Polymorphism of Selected Regions of Ovar-MHC and the Health Status of the Ovine Mammary Gland
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samplings
2.2. Somatic Cell Count
2.3. Lymphocyte Subpopulation Level Determination
2.4. Genomic DNA Extraction and Molecular Analysis
2.5. Statistics
3. Results
4. Discussion
4.1. Microsatellite Polymorphism of the MHC Genes
4.2. MHC Genes Polymorphism and Its Relations to SCC and ISCs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Watson, D.J. Sheep mastitis. Ann. Proceed. Sheep. Vet. Soc. 1982, 6, 88–92. [Google Scholar]
- Mackie, D.P.; Rodgers, S.P. Mastitis and cell content in milk from Scottish blackface ewes. Veter. Rec. 1986, 118, 20–21. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, F.D.; Lindsey, J.B.; Gore, M.T.; Notter, D.R. Incidence and control of subclinical mastitis in intensively managed ewes. J. Anim. Sci. 1988, 66, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C.; Jones, J.E.T. The effect of inoculation of coagulase–negative staphylococci (CNS) the ovine mammary gland. J. Comp. Pathol. 1990, 102, 211–219. [Google Scholar] [CrossRef]
- Bergonier, D.; De Cremoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy ruminants. Vet. Res. 2003, 34, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Shoop, D.S.; Myers, L.L. Serologic analysis of isolates of Pasteurella haemolytica and Staphylococcus aureus from mastitis ewe. Am. J. Vet. Res. 1984, 45, 1944–1946. [Google Scholar] [PubMed]
- Char, N.L.; Rao, M.R.K. Studies on prevalence of staphylococcus infections in animals. Livestock Advis. 1990, 15, 7–11. [Google Scholar]
- Watkins, G.; Burriel, A.R.; Jones, J. A field investigation of subclinical mastitis in sheep in southern England. Br. Veter. J. 1991, 147, 413–420. [Google Scholar] [CrossRef]
- Mavrogenis, A.; Koumas, A.; Kakoyiannis, C.; Taliotis, C. Use of somatic cell counts for the detection of subclinical mastitis in sheep. Small Rumin. Res. 1995, 17, 79–84. [Google Scholar] [CrossRef]
- González-Rodríguez, M.C.; Gonzalo, C.; Primitivo, F.S.; Cármenes, P. Relationship between somatic cell count and intramammary infection of the half udder in dairy ewes. J. Dairy Sci. 1995, 78, 2753–2759. [Google Scholar] [CrossRef]
- Piccinini, R.; Bronzo, V.; Moroni, P.; Luzzago, C.; Zecconi, A. Study on the relationship between milk immune factors and Staphylococcus aureus intramammary infections in dairy cows. J. Dairy Res. 1999, 66, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.; Chaffer, M.; Zamir, S.; Mor, T.; Glickman, A.; Winkler, M.; Weisblit, L.; Saran, A. Udder disease etiology, milk somatic cell caunt and NAGase activity in Israeli Assaf throughout lactation. Small Rumin. Res. 2001, 39, 107–112. [Google Scholar] [CrossRef]
- Pengov, A. The role of coagulase-negative Staphylococcus spp. and associated somatic cell counts in the ovine mammary gland. J. Dairy Sci. 2001, 84, 572–574. [Google Scholar] [CrossRef]
- Bergonier, D.; Berthelot, X. New advances in epizoology and control of ewe mastitis. Livestock Prod. Sci. 2003, 79, 1–16. [Google Scholar] [CrossRef]
