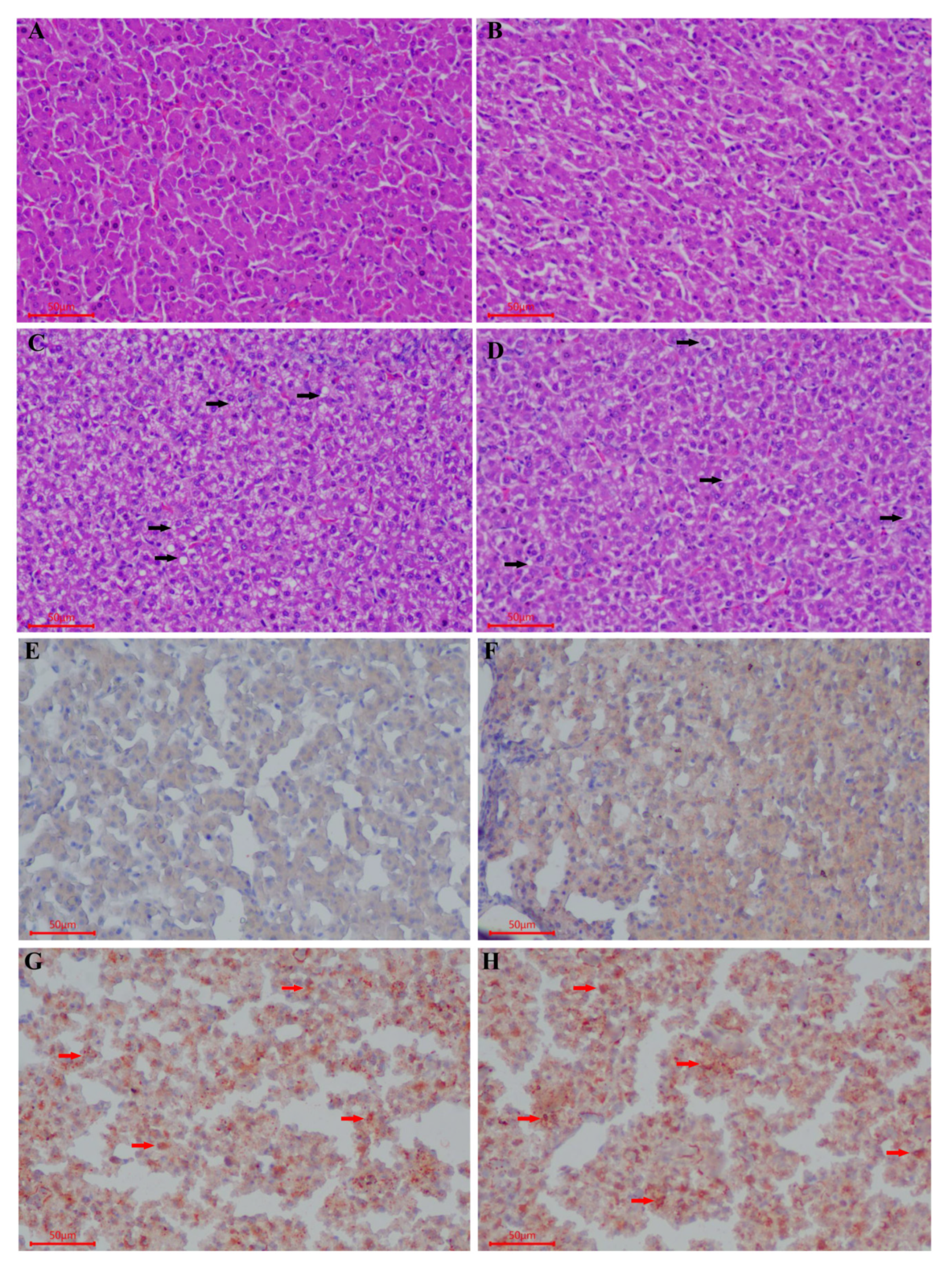
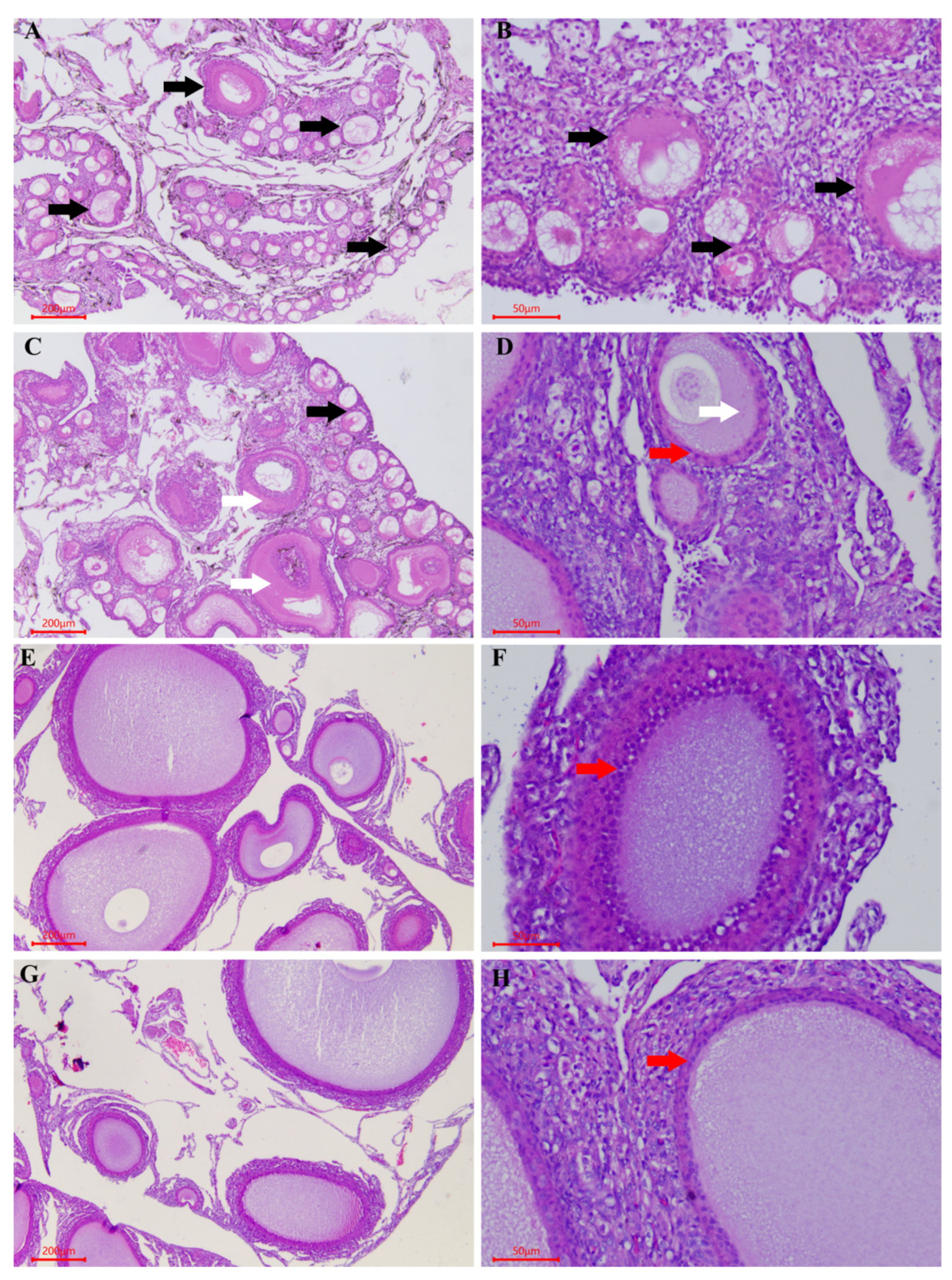
3.2. Morphological and Histological Analyses
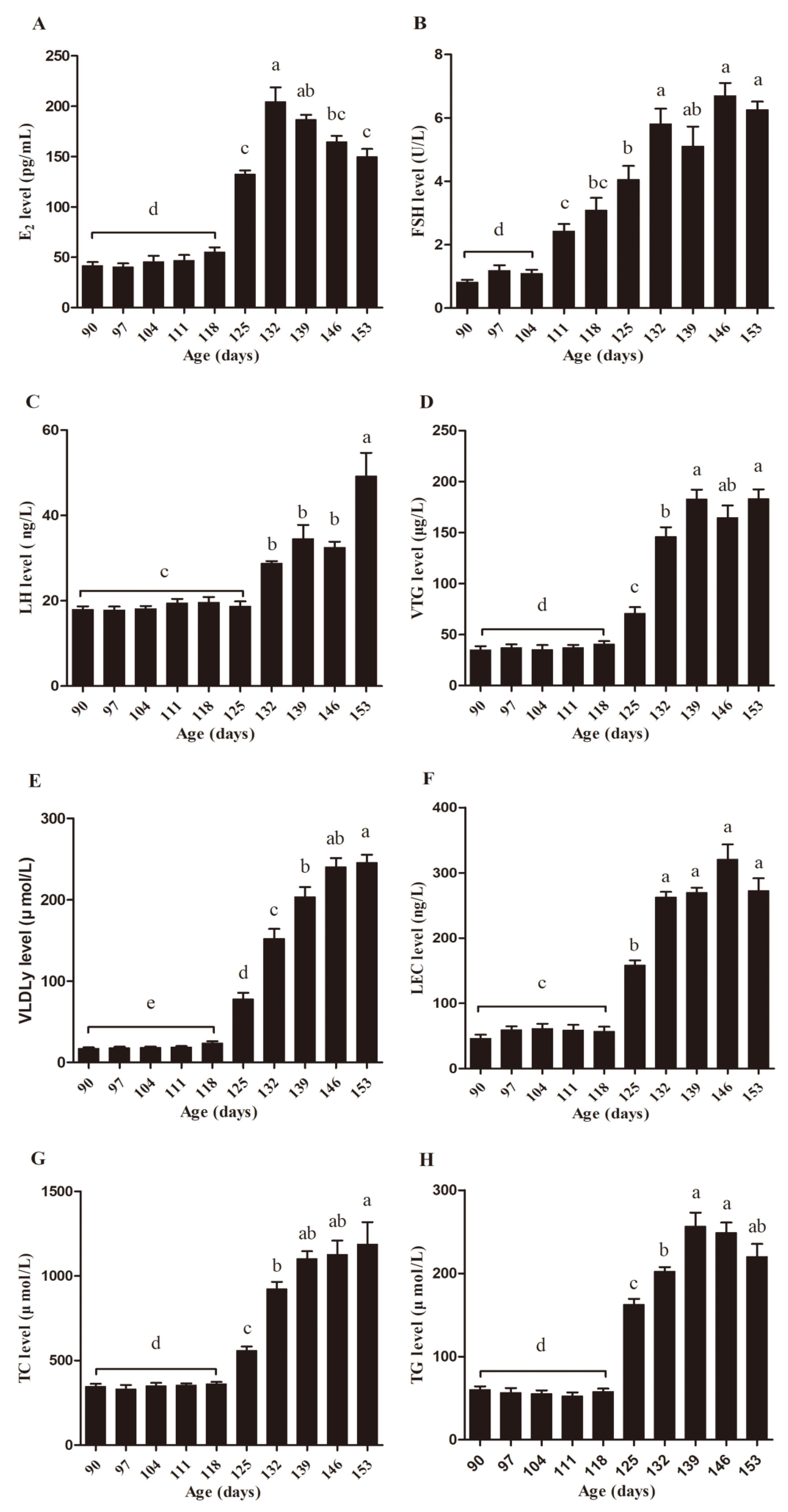
3.3. Serum Biochemical Parameter Assay
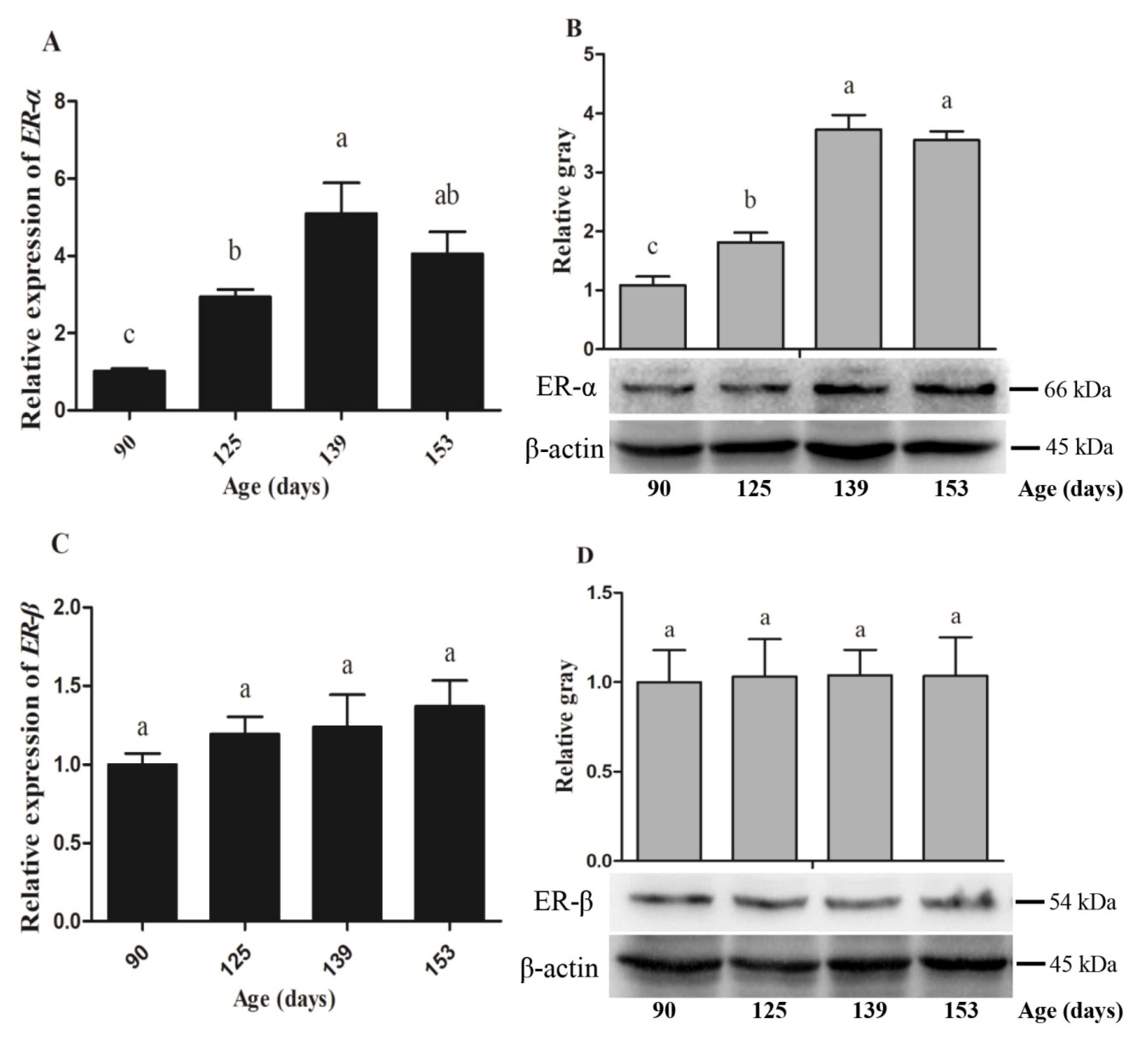
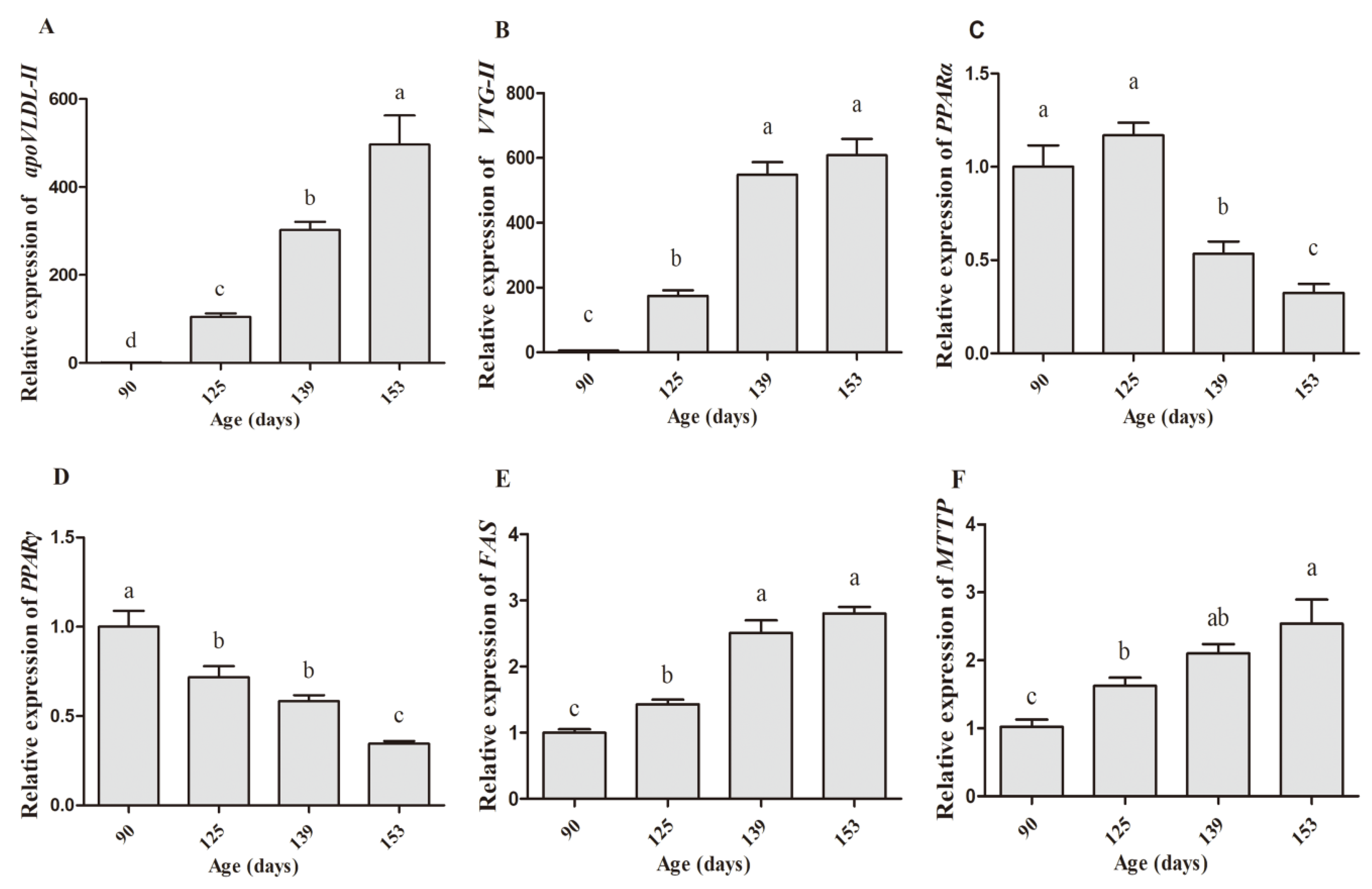
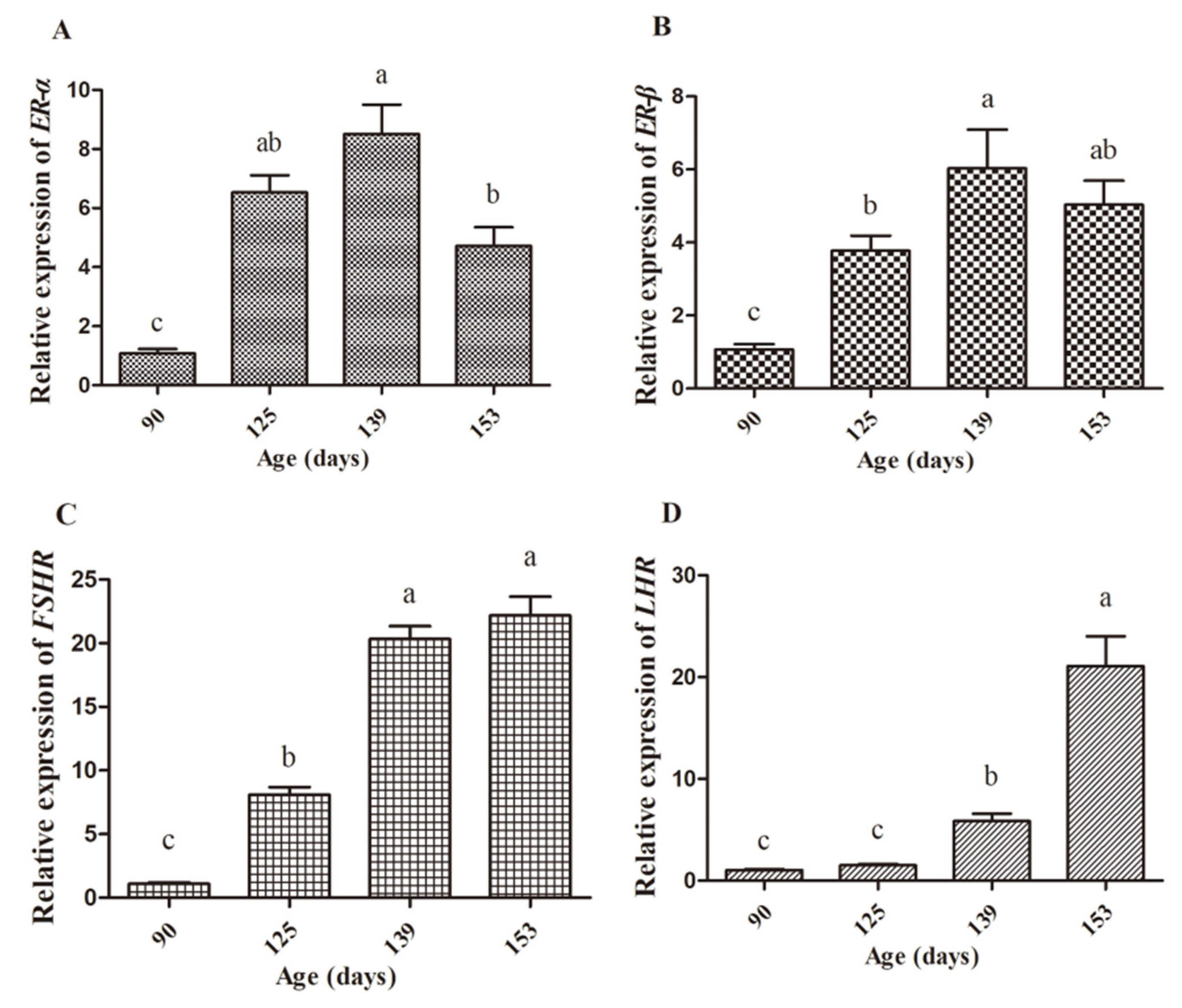
3.4. Expression Abundance of Hormone Receptors and Yolk Precursor Synthesis-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li-Chan, E.C.; Powrie, W.D.; Nakai, S. The Chemistry of Eggs and Egg Products; CRC Press: Boca Raton, FL, USA, 2017; pp. 105–175. [Google Scholar]
- Yamamoto, T.; Juneja, L.; Hatta, H.; Kim, M. General Chemical Composition of Hen Eggs; CRC Press: Boca Raton, FL, USA, 1997; pp. 13–25. [Google Scholar]
- Kuksis, A. Yolk lipids. BBA-Mol. Cell Biol. Lipids 1992, 1124, 205–222. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Avian egg antibodies: Basic and potential applications. Avian Poult. Biol. Rev. 2004, 15, 25–46. [Google Scholar] [CrossRef]
- Sim, J.; Sunwoo, H.; Lee, E. Ovoglobulin IgY; CRC Press: Boca Raton, FL, USA, 2000; pp. 240–265. [Google Scholar]
- Gadde, U.; Rathinam, T.; Lillehoj, H.S. Passive immunization with hyperimmune egg-yolk IgY as prophylaxis and therapy for poultry diseases—A review. Anim. Health Res. Rev. 2015, 16, 163–176. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sogo, N.; Iwao, R.; Miyamoto, T. Antioxidant Effect of Egg Yolk on Linoleate in Emulsions. Biol. Chem. 2014, 54, 3099–3104. [Google Scholar]
