Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Post-Mortem Analysis
2.2. Histolopathological Evaluation
2.3. Immunohistochemistry
2.4. Detection of EcPV2 and Evaluation of Oncogene Expression
3. Results
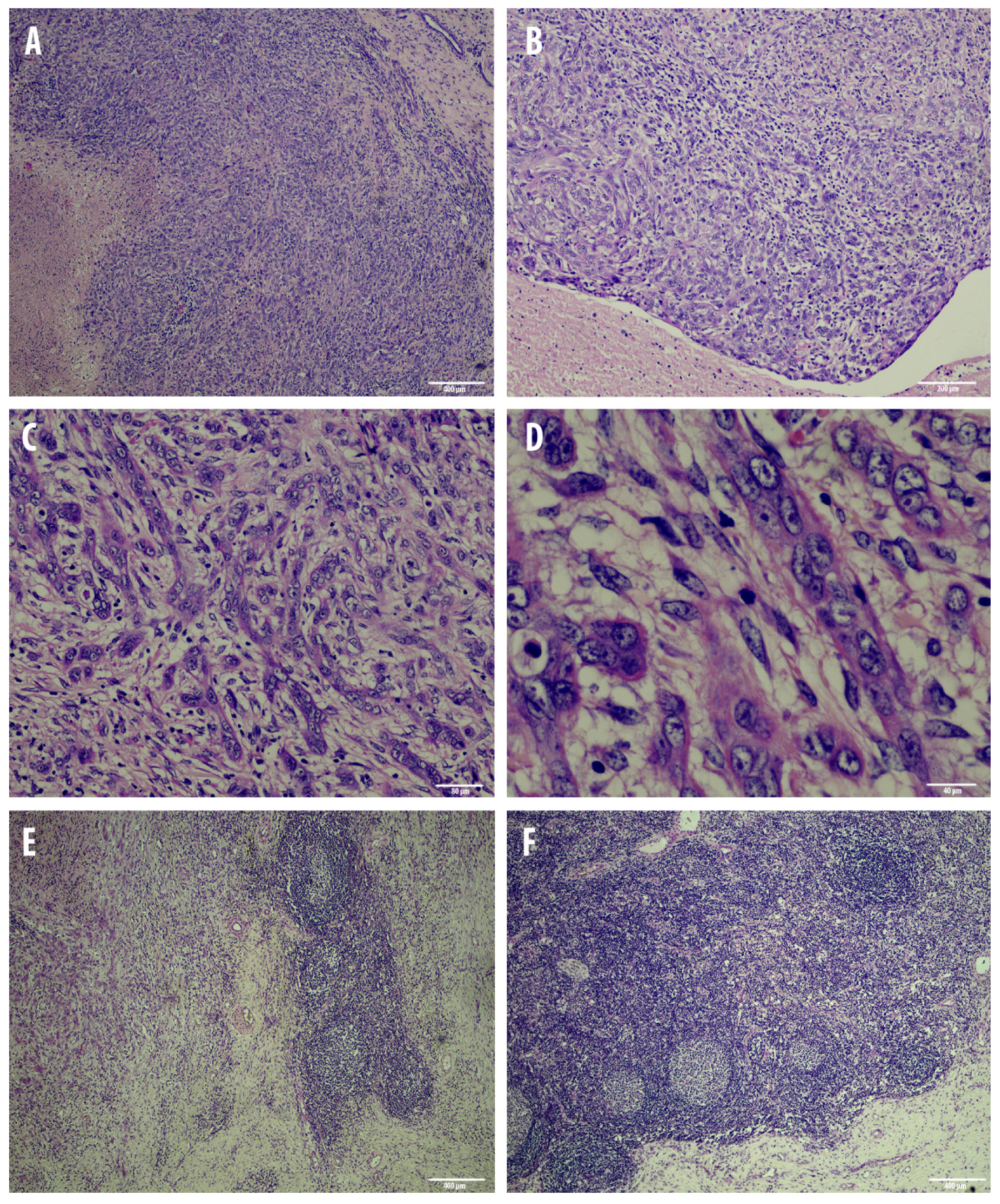
3.1. Post-Mortem and Microscopical Analysis
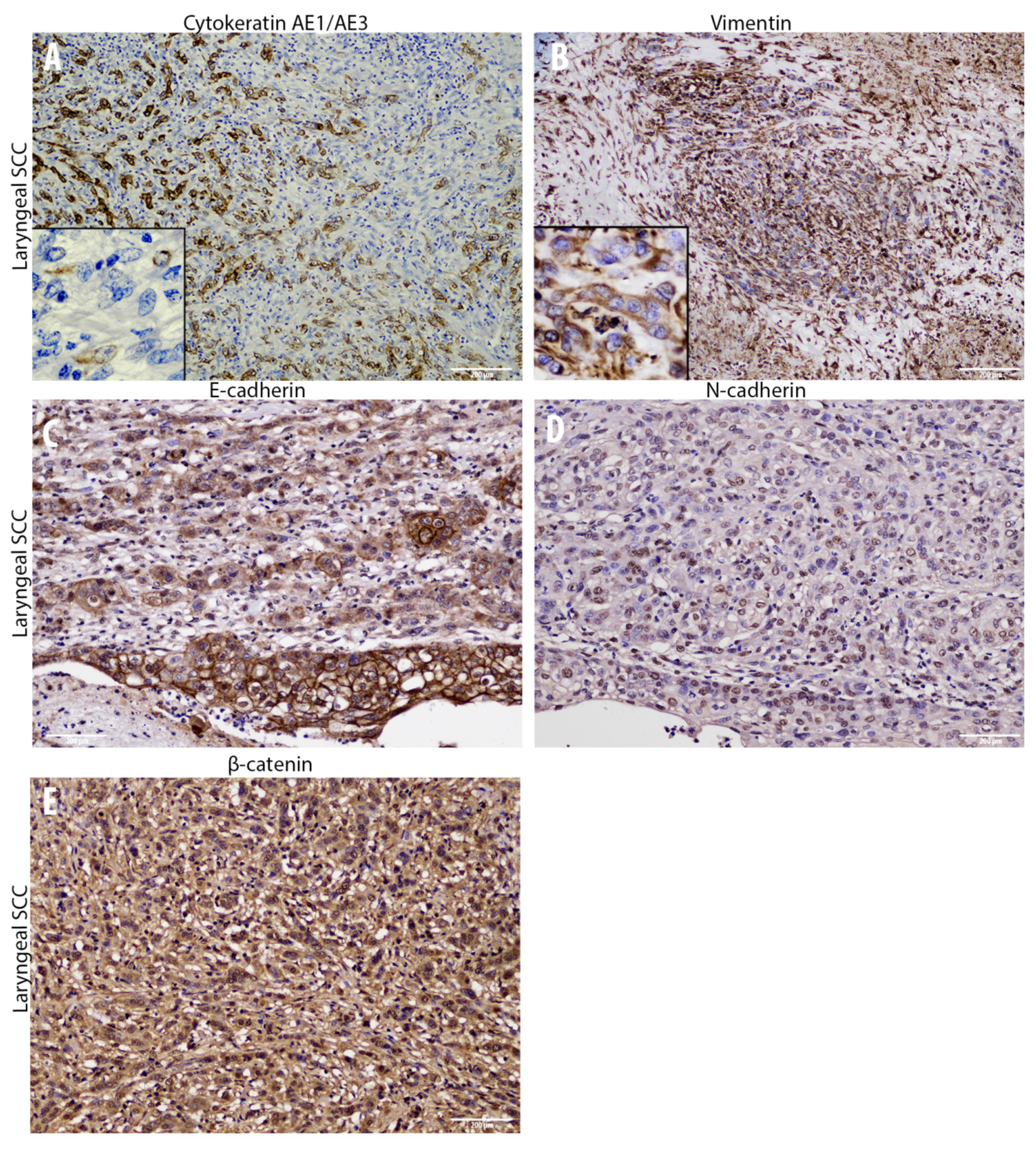
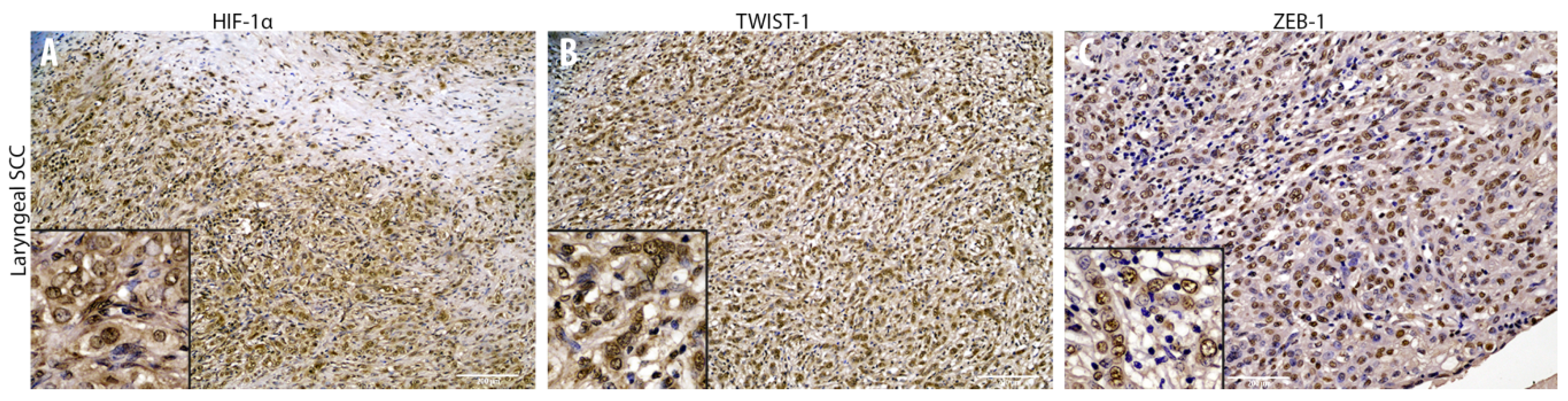
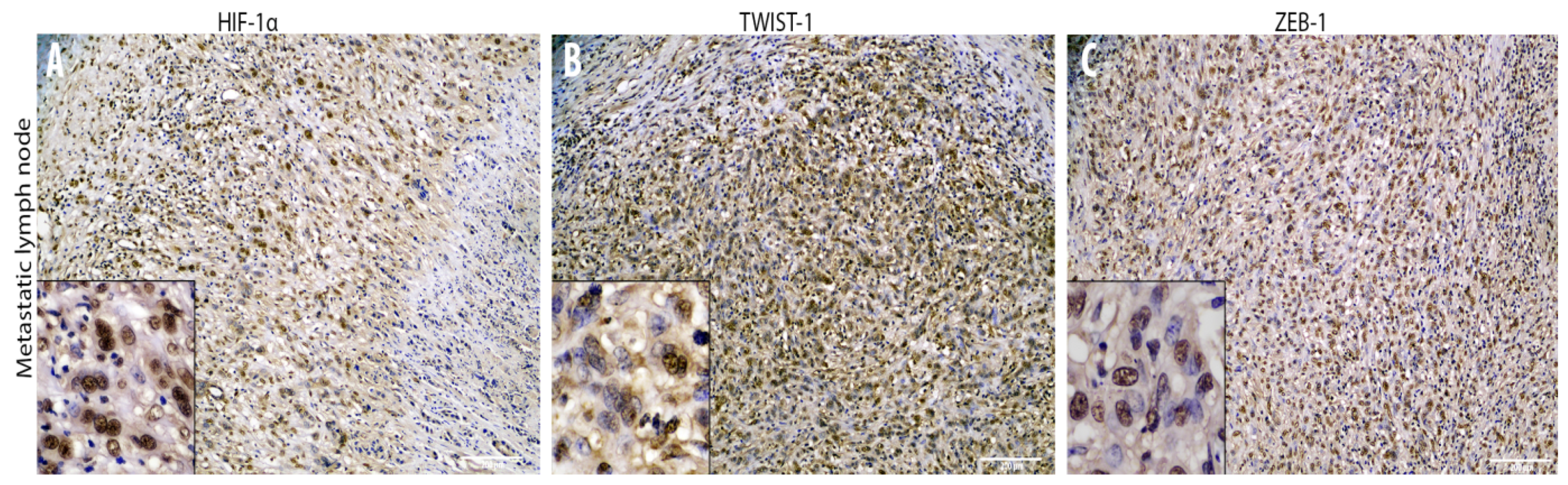
3.2. Cadherin Switch and Intermediate Filaments Rearrangements Suggest an EMT Phenomenon in Equine Laryngeal Squamous Cell Carcinomas
3.3. EcPV2 Detection Suggests a Potential Role in Equine Laryngeal Squamous Cell Carcinomas
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sykora, S.; Brandt, S. Papillomavirus infection and squamous cell carcinoma in horses. Vet. J. 2017, 223, 48–54. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H.J. Squamous cell carcinoma. In Equine Dermatology, 1st ed.; W.B. Saunders: St. Louis, MO, USA, 2003; pp. 707–712. [Google Scholar]
- Van den Top, J.G.; de Heer, N.; Klein, W.R.; Ensink, J.M. Penile and preputial squamous cell carcinoma in the horse: A retrospective study of treatment of 77 affected horses. Equine Vet. J. 2008, 40, 533–537. [Google Scholar] [PubMed]
- Jones, D.L. Squamous cell carcinoma of the larynx and pharynx in horses. Cornell Vet. 1994, 84, 15–24. [Google Scholar]
- Schultz, P. Vocal fold cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, L.; Li, H.; Gao, J.; Yang, Y.; Zhou, F.; Gao, C.; Li, M.; Jin, Q. Human papillomavirus infection and laryngeal cancer risk: A systematic review and meta-analysis. J. Infect. Dis. 2013, 207, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Porcellato, I.; Modesto, P.; Cappelli, K.; Varello, K.; Peletto, S.; Brachelente, C.; Martini, I.; Mechelli, L.; Ferrari, A.; Ghelardi, A.; et