- Mørk, T.; Waage, S.; Tollersrud, T.; Kvitle, B.; Sviland, S. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Acta Veter. Scand. 2007, 49, 23. [Google Scholar] [CrossRef] [PubMed]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Ruggieri, D.; Ciliberti, M.; Sevi, A. Immune competence of the mammary gland as affected by somatic cell and pathogenic bacteria in ewes with subclinical mastitis. J. Dairy Sci. 2012, 95, 3877–3887. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, C. Milk hygiene in small ruminants: A review. Span. J. Agric. Res. 2018, 15, e05R02. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Veter. World 2018, 11, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Tvarožková, K.; Tančin, V.; Holko, I.; Uhrinčať, M.; Mačuhová, L. Mastitis in ewes: Somatic cell counts, pathogens and antibiotic resistance. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 661–670. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Politis, A.P.; Mavrogianni, V.S.; Barbagianni, M.S.; Vasileiou, N.G.C.; Fthenakis, G.C.; Fragkou, I.A. Mammary defences and immunity against mastitis in sheep. Animals 2019, 9, 726. [Google Scholar] [CrossRef]
- Winter, P.; Colditz, I.G. Immunological response of the lactating ovine udder following experimental challenge with z Staphylococcus epidermidis. Vet. Immunol. Immunopathol. 2002, 89, 57–65. [Google Scholar] [CrossRef]
- Waller, K.P.; Colditz, I.G. The effect of experimental infectious mastitis on leukocyte subpopulations and cytokine production in non-lactating ewes. J. Veter. Med. Ser. B 1999, 46, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.P.; Colditz, I.G. Effect of intramammary infusion of beta-1,3-glucan or interleukin-2 on leukocyte subpopulations in mammary glands of sheep. Am. J. Vet. Res. 1999, 60, 703–707. [Google Scholar] [PubMed]
- Lee, J.-W.; O’Brien, C.N.; Guidry, A.J.; Paape, M.J.; Shafer-Weaver, K.A.; Zhao, X. Effect of a trivalent vaccine against Staphylococcus aureus mastitis lymphocyte subpopulations, antibody production, and neutrophil phagocytosis. Can. J. Veter. Res. 2005, 69, 11–18. [Google Scholar]
- Taylor, B.C.; Dellinger, J.D.; Cullor, J.S.; Stott, J.L. Bovine milk lymphocytes display the phenotype of memory T cells and are predominantly CD8+. Cell. Immunol. 1994, 156, 245–253. [Google Scholar] [CrossRef]
- Rivas, A.L.; Quimby, F.W.; Coksaygan, O.; Olmstead, L.; Lein, D.H. Longitudinal evaluation of CD4+ and CD8+ peripheral blood and mammary gland lymphocytes in cows experimentally inoculated with Staphylococcus aureus. Can. J. Veter. Res. 2000, 64, 232–237. [Google Scholar]
- Riollet, C.; Rainard, P.; Poutrel, B. Cells and cytokines in inflammatory secretions of bovine mammary gland. Biol. Mammary Gland 2000, 480, 247–258. [Google Scholar] [CrossRef]
- Blattman, A.N.; Beh, K.J. Dinucleotide repeat polymorphism within the ovine major histocompatibility complex. Anim. Genet. 2009, 23, 392. [Google Scholar] [CrossRef]
- Boyce, W.M.; Hedrick, P.W.; Muggli-Cockett, N.E.; Kalinowski, S.; Penedo, M.C.T.; Ramey, R.R. Genetic variation of major histocompatibilty complex and microsatellite loci: A comparison in bighorn sheep. Genetics 1997, 145, 421–433. [Google Scholar]
- Dicks, K.L.; Pemberton, J.M.; Ballingall, K.T. Characterisation of major histocompatibility complex class IIa haplotypes in an island sheep population. Immunogenetics 2019, 71, 383–393. [Google Scholar] [CrossRef]
- Paterson, S. Evidence for balancing selection at the major histocompatibility complex in a free-living ruminant. J. Hered. 1998, 89, 289–294. [Google Scholar] [CrossRef][Green Version]
