Global Methylation and Protamine Deficiency in Ram Spermatozoa Correlate with Sperm Production and Quality but Are Not Influenced by Melatonin or Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Semen Collection and Preparation
2.4. Chemicals
2.5. Flow Cytometry
2.5.1. Protamine Deficiency
2.5.2. Global Methylation
2.6. Statistical Analysis
3. Results
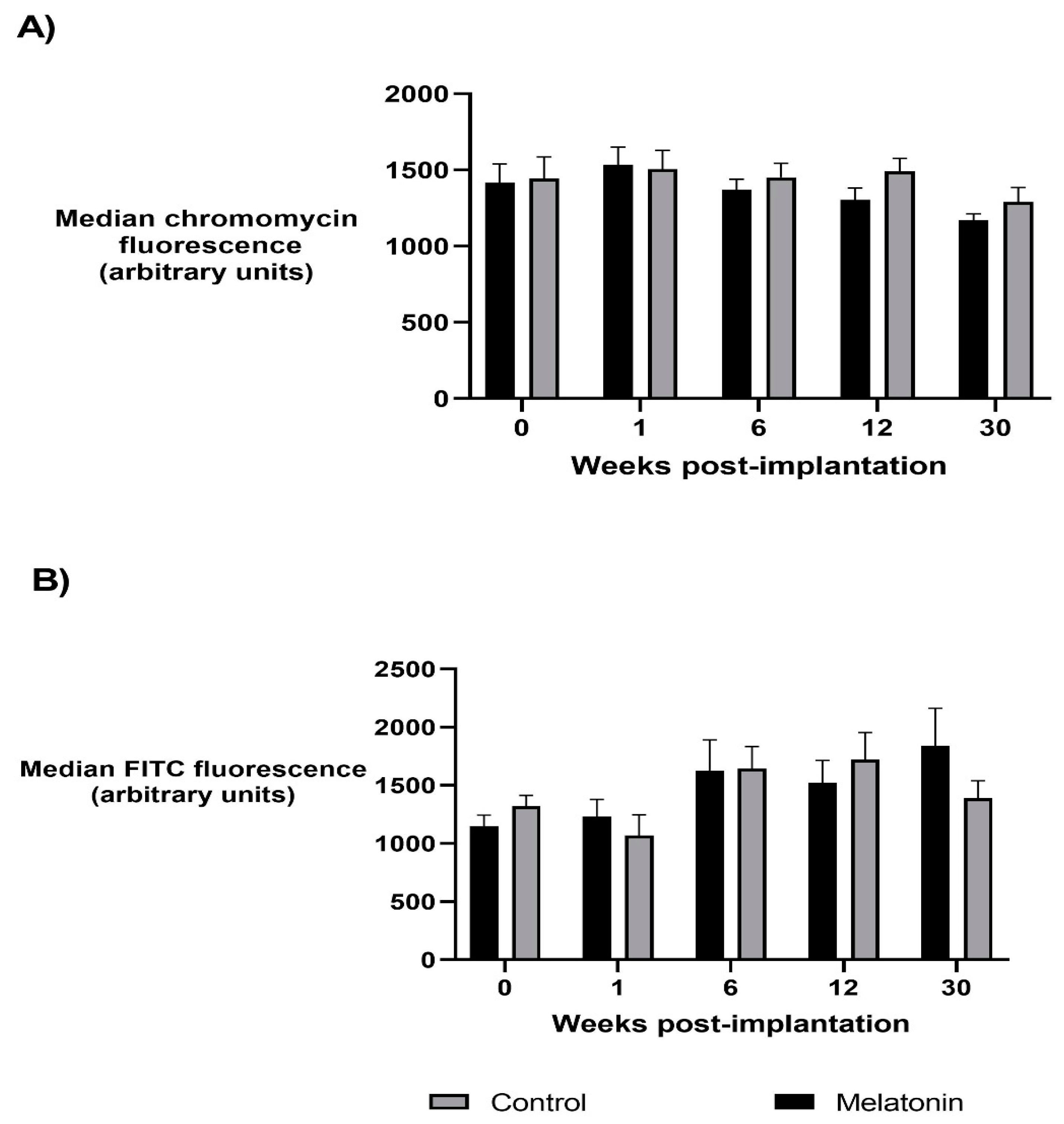
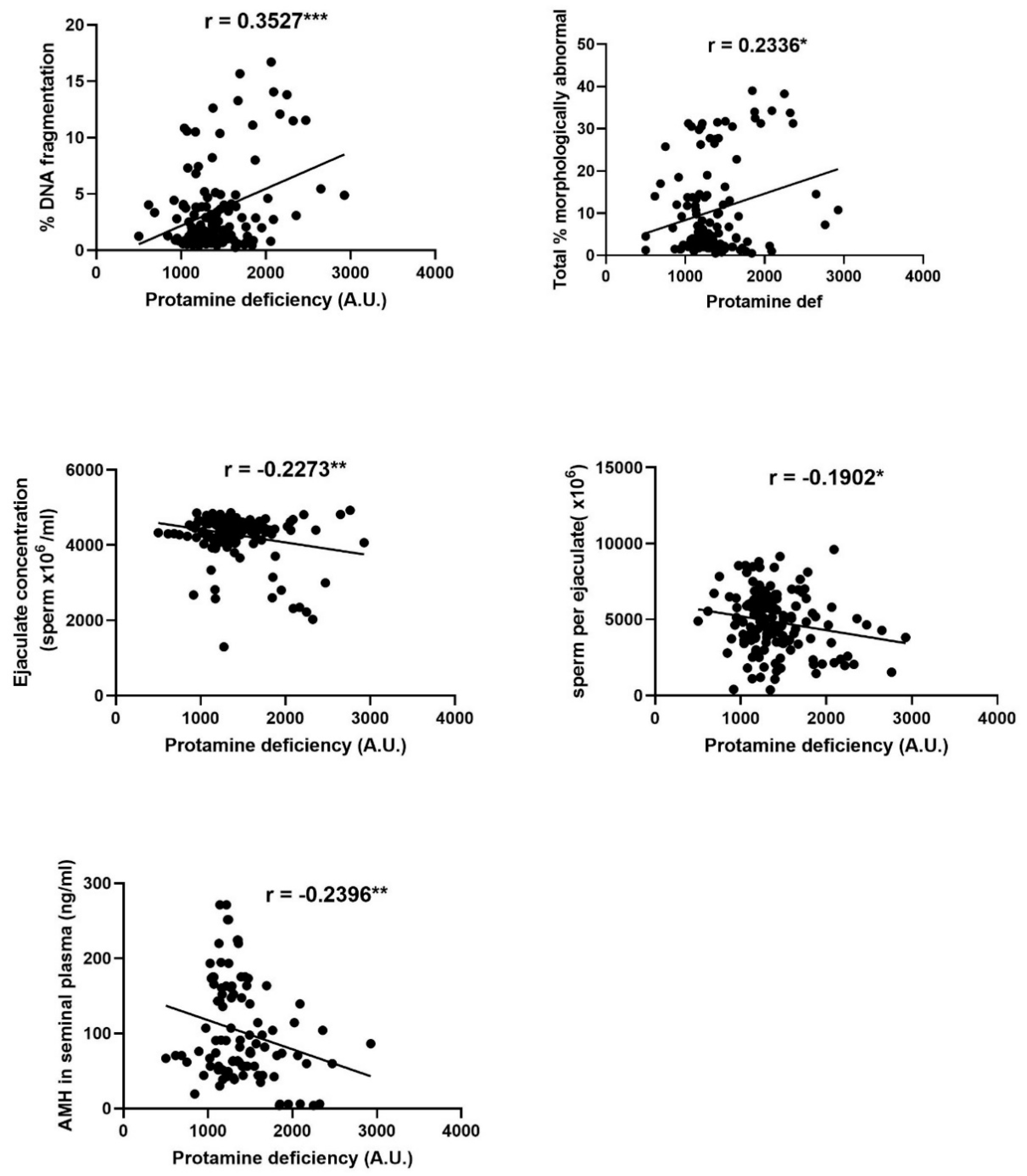
3.1. Protamine Deficiency
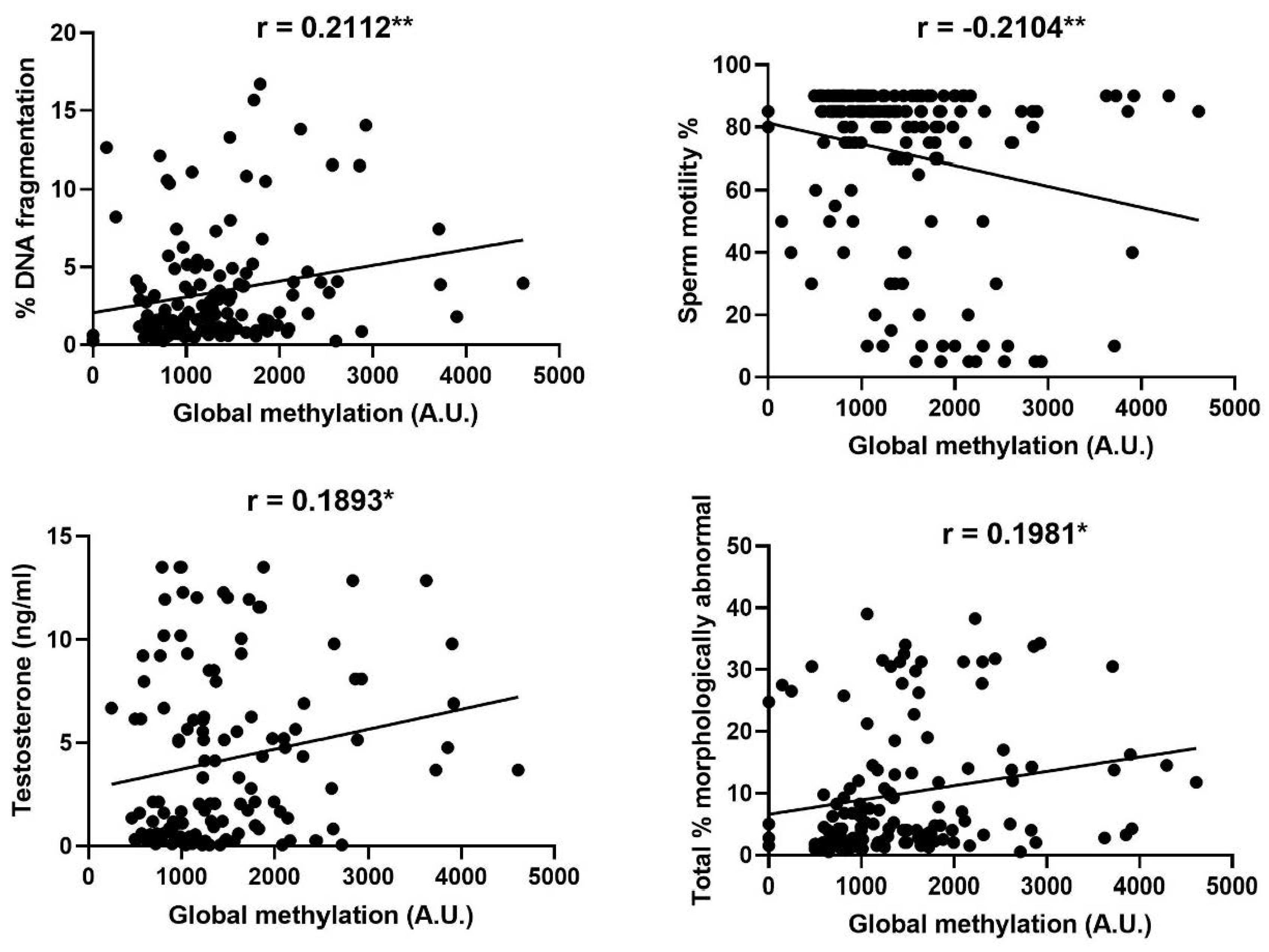
3.2. Sperm Global Methylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; O’Brien, B.J.; Harvey, J.T.; Charchar, F.J. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics 2015, 7, 717–731. [Google Scholar] [CrossRef]
- Radford, E.J.; Ito, M.; Shi, H.; Corish, J.A.; Yamazawa, K.; Isganaitis, E.; Seisenberger, S.; Hore, T.A.; Reik, W.; Erkek, S.; et al. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 2014, 345, 1255903. [Google Scholar] [CrossRef] [PubMed]
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The preconception environment and sperm epigenetics. Andrology 2019. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; Brundo, M.V.; Basile, A.; et al. Protamine-like proteins’ analysis as an emerging biotechnique for cadmium impact assessment on male mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Weems, P.W.; Goodman, R.L.; Lehman, M.N. Neural mechanisms controlling seasonal reproduction: Principles derived from the sheep model and its comparison with hamsters. Front. Neuroendocrinol. 2015, 37, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A.; Clarke, I.J. Photoperiodically-lnduced Cycles in the Secretion of Prolactin in Hypothalamo-Pituitary Disconnected Rams: Evidence for Translation of the Melatonin Signal in the Pituitary Gland. J. Neuroendocrinol. 1994, 6, 251–260. [Google Scholar] [CrossRef]
- Rekik, M.; Taboubi, R.; Ben Salem, I.; Fehri, Y.; Sakly, C.; Lassoued, N.; Hilali, E.M. Melatonin administration enhances the reproductive capacity of young rams under a southern Mediterranean environment. Anim. Sci. J. 2015, 86, 666–672. [Google Scholar] [CrossRef]
- Kaya, A.; Baspinar, N.; Yildiz, C.; Kurtoglu, F.; Ataman, M.; Haliloglu, S. Influence of melatonin implantation on sperm quality, biochemical composition of the seminal plasma and plasma testosterone levels in rams. Rev. Med. Vet. (Toulouse) 2000, 151, 1143–1146. [Google Scholar]