al. Equus caballus papillomavirus type 2 (EcPV2) in co-occurring vulvar and gastric lesions of a pony. Res. Vet. Sci. 2020, 132, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Porcellato, I.; Mecocci, S.; Mechelli, L.; Cappelli, K.; Brachelente, C.; Pepe, M.; Orlandi, M.; Gialletti, R.; Passeri, B.; Ferrari, A.; et al. Equine Penile Squamous Cell Carcinomas as a Model for Human Disease: A Preliminary Investigation on Tumor Immune Microenvironment. Cells 2020, 9, 2364. [Google Scholar] [CrossRef] [PubMed]
- Alloway, E.; Linder, K.; May, S.; Rose, T.; DeLay, J.; Bender, S.; Tucker, A.; Luff, J. A Subset of Equine Gastric Squamous Cell Carcinomas Is Associated With Equus Caballus Papillomavirus–2 Infection. Vet. Path. 2020, 57, 427–431. [Google Scholar] [CrossRef]
- Torres, S.M.F.; Koch, S.N. Papillomavirus-associated diseases. Vet. Clin. N. Am. Equine Pract. 2013, 29, 643–655. [Google Scholar] [CrossRef]
- Hibi, H.; Hatama, S.; Obata, A.; Shibahara, T.; Kadota, K. Laryngeal squamous cell carcinoma and papilloma associated with Equus caballus papillomavirus 2 in a horse. J. Vet. Med. Sci. 2019, 81, 1029–1033. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Farabaugh, S.M.; Ford, H.L. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J. Mammary Gland Biol. Neoplasia 2010, 15, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Review article: Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.S.; Willis, C.; Pittaway, R.; Smith, K.; Mair, T.; Priestnall, S.L. Molecular carcinogenesis in equine penile cancer: A potential animal model for human penile cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2018; Volume 36, pp. 532.e9–532.e18. [Google Scholar]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Liu, Y.; Xue, M.; Liu, H.; Du, S.; Zhang, L.; Wang, P. Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res. 2016, 44, 2514–2527. [Google Scholar] [CrossRef] [PubMed]