- Walling, G.A.; Visscher, P.M.; Wilson, A.D.; McTeir, B.L.; Simm, G.; Bishop, S.C. Mapping of quantitative trait loci for growth and carcass traits in commercial sheep populations1. J. Anim. Sci. 2004, 82, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Worley, K.; Carey, J.; Veitch, A.; Coltman, D.W. Detecting the signature of selection on immune genes in highly structured populations of wild sheep (Ovis dalli). Mol. Ecol. 2006, 15, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Geldermann, H.; Mir, M.R.; Kuss, A.W.; Bartenschlager, H. OLA-DRB1 microsatellite variants are associated with ovine growth and reproduction traits. Genet. Sel. Evol. 2006, 38, 431–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santucci, F.; Ibrahim, K.M.; Bruzzone, A.; Hewit, G.M. Selection on MHC-linked microsatellite loci in sheep populations. Heredity 2007, 99, 340–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ballingall, K.; Tassi, R. Sequence-based genotyping of the sheep MHC class II DRB1 locus. Immunogenetics 2009, 62, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Atlija, M.; Gutiérrez-Gil, B.; Arranz, J.J.; Semmer, J.; Stear, M.; Buitkamp, J. Major Histocompatibility Complex class IIB polymorphism in an ancient Spanish breed. Immunogenetics 2015, 67, 531–537. [Google Scholar] [CrossRef]
- Preuss, S.; Kuss, A.W.; He, H.; Bartenschlager, H.; Geldermann, H. Analysis of polymorphic PRNP microsatellite and ORF sites in German sheep breeds. J. Anim. Breed. Genet. 2005, 122, 64–70. [Google Scholar] [CrossRef]
- Nagaoka, Y.; Kabeya, H.; Onuma, M.; Kasai, N.; Okada, K.; Aida, Y. Ovine MHC class II DRB1 alleles associated with resistance or susceptibility to development of bovine leukemia virus-induced ovine lymphoma. Cancer Res. 1999, 59, 975–981. [Google Scholar]
- Schwaiger, F.W.; Gostomski, D.; Stear, M.J.; Duncan, J.L.; Mckellar, Q.A.; Epplen, J.T.; Buitkamp, J. An ovine major histocompatability complex DRB1 allele is associated with low faecal egg counts following natural, predominantly Ostertagia circucincta infection. Intern. J. Parasit. 1995, 25, 815–822. [Google Scholar] [CrossRef]
- Outteridge, P.M.; Andersson, L.; Duoch, P.G.C.; Green, R.S.; Gawakisa, P.S.; Hohenhaus, M.A.; Mikko, S. The PCR typing of MHC-DRB gens in the sheep using primers for an intronic microsatellite: Application nematode parasite resistance. Immunol. Cell Biol. 1996, 74, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Charon, K.M.; Moskwa, B.; Rutkowski, R.; Gruszczyńska, J.; Świderek, W. Microsatellite polymorphism in DRB1 gene (MHC class II) and its relation to nematode faecal egg count in Polish Heath Sheep. J. Anim. Feed. Sci. 2002, 11, 47–58. [Google Scholar] [CrossRef]
- Davies, G.; Stear, M.; Benothman, M.; Abuagob, O.; Kerr, A.; Mitchell, S.; Bishop, S.C. Quantitative trait loci associated with parasitic infection in Scottish blackface sheep. Heredity 2006, 96, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hajializadeh Valilou, R.; Rafat, S.; Firouzamandi, M.; Ebrahimi, M. Use of microsatellite polymorphisms in Ovar-DRB1 gene for identifying genetic resistance in fat-tailed Ghezel Sheep to gastrointestinal nematodes. Iran. J. Appl. Anim. Sci. 2016, 6, 879–886. [Google Scholar]
- Gruszczyńska, J.; Charon, K.M.; Kitlińska, J.; Szydłowski, M. The influence of OLA-DRB1 (MHC class II) gene polymorphism on lamb body weight and weight gain in Polish Heath Sheep. J. Appl. Genet. 2000, 41, 101–112. [Google Scholar]
- Barillet, F.; Arranz, J.-J.; Carta, A. Mapping quantitative trait loci for milk production and genetic polymorphisms of milk proteins in dairy sheep. Genet. Sel. Evol. 2005, 37, S109–S123. [Google Scholar] [CrossRef]