- Sugino, H.; Ishikawa, M.; Nitoda, T.; Koketsu, M.; Juneja, L.R.; Kim, M.; Yamamoto, T. Antioxidative activity of egg yolk phospholipids. J. Agric. Food Chem. 1997, 45, 551–554. [Google Scholar] [CrossRef]
- Juneja, L. Egg Yolk Lipids; CRC Press: Boca Raton, FL, USA, 1997; pp. 73–98. [Google Scholar]
- Sheppard, E.H. Specialty Eggs: N-3 Fatty Acid-Enriched Eggs; Wiley Online Library: Hoboken, NJ, USA, 2002; pp. 37–44. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, E.; Cieślik, I.; Molina-Ruiz, J.; Walkowska, I.; Migdal, W. The content of fat and fatty acids composition in chicken liver. Anim. Biotechnol. 2011, 27, 1855–1856. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.Z.; Leng, L.; Na, W.; Wang, Q.G.; Li, H. Differentially expressed genes in the liver of lean and fat chickens. Genet. Mol. Res. 2014, 13, 10823–10828. [Google Scholar] [CrossRef]
- Deeley, R.G.; Mullinix, D.P.; Wetekam, W.; Kronenberg, H.M.; Meyers, M.; Eldridge, J.D.; Goldberger, R.F. Vitellogenin synthesis in the avian liver. Vitellogenin is the precursor of the egg yolk phosphoproteins. J. Biol. Chem. 1975, 250, 9060–9066. [Google Scholar]
- Cochrane, A.; Deeley, R. Estrogen-dependent activation of the avian very low density apolipoprotein II and vitellogenin genes: Transient alterations in mRNA polyadenylation and stability early during induction. J. Mol. Biol. 1988, 203, 555–567. [Google Scholar] [CrossRef]