- Savagner, P. The epithelial–mesenchymal transition (EMT) phenomenon. Ann. Oncol. 2010, 21, 89–92. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell. Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef]
- O’Connor, J.W.; Gomez, E.W. Biomechanics of TGFβ-induced epithelial-mesenchymal transition: Implications for fibrosis and cancer. Clin. Transl. Med. 2014, 3, 23. [Google Scholar] [CrossRef]
- Santamaria, P.G.; Moreno-Bueno, G.; Portillo, F.; Cano, A. EMT: Present and future in clinical oncology. Mol. Oncol. 2017, 11, 718–738. [Google Scholar] [CrossRef]
- Armando, F.; Gambini, M.; Corradi, A.; Becker, K.; Marek, K.; Pfankuche, V.M.; Mergani, A.E.; Brogden, G.; de Buhr, N.; von Köckritz-Blickwede, M.; et al. Mesenchymal to epithelial transition driven by canine distemper virus infection of canine histiocytic sarcoma cells contributes to a reduced cell motility in vitro. J. Cell. Mol. Med. 2020, 24, 9332–9348. [Google Scholar] [CrossRef]
- Leonardi, F.; Costa, G.L.; Dubau, M.; Sabbioni, A.; Simonazzi, B.; Angelone, M. Effects of intravenous romifidine, detomidine, detomidine combined with butorphanol, and xylazine on tear production in horses. Equine Vet. Educ. 2018, 32, 53–57. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Grzegrzolka, J.; Biala, M.; Wojtyra, P.; Kobierzycki, C.; Olbromski, M.; Gomulkiewicz, A.; Piotrowska, A.; Rys, J.; Podhorska-Okolow, M.; Dziegiel, P. Expression of EMT markers SLUG and TWIST in breast cancer. Anticancer Res. 2015, 35, 3961–3968. [Google Scholar] [PubMed]