- Rosa, H.; Juniper, D.; Bryant, M. Effects of recent sexual experience and melatonin treatment of rams on plasma testosterone concentration, sexual behaviour and ability to induce ovulation in seasonally anoestrous ewes. J. Reprod. Fertil. 2000, 120, 169–176. [Google Scholar] [CrossRef][Green Version]
- Boland, M.P.; Al-Kamali, A.A.; Crosby, T.F.; Haynes, N.B.; Howles, C.M.; Kelleher, D.L.; Gordon, I. The influence of breed, season and photoperiod on semen characteristics, testicular size, libido and plasma hormone concentrations in rams. Anim. Reprod. Sci. 1985, 9, 241–252. [Google Scholar] [CrossRef]
- Belkadi, S.; Safsaf, B.; Heleili, N.; Tlidjane, M.; Belkacem, L.; Oucheriah, Y. Seasonal influence on sperm parameters, scrotal measurements, and serum testosterone in Ouled Djellal breed rams in Algeria. Vet. World 2017, 10, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Abella, D.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Villegas, N.; Bentancur, O. Sperm production, testicular size, serum gonadotropins and testosterone levels in Merino and Corriedale breeds. Reprod. Nutr. Dev. 1999, 39, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, M.J.; Khodaei, H.R. Seasonal thyroidal activity and reproductive characteristics of Iranian fat-tailed rams. Anim. Reprod. Sci. 2005, 88, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Pool, K.R.; Rickard, J.P.; Pini, T.; de Graaf, S.P. Exogenous melatonin advances the ram breeding season and increases testicular function. Sci. Rep. 2020, 10, 9711. [Google Scholar] [CrossRef]
- Casao, V.S.; Palacin, I.; Perez-Pe, R.; Lavina, A.; Quintin, F.J.; Sevilla, E.; Abecia, J.A.; Cebrian-Perez, J.A.; Forcada, F. Effects of melatonin implants during non-breeding season on sperm motility and reproductive parameters in rasa aragonesa rams. Reprod. Domest. Anim. 2008, 45, 425–432. [Google Scholar] [CrossRef]
- Buffoni, A.; Vozzi, A.; Gonzalez, D.M.; Viegas, H.; LaTorraca, A.; Hozbor, F.; Ledesma, A.; Abecia, J.A. Melatonin modifies scrotal circumference but not plasma testosterone concentrations and semen quality of rams during the seasonal anestrus at 43°S. Biol. Rhythm. Res. 2015, 46, 785–795. [Google Scholar] [CrossRef]
- Cevik, M.; Yilmazer, C.; Kocyigit, A. Effects of melatonin implantation on the fertility potentials of Kivircik and Charollais ewes and rams during the non-breeding season. Pol. J. Vet. Sci 2017, 20, 501–506. [Google Scholar] [CrossRef]