- Bergink, E.W.; Wallace, R.A.; Van de Berg, J.A.; Bos, E.S.; Gruber, M.; AB, G. Estrogen-induced synthesis of yolk proteins in roosters. Am. Zool. 1974, 14, 1177–1193. [Google Scholar] [CrossRef]
- Capony, F.; Williams, D.L. Apolipoprotein B of avian very low density lipoprotein: Characteristics of its regulation in nonstimulated and estrogen-stimulated rooster. Biochemistry 1980, 19, 2219–2226. [Google Scholar] [CrossRef]
- Ratna, W.N.; Bhatt, V.D.; Chaudhary, K.; Ariff, A.B.; Bavadekar, S.A.; Ratna, H.N. Estrogen-responsive genes encoding egg yolk proteins vitellogenin and apolipoprotein II in chicken are differentially regulated by selective estrogen receptor modulators. Theriogenology 2016, 85, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Montorzi, M.; Falchuk, K.H.; Vallee, B.L. Vitellogenin and lipovitellin: Zinc proteins of Xenopus laevis oocytes. Biochemistry 1995, 34, 10851–10858. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; Hansen, R.J.; Williams, D.L.; Hamilton, R.L. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 1999, 129, 467S–472S. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.; Davis, P.; Hansen, R. Overfeeding increases very low density lipoprotein diameter and causes the appearance of a unique lipoprotein particle in association with failed yolk deposition. J. Lipid Res. 1994, 35, 1354–1366. [Google Scholar]
- Perry, M.; Gilbert, A.; Evans, A. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J. Anat. 1978, 125, 481. [Google Scholar]