- Armando, F.; Ferrari, L.; Arcari, M.L.; Azzali, G.; Dallatana, D.; Ferrari, M.; Lombardi, G.; Zanfabro, M.; Di Lecce, R.; Lunghi, P.; et al. Endocanalicular transendothelial crossing (ETC): A novel intravasation mode used by HEK-EBNA293-VEGF-D cells during the metastatic process in a xenograft model. PLoS ONE 2020, 15, e0239932. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Lewis-Tuffin, L.J.; Anastasiadis, P.Z. E-cadherin’s dark side: Possible role in tumor progression. Biochim. Biophys. Acta 2012, 1826, 23–31. [Google Scholar] [CrossRef]
- Kourtidis, A.; Lu, R.; Pence, L.J.; Anastasiadis, P.Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 2017, 358, 78–85. [Google Scholar] [CrossRef]
- Luo, W.R.; Wu, A.B.; Fang, W.Y.; Li, S.Y.; Yao, K.T. Nuclear expression of N-cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology 2012, 61, 237–246. [Google Scholar] [CrossRef]
- Wang, W.; Wen, Q.; Luo, J.; Chu, S.; Chen, L.; Xu, L.; Zang, H.; Alnemah, M.M.; Li, J.; Zhou, J.; et al. Suppression of β-catenin nuclear translocation by CGP57380 decelerates poor progression and potentiates radiation-induced apoptosis in nasopharyngeal carcinoma. Theranostics 2017, 7, 2134–2149. [Google Scholar] [CrossRef]
- Marie-Egyptienne, D.T.; Lohse, I.; Hill, R.P. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013, 341, 63–72. [Google Scholar] [CrossRef]
- Cannito, S.; Novo, E.; Compagnone, A.; Valfrè di Bonzo, L.; Busletta, C.; Zamara, E.; Paternostro, C.; Povero, D.; Bandino, A.; Bozzo, F.; et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 2008, 29, 2267–2278. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell. Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, S.; Chow-Lockerbie, B.; Epp, T.; Knight, C.; Wachoski-Dark, G.; MacDonald-Dickinson, V.; Wobeser, B. Prevalence and Prognostic Impact of Equus caballus Papillomavirus Type 2 Infection in Equine Squamous Cell Carcinomas in Western Canadian Horses. Vet. Path. 2020, 57, 623–631. [Google Scholar] [CrossRef]