- Rosa, H.J.D.; Silva, C.C.; Bryant, M.J. The effect of melatonin treatment in rams on seasonal variation of testicular size and semen production parameters. Small Rumin. Res. 2012, 102, 197–201. [Google Scholar] [CrossRef]
- Deng, S.-L.; Wang, Z.-P.; Jin, C.; Kang, X.-L.; Batool, A.; Zhang, Y.; Li, X.; Wang, X.; Chen, S.; Chang, C.; et al. Melatonin promotes sheep Leydig cell testosterone secretion in a co-culture with Sertoli cells. Theriogenology 2018, 106, 170–177. [Google Scholar] [CrossRef]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrian-Perez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Serna, M.; Torres-Ruda, F.; Cardozo, J.A.; Grajales-Lombana, H.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Perez-Pe, R.; Casao, A. Changes in melatonin concentrations in seminal plasma are not correlated with testosterone or antioxidant enzyme activity when rams are located in areas with an equatorial photoperiod. Anim. Reprod. Sci. 2019, 200, 22–30. [Google Scholar] [CrossRef]
- Dada, R.; Kumar, M.; Jesudasan, R.; Fernández, J.L.; Gosálvez, J.; Agarwal, A. Epigenetics and its role in male infertility. J. Assist. Reprod. Genet. 2012, 29, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Giacone, F.; Cannarella, R.; Mongioì, L.M.; Alamo, A.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Epigenetics of male fertility: Effects on assisted reproductive techniques. World J. Men’s Health 2019, 37, 148–156. [Google Scholar] [CrossRef]
- Kutchy, N.A.; Menezes, E.S.B.; Chiappetta, A.; Tan, W.; Wills, R.W.; Kaya, A.; Topper, E.; Moura, A.A.; Perkins, A.D.; Memili, E. Acetylation and methylation of sperm histone 3 lysine 27 (H3K27ac and H3K27me3) are associated with bull fertility. Andrologia 2018, 50, e12915. [Google Scholar] [CrossRef]
- Kaneda, M.; Okano, M.; Hata, K.; Sado, T.; Tsujimoto, H.; Li, E.; Sasaki, H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004, 429, 900–903. [Google Scholar] [CrossRef]
- Marques, C.J.; Francisco, T.; Sousa, S.; Carvalho, F.; Barros, A.; Sousa, M. Methylation defects of imprinted genes in human testicular spermatozoa. Fertil. Steril. 2010, 94, 585–594. [Google Scholar] [CrossRef]
- Godmann, M.; Lambrot, R.; Kimmins, S. The dynamic epigenetic program in male germ cells: Its role in spermatogenesis, testis cancer, and its response to the environment. Microsc. Res. Tech. 2009, 72, 603–619. [Google Scholar] [CrossRef]