- Griffin, H. Manipulation of egg yolk cholesterol: A physiologist’s view. World Poult. Sci. J. 1992, 48, 101–112. [Google Scholar] [CrossRef]
- Evans, M.I.; Chu, W.W.; Berkowitz, E.A. Regulation of the Chicken Very Low Density Apolipoprotein II Gene: Interaction of Estrogen and Insulin. Recent Prog. Horm. Res. 1994, 49, 335–339. [Google Scholar]
- Yilmaz, O.; Prat, F.; Ibañez, A.J.; Amano, H.; Koksoy, S.; Sullivan, C.V. Estrogen-induced yolk precursors in European sea bass, Dicentrarchus labrax: Status and perspectives on multiplicity and functioning of vitellogenins. Gen. Comp. Endocr. 2015, 221, 16–22. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, L.; Zhao, X.; Ran, J.; Wang, Y.; Yin, H.; Li, D.; Zhu, Q. Analysis of Expression and Single Nucleotide Polymorphisms of INHA Gene Associated with Reproductive Traits in Chickens. BioMed Res. Int. 2019, 2019, 8572837. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Wang, D.; Stewart, C.N., Jr. Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol. J. 2008, 3, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Carlander, D.; Stålberg, J.; Larsson, A. Chicken antibodies: A clinical chemistry perspective. Ups. J. Med. Sci. 1999, 104, 179–189. [Google Scholar] [CrossRef]
- Johnson, A.L. Reproduction in the Female; Elsevier: Amsterdam, The Netherlands, 2015; pp. 635–665. [Google Scholar]