- Charon, K.M.; Skolasiński, W.; Świderek, W.P. The effect of management and the way of productive use of sheep on the status of their udders. Ann. Warsaw Agricult. Univ. SGGW Anim. Sci. 1994, 31, 75–81. [Google Scholar]
- Świderek, W.P. A trial at evaluating biological and environmental factors on the health state of the udders in sheep. Ann. Warsaw Agricult. Univ. SGGW Anim. Sci. 1996, 32, 55–64. [Google Scholar]
- Paape, M.; Wiggans, G.; Bannerman, D.; Thomas, D.; Sanders, A.; Contreras, A.; Moroni, P.; Miller, R. Monitoring goat and sheep milk somatic cell counts. Small Rumin. Res. 2007, 68, 114–125. [Google Scholar] [CrossRef]
- Ruegg, P.L. Mastitis in small ruminants. In Proceedings of the 44th Annual Conference of the American Association of Bovine Practitioners, Small Ruminant Session, St. Louis, MO, USA, 22–25 September 2011; pp. 1–26. [Google Scholar]
- Alnakip, M.E.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Caamaño-Antelo, S.; Calo-Mata, P.; Barros-Velázquez, J. The immunology of mammary gland of dairy ruminants between healthy and inflammatory conditions. J. Veter. Med. 2014, 2014, 659801. [Google Scholar] [CrossRef]
- Knuth, R.M.; Stewart, W.C.; Taylor, J.B.; Yeoman, C.J.; Bisha, B.; Page, C.M.; Rowley, C.M.; Lindsey, B.C.; Van Emon, M.L.; Murphy, T.W. Subclinical mastitis in sheep: Etiology and association with milk somatic cell count and ewe productivity in three research flocks in the Western United States. Transl. Anim. Sci. 2019, 3, 1739–1743. [Google Scholar] [CrossRef]
- Świderek, W.P.; Charon, K.M.; Winnicka, A.; Gruszczyńska, J.; Pierzchała, M. Physiological threshold of somatic cell count in milk of polish heath sheep and Polish lowland sheep. Ann. Anim. Sci. 2016, 16, 155–170. [Google Scholar] [CrossRef]
- Winnicka, A.; Kluciński, W.; Hoser, G.; Sikora, J.; Kawiak, J. Flow cytometry analysis of milk and peripheral blood cells from goats during lactation. Zentralbl. Veterinarmed. A 1999, 46, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Świderek, W.P.; Winnicka, A.; Kluciński, W.; Charon, K.M. Cytometric analysis of peripheral blood in sheep of Wrzosówka breed and Polish Lowland Sheep of Żelazna variety. Ann. Warsaw Agricult. Univ. SGGW Anim. Sci. 1999, 35, 119–124. [Google Scholar]
- Gruszczyńska, J.; Świderek, W.P.; Charon, K.M.; Kurył, J.; Cieślak, D.; Pierzchała, M. Microsatellite polymorphism of pseudo-gene OLA-DRB2 in intron 5 in two Polish sheep breeds. Anim. Sci. Pap. Rep. 2002, 20, 67–72. [Google Scholar]
- Gruszczyńska, J.; Charon, K.M.; Świderek, W.P.; Sawera, M. Microsatellite polymorphism in OMHC1 (MHC class I) locus in Polish heath sheep and Polish lowland sheep (Żelazna variety). J. Appl. Genet. 2002, 43, 217–222. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Tereba, A.; Tereba, Z. Program. ALFreq 1.03.; [CD-ROM]; Tereba: Warsaw, Poland, 2005. [Google Scholar]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar]
- Robertson, A.; Hill, W.G. Deviations from Hardy-Weinberg proportions: Sampling variances and use in estimation of inbreeding coeficients. Genetics 1984, 107, 703–718. [Google Scholar]
- Belkhir, K.; Borsa, P.; Chikhi, L.; Raufaste, N.; Bonhomme, F. GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations; Laboratoire Génome, Populations, Interactions, CNRS UMR 5171; Université de Montpellier II: Montpellier, France, 1996–2004; Available online: https://kimura.univ-montp2.fr/genetix/ (accessed on 25 October 2019).