- Knight, C.G.; Munday, J.S.; Rosa, B.V.; Kiupel, M. Persistent, widespread papilloma formation on the penis of a horse: A novel presentation of equine papillomavirus type 2 infection. Vet. Dermatol. 2011, 22, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.R.; James, C.D.; Bristol, M.L.; Nulton, T.J.; Wang, X.; Kaur, N.; White, E.A.; Windle, B.; Morgan, I.M. Human papillomavirus 16 E2 regulates keratinocyte gene expression relevant to cancer and the viral life cycle. J. Virol. 2019, 93, e01918–e01941. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, B.; Xiu, Z.; Zhou, Z.; Liu, J.; Li, X.; Tang, X. PI3K/Akt/HIF-1α signaling pathway mediates HPV-16 oncoprotein-induced expression of EMT-related transcription factors in non-small cell lung cancer cells. J. Cancer 2018, 9, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Mostafaei, S.; Aghaei, A.; Hosseini, N.; Darabi, H.; Nouri, M.; Etemadi, A.; Neill, A.O.; Nahand, J.S.; Mirzaei, H.; et al. The association between HPV gene expression, inflammatory agents and cellular genes involved in EMT in lung cancer tissue. BMC Cancer 2020, 20, 916. [Google Scholar] [CrossRef]


| Target Antigen | Antibody Details/Clone | Blocking Serum | Heat Induced Epitope Retrieval (HIER) | Primary Antibody Dilution | Secondary Antibody (1:200) | Positive Control |
|---|---|---|---|---|---|---|
| E-cadherin | Monoclonal mouse anti-human, IgG2a, clone 36/E-Cadherin BD 610181 (BD transduction laboratories) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:100 | biotinylated goat anti-mouse IgG (BA-1000-Vector Labs) | Horse, skin Dog, skin |