- Griffin, H.; Grant, G.; Perry, M. Hydrolysis of plasma triacylglycerol-rich lipoproteins from immature and laying hens (Gallus domesticus) by lipoprotein lipase in vitro. Biochem. J. 1982, 206, 647–654. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Mi, Y.; Zeng, W.; Zhang, C. Age-related changes of yolk precursor formation in the liver of laying hens. J. Zhejiang Univ. Sci. B 2018, 19, 390–399. [Google Scholar] [CrossRef]
- Cohen, A.; Smith, Y. Estrogen regulation of microRNAs, target genes, and microRNA expression associated with vitellogenesis in the zebrafish. Zebrafish 2014, 11, 462–478. [Google Scholar] [CrossRef]
- Hall, J.M.; Couse, J.F.; Korach, K.S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001, 276, 36869–36872. [Google Scholar] [CrossRef]
- Li, J.; Leghari, I.H.; He, B.; Zeng, W.; Mi, Y.; Zhang, C. Estrogen stimulates expression of chicken hepatic vitellogenin II and very low-density apolipoprotein II through ER-α. Theriogenology 2014, 82, 517–524. [Google Scholar] [CrossRef]
- Luskey, K.L.; Brown, M.S.; Goldstein, J.L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J. Biol. Chem. 1974, 249, 5939–5947. [Google Scholar]
- Williams, D.L. Apoproteins of avian very low density lipoprotein: Demonstration of a single high molecular weight apoprotein. Biochemistry 1979, 18, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Dashti, N.; Kelley, J.L.; Thayer, R.H.; Ontko, J.A. Concurrent inductions of avian hepatic lipogenesis, plasma lipids, and plasma apolipoprotein B by estrogen. J. Lipid Res. 1983, 24, 368–380. [Google Scholar] [PubMed]