- O’doherty, A.M.; McGettigan, P.A. Epigenetic processes in the male germline. Reprod. Fertil. Dev. 2015, 27, 725–738. [Google Scholar] [CrossRef]
- Laqqan, M.; Ahmed, I.; Yasin, M.; Hammadeh, M.E.; Yassin, M. Influence of variation in global sperm DNA methylation level on the expression level of protamine genes and human semen parameters. Andrologia 2020, 52. [Google Scholar] [CrossRef]
- Benchaib, M.; Ajina, M.; Lornage, J.; Niveleau, A.; Durand, P.; Guérin, J.F. Quantitation by image analysis of global DNA methylation in human spermatozoa and its prognostic value in in vitro fertilization: A preliminary study. Fertil. Steril. 2003, 80, 947–953. [Google Scholar] [CrossRef]
- Khezri, A.; Narud, B.; Stenseth, E.B.; Johannisson, A.; Myromslien, F.D.; Gaustad, A.H.; Wilson, R.C.; Lyle, R.; Morrell, J.M.; Kommisrud, E.; et al. DNA methylation patterns vary in boar sperm cells with different levels of DNA fragmentation. BMC Genom. 2019, 20, 897. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.; Elmhiri, G.; Gloaguen, C.; Magneron, V.; Kereselidze, D.; Saci, N.; Elie, C.; Vaysset, E.; Benadjaoud, M.M.; Tack, K.; et al. Multigenerational exposure to uranium changes morphometric parameters and global DNA methylation in rat sperm. C. R. Biol. 2019, 342, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, F.; Razavi, S.; Hashemi, F. Protective effects of zinc on rat sperm chromatin integrity involvement: DNA methylation, DNA fragmentation, ubiquitination and protamination after bleomycin etoposide and cis-platin treatment. Theriogenology 2020, 142, 177–183. [Google Scholar] [CrossRef] [PubMed]
- González-Marín, C.; Gosálvez, J.; Roy, R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 2012, 13, 14026–14052. [Google Scholar] [CrossRef]
- Lewis, J.D.; Song, Y.; De Jong, M.E.; Bagha, S.M.; Ausió, J. A walk though vertebrate and invertebrate protamines. Chromosoma 2003, 111, 473–482. [Google Scholar] [CrossRef]
- Jodar, M.; Oliva, R. Protamine alterations in human spermatozoa. Adv. Exp. Med. Biol. 2014, 791, 83–102. [Google Scholar] [CrossRef]
- Cho, C.; Willis, W.D.; Goulding, E.H.; Jung-Ha, H.; Choi, Y.-C.; Hecht, N.B.; Eddy, E.M. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat. Genet. 2001, 28, 82–86. [Google Scholar] [CrossRef]
- Fortes, M.R.S.; Satake, N.; Corbet, D.H.; Corbet, N.J.; Burns, B.M.; Moore, S.S.; Boe-Hansen, G.B. Sperm protamine deficiency correlates with sperm DNA damage in Bos indicus bulls. Andrology 2014, 2, 370–378. [Google Scholar] [CrossRef]