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–291. [Google Scholar] [CrossRef]
- Benzécri, J.P. Analyse des correspondances. In L’Analyse des Donnees; Dunod, l`Université de Paris: Paris, France, 1973; Volume 2, pp. 1–619. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Earl, D.A.; Vonholdt, B.M. Structure harvester: A website and program for visualizing structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Rosenberg, N.A. Distruct: A program for the graphical display of population structure. Mol. Ecol. Notes 2003, 4, 137–138. [Google Scholar] [CrossRef]
- Dukkipati, V.S.R.; Blair, H.T.; Garrick, D.J.; Murray, A. ‘Ovar-Mhc’-ovine major histocompatibility complex: Structure and gene polymorphisms. Genet. Mol. Res. 2006, 5, 581–608. [Google Scholar] [PubMed]
- Gowane, G.R.; Akram, N.; Misra, S.S.; Ved Prakash, V.; Kumar, A. Genetic diversity of Cahi DRB and DQB genes of caprine MHC class II in Sirohi goat. J. Gnet. 2018, 97, 483–492. [Google Scholar] [CrossRef]
- Groth, D.M.; Wetherall, J.D. Dinucleotide repeat polymorphism within the ovine major histocompatibility complex class I region. Anim. Genet. 1994, 25, 61. [Google Scholar] [CrossRef]
- Chaffer, M.; Leitner, G.; Winkler, M.; Glickman, A.; Krifucks, O.; Ezra, E.; Saran, A. Coagulase-negative staphylococci and mammary gland infections in cows. J. Vet. Med. Ser. B 1999, 46, 707–712. [Google Scholar] [CrossRef]
- Soltys, J.; Quinn, M.T. Selective recruitment of T-cell subsets to the udder during staphylococcal and streptococcal mastitis: Analysis of lymphocyte subsets and adhesion molecule expression. Infect. Immun. 1999, 67, 6293–6302. [Google Scholar] [CrossRef]