- Reddy, I.; David, C.; Sarma, P.; Singh, K. The possible role of prolactin in laying performance and steroid hormone secretion in domestic hen (Gallus domesticus). Gen. Comp. Endocr. 2002, 127, 249–255. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Xu, C.; Wang, D.; Ren, J.; Li, Y.; Tian, Y.; Wang, Y.; Jiao, Y.; Kang, X. Transcriptome profile of liver at different physiological stages reveals potential mode for lipid metabolism in laying hens. BMC Genom. 2015, 16, 763. [Google Scholar] [CrossRef]
- Johnson, A.L. Regulation of follicle differentiation by gonadotropins and growth factors. Poult. Sci. 1993, 72, 867–873. [Google Scholar] [CrossRef]
- Palmer, S.S. Follicle Stimulating Hormone and Steroidogenesis in Ovarian Granulosa Cells during Aging in the Domestic Hen. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 1989. [Google Scholar]
- Hammond, R.; Burke, W.; Hertelendy, F. Influence of follicular maturation on progesterone release in chicken granulosa cells in response to turkey and ovine gonadotropins. Biol. Reprod. 1981, 24, 1048–1055. [Google Scholar] [CrossRef]

| Gene | Sequence (5’-3’) | Product Length (bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|
| ER-α | F: TGTGCTGTGTGCAACGACTA | 167 | 57 | NM_205183.2 |
| R: CAGGCCTGGCAACTCTTTCT | ||||
| ER-β | F: GGCTGCAACCCGTGTAAAAG | 189 | 58 | NM_204794.2 |
| R: GCCCAGCCAATCATGTGAAC | ||||
| apoVLDL-II | F: CCTTAGCACCACTGTCCCTG | 130 | 58 | NM_205483.2 |
| R: AGCTCTAGGGGACACCTTGT | ||||