- Simes, R.; Feitosa, W.B.; Mendes, C.M.; Marques, M.G.; Nicacio, A.C.; De Barros, F.R.O.; Visintin, J.A.; Assumpccao, M. Use of chromomycin A3 staining in bovine sperm cells for detection of protamine deficiency. Biotech. Histochem. 2009, 84, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Bahreinian, M.; Tavalaee, M.; Abbasi, H.; Kiani-Esfahani, A.; Shiravi, A.H.; Nasr-Esfahani, M.H. DNA hypomethylation predisposes sperm to DNA damage in individuals with varicocele. Syst. Biol. Reprod. Med. 2015, 61, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Benchaib, M.; Braun, V.; Ressnikof, D.; Lornage, J.; Durand, P.; Niveleau, A.; Guerin, J.F. Influence of global sperm DNA methylation on IVF results. Hum. Reprod. 2005, 20, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Lolis, D.; Georgiou, I.; Syrrou, M.; Zikopoulos, K.; Konstantelli, M.; Messinis, I. Chromomycin A3-staining as an indicator of protamine deficiency and fertilization. Int. J. Androl. 1996, 19, 23–27. [Google Scholar] [CrossRef]
- Zandemami, M.; Qujeq, D.; Akhondi, M.M.; Kamali, K.; Raygani, M.; Lakpour, N.; Shiraz, E.S.; Sadeghi, M.R. Correlation of CMA3 Staining with Sperm Quality and Protamine Deficiency. Lab. Med. 2012, 43, 262–267. [Google Scholar] [CrossRef]
- Ni, K.; Spiess, A.N.; Schuppe, H.C.; Steger, K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: A systematic review and meta-analysis. Andrology 2016, 4, 789–799. [Google Scholar] [CrossRef]
- Steger, K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat. Embryol. (Berl.) 1999, 199, 471–487. [Google Scholar] [CrossRef]
- Francis, S.; Yelumalai, S.; Jones, C.; Coward, K. Aberrant protamine content in sperm and consequential implications for infertility treatment. Hum. Fertil. 2014, 17, 80–89. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Riel, J.M.; Ward, M.A. Paternal DNA damage resulting from various sperm treatments persists after fertilization and is similar before and after DNA replication. J. Androl. 2012, 33, 229–238. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Lorena, F.B. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- Malama, E.; Bollwein, H.; Taitzoglou, I.A.; Theodosiou, T.; Boscos, C.M.; Kiossis, E. Chromatin integrity of ram spermatozoa. Relationships to annual fluctuations of scrotal surface temperature and temperature-humidity index. Theriogenology 2013, 80, 533–541. [Google Scholar] [CrossRef] [PubMed]