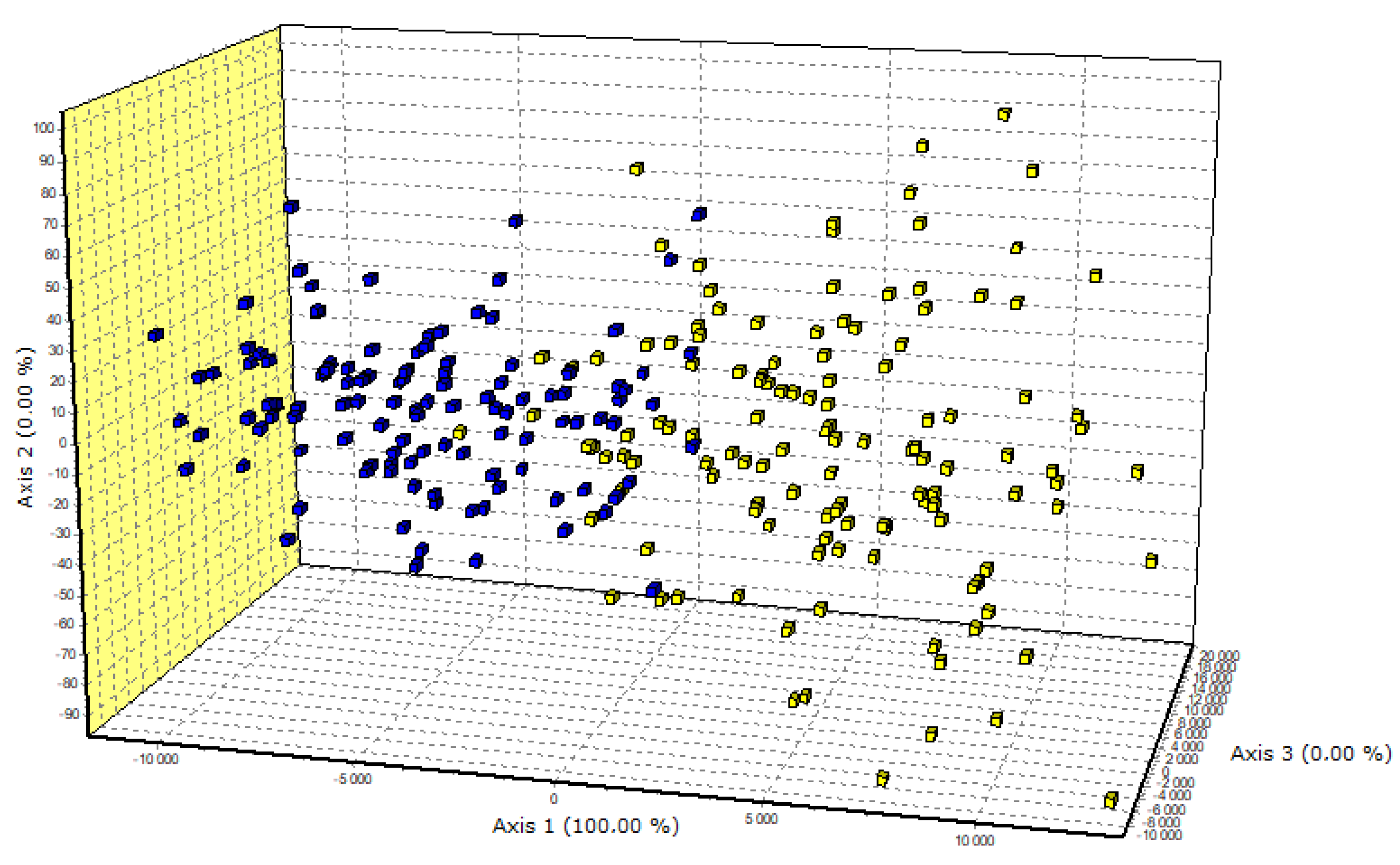

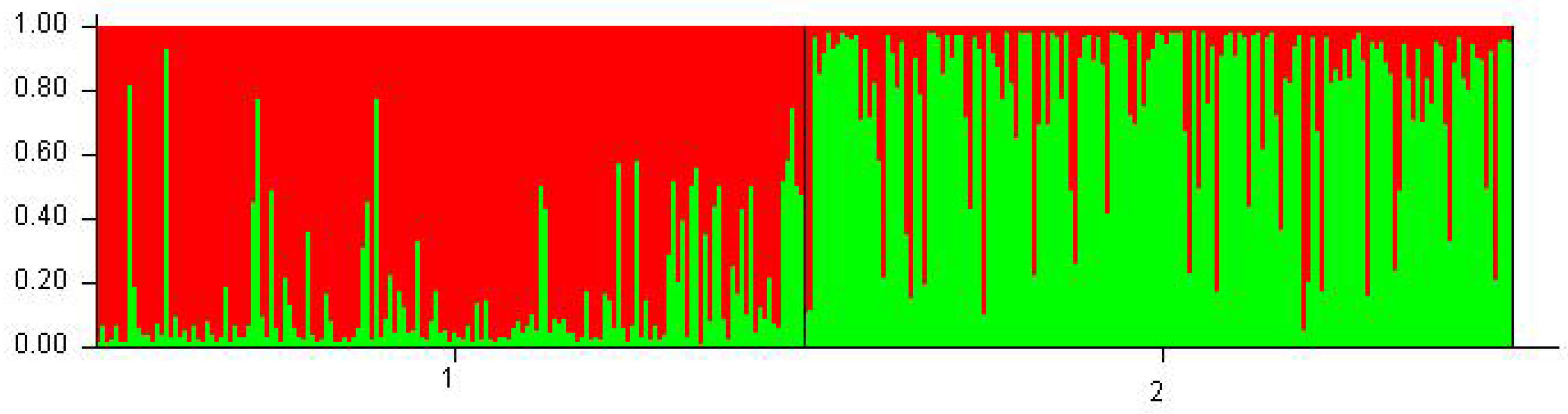
| Locus/Breed | Allele Frequency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ovar-DRB1 (bp) | 488 | 508 | 516 | 520 | 526 | 530 | 540 | 566 | 590 |
| PHS | 46.1 | 11.0 | 10.2 | 1.6 | 7.5 | 1.6 | 5.1 | 13.4 | 3.5 |
| PLS | 6.1 | 32.0 | 15.0 | 3.7 | 4.4 | 1.4 | 8.8 | 28.6 | - |
| OMHC1 (bp) | 188 | 190 | 192 | 194 | 196 | 202 | 208 | ||
| PHS | 8.3 | 8.7 | 32.3 | 29.0 | 4.0 | 10.0 | 7.7 | ||
| PLS | 4.9 | 12.7 | 6.8 | 35.7 | 25.6 | 14.3 | - | ||
| Ovar-DRB2 (bp) | 262 | 268 | 272 | 274 | 276 | 284 | 290 | ||
| PHS | 17.2 | 32.8 | - | 20.9 | - | 22.0 | 7.1 | ||
| PLS | 7.1 | 38.8 | 25.9 | 12.9 | 12.9 | - | 2.4 | ||
| Breed. | PHS | PLS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | n | No. of Alleles | HO | HE | PIC | HW b | Fis | n | No. of Alleles | HO | HE | PIC | HW b | Fis |
| Ovar-DRB1 | 126 | 9 | 0.75 | 0.74 | 0.71 | NS | −0.01060 | 147 | 8 | 0.58 | 0.78 | 0.75 | *** | 0.25184 |
| OMHC1 | 149 | 7 | 0.80 | 0.78 | 0.75 | NS | −0.02306 | 154 | 6 | 0.84 | 0.77 | 0.73 | ** | −0.10286 |
| Ovar-DRB2 | 133 | 5 | 0.74 | 0.77 | 0.73 | NS | 0.03869 | 147 | 6 | 0.70 | 0.75 | 0.71 | NS | 0.70440 |
| Mean | 7 | 0.76 | 0.76 | 0.73 | 0.00169 a | 6.67 | 0.71 | 0.77 | 0.73 | 0.07445 a | ||||
| ±SD | 1.63 | 0.03 | 0.02 | 0.02 | 0.94 | 0.11 | 0.01 | 0.02 | ||||||
| Breed | Median | Median of Immune Cells (%) | ||||