| VTG-II | F: AACTACTCGATGCCCGCAAA | 179 | 58 | NM_001031276.1 |
| R: ACCAGCAGTTTCACCTGTCC | ||||
| FAS | F: TGCTATGCTTGCCAACAGGA | 128 | 59 | NM_205155.3 |
| R: ACTGTCCGTGACGAATTGCT | ||||
| PPARα | F: AGGCCAAGTTGAAAGCAGAA | 155 | 60 | NM_001001464.1 |
| R: TTTCCCTGCAAGGATGACTC | ||||
| PPARγ | F: TGACAGGAAAGACGACAGACA | 164 | 59 | NM_001001460.1 |
| R: CTCCACAGAGCGAAACTGAC | ||||
| MTTP | F: GTTCTGAAGGACATGCGTGC | 120 | 58 | NM_001109784.2 |
| R: GATGTCTAGGCCGTACGTGG | ||||
| GAPDH | F: TCCTCCACCTTTGATGCG | 144 | 60 | NM_204305.1 |
| R: GTGCCTGGCTCACTCCTT |
| Age (d) | Body Weight (g) | Liver Weight (g) | Ovary Weight (g) | Follicle Numbers (Diameter > 12 mm) |
|---|---|---|---|---|
| 90 | 1085.33 ± 71.39 b | 21.46 ± 0. 60 b | 0.45 ± 0.03 c | - |
| 97 | 1136.20 ± 24.44 b | 22.52 ± 2.59 b | 0.47 ± 0.01 c | - |
| 104 | 1084.97 ± 48.56 b | 22.21 ± 2.76 b | 0.48 ± 0.01 c | - |
| 111 | 1127.59 ± 142.19 b | 23.30 ± 4.46 b | 0.52 ± 0.04 c | - |
| 118 | 1127.59 ± 142.19 b | 24.79 ± 4.39 b | 0.62 ± 0.12 c | - |
| 125 | 1338.51 ± 138.49 b | 22.92 ± 3.29 b | 0.74 ± 0.17 c | - |
| 132 | 1713.40 ± 131.03 a | 39.82 ± 7.78 a | 1.50 ± 0.17 c | 1.00 ± 0.23 c |
| 139 | 1760.00 ± 101.76 a | 37.13 ± 7.73 a | 17.80 ± 10.96 b | 4.44 ± 0.28 b |
| 146 | 1731.12 ± 176.08 a | 36.69 ± 6.83 a | 36.47 ± 5.41 a | 6.00 ± 0.24 a |
| 153 | 1794.00 ± 228.54 a | 36.64 ± 4.99 a | 47.68 ± 13.64 a | 6.44 ± 0.17 a |
| Variable | E2 | FSH | LH | VTG | VLDLy | LEC | TC | TG |
|---|---|---|---|---|---|---|---|---|