- García-Macías, V.; Martínez-Pastor, F.; Álvarez, M.; Borragan, S.; Chamorro, C.A.; Soler, A.J.; Anel, L.; de Paz, P. Seasonal changes in sperm chromatin condensation in ram (Ovis aries), Iberian red deer (Cervus elaphus hispanicus), and brown bear (Ursus arctos). J. Androl. 2006, 27, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M. Seasonal dependence of cadmium molecular effects on Mytilus galloprovincialis (Lamarck, 1819) protamine-like protein properties. Mol. Reprod. Dev. 2019, 86, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Semercioz, A.; Baltaci, A.K.; Mogulkoc, R.; Avunduk, M.C. Effect of Zinc and Melatonin on Oxidative Stress and Serum Inhibin-B Levels in a Rat Testicular Torsion–Detorsion Model. Biochem. Genet. 2017, 55, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Pierik, F.H.; Vreeburg, J.T.M.; Stijnen, T.; De Jong, F.H.; Weber, R.F.A. Serum inhibin B as a marker of spermatogenesis. J. Clin. Endocrinol. Metab. 1998, 83, 3110–3114. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A. Protective Effects of Umbelliferone in Experimental Testicular Ischaemia/Reperfusion Injury in Rats. Anat. Physiol. 2016, 6. [Google Scholar] [CrossRef]
- Barranco, I.; Fernandez-Fuertes, B.; Padilla, L.; Delgado-Bermúdez, A.; Tvarijonaviciute, A.; Yeste, M. Seminal Plasma Anti-Müllerian Hormone: A Potential AI-Boar Fertility Biomarker? Biology 2020, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Nery, S.F.; Vieira, M.A.F.; Dela Cruz, C.; Lobach, V.N.M.; Del Puerto, H.L.; Torres, P.B.; Rocha, A.L.L.; Reis, A.B.; Reis, F.M. Seminal plasma concentrations of Anti-Müllerian hormone and inhibin B predict motile sperm recovery from cryopreserved semen in asthenozoospermic men: A prospective cohort study. Andrology 2014, 2, 918–923. [Google Scholar] [CrossRef]
- La Marca, A.; Sighinolfi, G.; Radi, D.; Argento, C.; Baraldi, E.; Artenisio, A.C.; Stabile, G.; Volpe, A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 2010, 16, 113–130. [Google Scholar] [CrossRef]
- Fujisawa, M.; Yamasaki, T.; Okada, H.; Kamidono, S. The significance of anti-Mullerian hormone concentration in seminal plasma for spermatogenesis. Hum. Reprod. 2002, 17, 968–970. [Google Scholar] [CrossRef]