| Pan-ckytokeratin AE3/AE1 | Monoclonal mouse anti-human IgG1 SC-81714 (Santa Cruz Biotechnology) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:100 | biotinylated goat anti-mouse IgG (BA-1000-Vector Labs) | Horse, skin Cow, skin |
| β-catenin | Polyclonal goat anti-human, IgG, AB0095-200 (Sicgen) | Rabbit | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:3000 | biotinylated rabbit anti-goat IgG (BA-1000-Vector Labs) | Horse, intestine Dog, intestine |
| N-cadherin | Polyclonal rabbit anti-human, IgG, 22018-1-AP (Proteintech) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:3000 | biotinylated goat anti-rabbit IgG (BA-1000-Vector Labs) | Horse, heart Cow, heart |
| Vimentin | Monoclonal mouse anti-human IgG1, Clone V9 (Dako) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:100 | biotinylated goat anti-mouse IgG (BA-1000-Vector Labs) | Horse, heart (vessels) |
| ZEB-1 | Polyclonal rabbit anti-human, IgG, LS-C31478 (LSBio) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:200 | biotinylated goat anti-rabbit IgG (BA-1000-Vector Labs) | Horse, kidney Dog, kidney |
| TWIST-1 | Polyclonal rabbit anti-human, IgG, Orb329955 (Biorbyt) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:800 | biotinylated goat anti-rabbit IgG (BA-1000-Vector Labs) | Horse, kidney |
| HIF-1α | Polyclonal rabbit anti-human, IgG, NB100-449 (NovusBio) | Goat | Microwave 400 W, 3 cycles, 5 min. each, sodium citrate buffer, pH 6.0 | 1:1000 | biotinylated goat anti-rabbit IgG (BA-1000-Vector Labs) | Horse, kidney Dog, kidney |
| Gene | Primers | Accession Number | Amplicon (Base Pairs) |
|---|---|---|---|
| Ec-PV2-E2 | For: 5’-AAAAGGGAGGGTACGTTGTC-3’ Rev: 5’-CCTGGTAGTAGACATGCTGC-3’ | NC_012123.1 | 90 |