|---|---|---|---|---|---|---|
| SCC × 103 | CD2+ | CD4+ | CD8+ | CD19+ | MHCII+ | |
| PHS | 142.0 A | 55.0 A | 22.8 | 31.0 A | 18.0 a | 58.5 A |
| PLS | 305.5 B | 43.5 B | 21.0 | 17.3 B | 14.0 b | 40.0 B |
| SCC × 103 | n | Median of Immune Cells (%) | ||||
|---|---|---|---|---|---|---|
| CD2+ | CD4+ | CD8+ | CD19+ | MHCII+ | ||
| <200 | 296 | 53.5 A | 24.0 A | 25.0 | 18.0 A | 45.0 |
| >200 | 312 | 47.0 B | 19.3 B | 22.0 | 14.0 B | 53.0 |
| Locus/Allele (bp) | n | Median | Median of the Immune Cells (%) | |||||
|---|---|---|---|---|---|---|---|---|
| SCC × 103 | CD2+ | CD4+ | CD8+ | CD19+ | MHCII+ | |||
| Ovar-DRB1 | 488 | 133 | 129.0 | 54.5 aA | 25.5 aA | 30.0 aA | 17.6 | 50.0 a |
| 508 | 122 | 232.5 | 44.5 B | 17.0 B | 18.8 B | 11.0 | 37.0 b | |
| 516 | 70 | 256.5 | 53.0 a | 21.3 a | 21.8 a | 19.0 | 38.3 | |
| 566 | 118 | 254.0 | 46.5 B | 20.0 a | 16.3 B | 18.0 | 46.0 | |
| OMHC1 | 192 | 118 | 171.0 | 52.0 | 21.5 | 26.0 | 15.0 | 47.0 |
| 194 | 195 | 211.0 | 53.5 | 22.8 | 25.0 | 17.0 | 52.0 | |
| 196 | 88 | 329.0 | 46.8 | 22.8 | 19.0 | 15.0 | 41.3 | |
| 202 | 74 | 193.0 | 49.3 | 19.8 | 21.8 | 14.0 | 48.0 | |
| Ovar-DRB2ps | 268 | 202 | 206.5 | 47.0 a | 21.5 a | 21.0 ab | 16.5 | 47.0 a |
| 272 | 72 | 277.5 | 37.0 bA | 15.0 A | 15.3 b | 13.5 | 30.0 bB | |
| 274 | 92 | 265.0 | 50.0 a | 19.0 a | 25.8 a | 18.0 | 59.5 aA | |
| 284 | 59 | 156.0 | 56.5 aB | 27.0 aB | 31.0 a | 16.5 | 45.0 a | |
| Genotype (bp/bp) | n | Median | Median of Immune Cells (%) | |||||
|---|---|---|---|---|---|---|---|---|
| SCC × 103 | CD2+ | CD4+ | CD8+ | CD19+ | MHCII+ | |||