- Mostafa, T.; Amer, M.K.; Abdel-Malak, G.; Nsser, T.A.; Zohdy, W.; Ashour, S.; El-Gayar, D.; Awad, H.H. Seminal plasma anti-Müllerian hormone level correlates with semen parameters but does not predict success of testicular sperm extraction (TESE). Asian J. Androl. 2007, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Duvilla, E.; Lejeune, H.; Trombert-Paviot, B.; Gentil-Perret, A.; Tostain, J.; Levy, R. Significance of inhibin B and anti-Müllerian hormone in seminal plasma: A preliminary study. Fertil. Steril. 2008, 89, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Capra, E.; Lazzari, B.; Turri, F.; Cremonesi, P.; Portela, A.M.R.; Ajmone-Marsan, P.; Stella, A.; Pizzi, F. Epigenetic analysis of high and low motile sperm populations reveals methylation variation in satellite regions within the pericentromeric position and in genes functionally related to sperm DNA organization and maintenance in Bos taurus. BMC Genom. 2019, 20, 940. [Google Scholar] [CrossRef] [PubMed]
- Rahiminia, T.; Yazd, E.F.; Fesahat, F.; Moein, M.R.; Mirjalili, A.M.; Talebi, A.R. Sperm chromatin and DNA integrity, methyltransferase mRNA levels, and global DNA methylation in oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2018, 45, 17–24. [Google Scholar] [CrossRef]
- Montjean, D.; Zini, A.; Ravel, C.; Belloc, S.; Dalleac, A.; Copin, H.; Boyer, P.; McElreavey, K.; Benkhalifa, M. Sperm global DNA methylation level: Association with semen parameters and genome integrity. Andrology 2015, 3, 235–240. [Google Scholar] [CrossRef]
- Houshdaran, S.; Cortessis, V.K.; Siegmund, K.; Yang, A.; Laird, P.W.; Sokol, R.Z. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE 2007, 2, e1289. [Google Scholar] [CrossRef]
- Verma, A.; Rajput, S.; De, S.; Kumar, R.; Chakravarty, A.K.; Datta, T.K. Genome-wide profiling of sperm DNA methylation in relation to buffalo (Bubalus bubalis) bull fertility. Theriogenology 2014, 82, 750–759. [Google Scholar] [CrossRef]
- Hamad, M.F.; Dayyih, W.A.A.; Laqqan, M.; AlKhaled, Y.; Montenarh, M.; Hammadeh, M.E. The status of global DNA methylation in the spermatozoa of smokers and non-smokers. Reprod. Biomed. Online 2018, 37, 581–589. [Google Scholar] [CrossRef]
- David, I.; Kohnke, P.; Lagriffoul, G.; Praud, O.; Plouarboué, F.; Degond, P.; Druart, X. Mass sperm motility is associated with fertility in sheep. Anim. Reprod. Sci. 2015, 161, 75–81. [Google Scholar] [CrossRef]