| Age | 0.824 ** | 0.874 ** | 0.765 ** | 0.867 ** | 0.913 ** | 0.872 ** | 0.861 ** | 0.863 ** |
| Variable | ER-α | ER-β | apoVLDL-II | VTG-II | FAS | PPARα | PPARγ | MTTP |
|---|---|---|---|---|---|---|---|---|
| Age | 0.73 ** | 0.319 | 0.925 ** | 0.889 ** | 0.873 ** | −0.63 ** | −0.808 ** | 0.763 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Amevor, F.K.; Feng, Q.; Kang, X.; Song, W.; Zhu, Q.; Wang, Y.; Li, D.; Zhao, X. Sexual Maturity Promotes Yolk Precursor Synthesis and Follicle Development in Hens via Liver-Blood-Ovary Signal Axis. Animals 2020, 10, 2348. https://doi.org/10.3390/ani10122348
Cui Z, Amevor FK, Feng Q, Kang X, Song W, Zhu Q, Wang Y, Li D, Zhao X. Sexual Maturity Promotes Yolk Precursor Synthesis and Follicle Development in Hens via Liver-Blood-Ovary Signal Axis. Animals. 2020; 10(12):2348. https://doi.org/10.3390/ani10122348
Chicago/Turabian StyleCui, Zhifu, Felix Kwame Amevor, Qian Feng, Xincheng Kang, Weizhen Song, Qing Zhu, Yan Wang, Diyan Li, and Xiaoling Zhao. 2020. "Sexual Maturity Promotes Yolk Precursor Synthesis and Follicle Development in Hens via Liver-Blood-Ovary Signal Axis" Animals 10, no. 12: 2348. https://doi.org/10.3390/ani10122348
APA StyleCui, Z., Amevor, F. K., Feng, Q., Kang, X., Song, W., Zhu, Q., Wang, Y., Li, D., & Zhao, X. (2020). Sexual Maturity Promotes Yolk Precursor Synthesis and Follicle Development in Hens via Liver-Blood-Ovary Signal Axis. Animals, 10(12), 2348. https://doi.org/10.3390/ani10122348