| Ovar-DRB1 | 488/488 | 19 | 124.0 | 55.5 | 31.0 | 26.0 | 21.0 | 60.0 |
| 508/508 | 24 | 219.0 | 37.5 | 17.0 | 17.5 | 10.0 | 32.5 | |
| 566/566 | 22 | 231.5 | 42.0 | 21.5 | 8.0 | 19.0 | 59.0 | |
| OMHC1 | 192/194 | 31 | 159.0 | 46.3 | 20.0 | 16.0 | 21.5 | 47.0 |
| 194/196 | 36 | 282.0 | 54.8 | 29.5 | 20.0 | 15.3 | 44.3 | |
| 194/202 | 23 | 226.0 | 50.0 | 20.0 | 25.0 | 14.0 | 49.0 | |
| Ovar-DRB2ps | 268/268 | 34 | 221.0 | 43.0 | 28.5 | 22.0 | 18.0 | 63.5 A |
| 268/272 | 17 | 1176.0 | 37.5 a | 15.0 | 8.0 | 13.0 | 23.5 B | |
| 284/284 | 10 | 132.0 | 68.0 b | 35.2 | 26.2 | 17.0 | 42.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderek, W.P.; Gruszczyńska, J.; Winnicka, A. Polymorphism of Selected Regions of Ovar-MHC and the Health Status of the Ovine Mammary Gland. Animals 2020, 10, 2325. https://doi.org/10.3390/ani10122325
Świderek WP, Gruszczyńska J, Winnicka A. Polymorphism of Selected Regions of Ovar-MHC and the Health Status of the Ovine Mammary Gland. Animals. 2020; 10(12):2325. https://doi.org/10.3390/ani10122325
Chicago/Turabian StyleŚwiderek, Wiesław Piotr, Joanna Gruszczyńska, and Anna Winnicka. 2020. "Polymorphism of Selected Regions of Ovar-MHC and the Health Status of the Ovine Mammary Gland" Animals 10, no. 12: 2325. https://doi.org/10.3390/ani10122325
APA StyleŚwiderek, W. P., Gruszczyńska, J., & Winnicka, A. (2020). Polymorphism of Selected Regions of Ovar-MHC and the Health Status of the Ovine Mammary Gland. Animals, 10(12), 2325. https://doi.org/10.3390/ani10122325