- David, I.; Kohnke, P.; Fehrenbach, J.; Lopes Simoes, A.R.; Debreuve, E.; Descombes, X.; Plouraboue, F.; Degond, P.; Druart, X. New objective measurements of semen wave motion are associated with fertility in sheep. Reprod. Fertil. Dev. 2018, 30, 889. [Google Scholar] [CrossRef]
- Aydos, O.S.; Yükselten, Y.; Kaplan, F.; Sunguroğlu, A.; Aydos, K. İnfertil erkeklerde sperm DNA bütünlüğü ve konvansiyonel semen parametreleri arasındaki korelasyonun analizi. Turk. Urol. Derg. 2015, 41, 191–197. [Google Scholar] [CrossRef]
- Elbashir, S.; Magdi, Y.; Rashed, A.; Ibrahim, M.A.; Edris, Y.; Abdelaziz, A.M. Relationship between sperm progressive motility and DNA integrity in fertile and infertile men. Middle East. Fertil. Soc. J. 2018, 23, 195–198. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, D.P.C.; Tsao, H.M.; Cheng, T.C.; Liu, C.H.; Lee, M.S. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil. Steril. 2005, 84, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J. Effects of Age on Testicular Function and Consequences of Testosterone Treatment. J. Clin. Endocrinol. Metab. 2001, 86, 2369–2372. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Olvera, S.F.; Rajmil, O.; Sanchez-Curbelo, J.-R.; Vinay, J.; Rodriguez-Espinosa, J.; Ruiz-Castañé, E. Association of serum testosterone levels and testicular volume in adult patients. Andrologia 2018, 50, e12933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pool, K.R.; Rickard, J.P.; de Graaf, S.P. Global Methylation and Protamine Deficiency in Ram Spermatozoa Correlate with Sperm Production and Quality but Are Not Influenced by Melatonin or Season. Animals 2020, 10, 2302. https://doi.org/10.3390/ani10122302
Pool KR, Rickard JP, de Graaf SP. Global Methylation and Protamine Deficiency in Ram Spermatozoa Correlate with Sperm Production and Quality but Are Not Influenced by Melatonin or Season. Animals. 2020; 10(12):2302. https://doi.org/10.3390/ani10122302
Chicago/Turabian StylePool, Kelsey R., Jessica P. Rickard, and Simon P. de Graaf. 2020. "Global Methylation and Protamine Deficiency in Ram Spermatozoa Correlate with Sperm Production and Quality but Are Not Influenced by Melatonin or Season" Animals 10, no. 12: 2302. https://doi.org/10.3390/ani10122302
APA StylePool, K. R., Rickard, J. P., & de Graaf, S. P. (2020). Global Methylation and Protamine Deficiency in Ram Spermatozoa Correlate with Sperm Production and Quality but Are Not Influenced by Melatonin or Season. Animals, 10(12), 2302. https://doi.org/10.3390/ani10122302