| Ec-PV2-E6 | For: 5’-CGTTGGCCTTCTTTGCATCT-3’ Rev: 5’-AGGTTCAGGTCTGCTGTGTT-3’ | NC_012123.1 | 81 |
| Ec-PV2-L1 | For: 5’-TTGTCCAGGAGAGGGGTTAG-3’ Rev: 5’-TGCCTTCCTTTTCTTGGTGG-3’ | NC_012123.1 | 81 |
| pEc-PV2-E2 | FAM-GCCAAGACAGCCACGACGCCAT-TAMRA | NC_012123.1 | 22 |
| pEc-PV2-E6 | FAM-CCGTGTGGCTATGCTGATGACATTTGG-TAMRA | NC_012123.1 | 27 |
| pEc-PV2-L1 | FAM-CGTCCAGCACCTTCGACCACCA-TAMRA | NC_012123.1 | 22 |
| Sample ID | Type of Sample | RT-PCR | RT-qPCR | ||
|---|---|---|---|---|---|
| B2M | L1 | E2 | E6 | ||
| 1 | Epiglottis tumor | 21.1 ± 0.62 | 26.5 ± 0.17 | >40 | 30.4 ± 0.24 |
| 2 | Kidney negative sample | 21.6 ± 0.27 | - | >40 | >40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armando, F.; Godizzi, F.; Razzuoli, E.; Leonardi, F.; Angelone, M.; Corradi, A.; Meloni, D.; Ferrari, L.; Passeri, B. Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives. Animals 2020, 10, 2318. https://doi.org/10.3390/ani10122318
Armando F, Godizzi F, Razzuoli E, Leonardi F, Angelone M, Corradi A, Meloni D, Ferrari L, Passeri B. Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives. Animals. 2020; 10(12):2318. https://doi.org/10.3390/ani10122318
Chicago/Turabian StyleArmando, Federico, Francesco Godizzi, Elisabetta Razzuoli, Fabio Leonardi, Mario Angelone, Attilio Corradi, Daniela Meloni, Luca Ferrari, and Benedetta Passeri. 2020. "Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives" Animals 10, no. 12: 2318. https://doi.org/10.3390/ani10122318
APA StyleArmando, F., Godizzi, F., Razzuoli, E., Leonardi, F., Angelone, M., Corradi, A., Meloni, D., Ferrari, L., & Passeri, B. (2020). Epithelial to Mesenchymal Transition (EMT) in a Laryngeal Squamous Cell Carcinoma of a Horse: Future Perspectives. Animals, 10(12), 2318. https://doi.org/10.3390/ani10122318








