Transient Pulmonary Artery Hypertension in Holstein Neonate Calves
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Sample Collection and Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paiano, R.B.; Birgel, D.B.; Birgel Junior, E.H. Influence of peripartum on the erythrogram of Holstein dairy cows. J. S. Afr. Vet. Assoc. 2020, 91, a1975. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Birgel, D.B.; Bonilla, J.; Birgel Junior, E.H. Evaluation of biochemical profile of dairy cows with metabolic diseases in tropical conditions. Reprod. Domest. Anim. 2020, 55, 1219–1228. [Google Scholar] [CrossRef]
- Paiano, R.B.; Lahr, F.C.; Silva, L.S.B.; Marques, D.S.; Ferreira, C.A.; Birgel, D.B.; Bisinotto, R.S.; Birgel Junior, E.H. Haematological and biochemical profiles during the puerperium in dairy cows. Acta Vet. Hung. 2019, 67, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Birgel, D.B.; Ollhoff, R.D.; Birgel Junior, E.H. Biochemical profile and productive performance in dairy cows with lameness during postpartum period. Acta Sci. Vet. 2019, 47, 1673. [Google Scholar] [CrossRef]
- Paiano, R.B.; Birgel, D.B.; Bonilla, J.; Birgel Junior, E.H. Alterations in biochemical profiles and reproduction performance in postpartum dairy cows with metritis. Reprod. Domest. Anim. 2020, 55, 1–8. [Google Scholar] [CrossRef]
- Vannucchi, C.I.; Silva, L.G.; Lúcio, C.F.; Veiga, G.A.L. Oxidative stress and acid–base balance during the transition period of neonatal Holstein calves submitted to different calving times and obstetric assistance. J. Dairy Sci. 2019, 102, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.P.; McGuirk, S.M. Respiratory disease of the bovine neonate. Vet. Clin. N. Am. Food Anim. Pract. 2009, 1, 121–137. [Google Scholar] [CrossRef]
- Dillane, P.; Krump, L.; Kennedy, A.; Sayers, R.G.; Sayers, G.P. Establishing blood gas ranges in healthy bovine neonates differentiated by age, sex, and breed type. J. Dairy Sci. 2018, 101, 3205–3212. [Google Scholar] [CrossRef]
- Murray, C.F.; Leslie, K.E. Newborn calf vitality: Risk factors, characteristics, assessment, resulting outcomes and strategies for improvement. Vet. J. 2013, 198, 322–328. [Google Scholar] [CrossRef]
- Benesi, F.J. Síndrome asfixia neonatal dos bezerros. Importância e avaliação crítica. Arq. Esc. Med. Vet. 1993, 16, 38–48. [Google Scholar]
- Sbano, J.C.N.; Tsutsui, J.M.; Filho, M.T.; Junior, W.M. The role of Doppler echocardiography in the evaluation of pulmonary hypertension. J. Bras. Pneumol. 2004, 30, 78–86. [Google Scholar] [CrossRef]
- Chavatte-Palmer, P.; Remy, D.; Cordonnier, N.; Richard, C.; Issenman, H.; Laigre, P.; Heyman, Y.; Mialot, J.P. Health status of cloned cattle at different ages. Cloning Stem Cells 2004, 6, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Bowdle, T.A. Complications of invasive monitoring. Anesthesiol. Clin. N. Am. 2002, 3, 571–588. [Google Scholar] [CrossRef]
- Amory, H.; Linden, A.; Desmecht, D.; Rollin, F.; Genicot, B.; Lekeux, P. Validation of the thermodilution techinique for the estimation of the cardiac output in the unsedated calf. J. Vet. Med. Ser. A 1991, 38, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Keegan, R.D.; Greene, S.A.; Valdez, R.A.; Knowles, D.K.l. Cardiovascular effects of desflurane in mechanically ventilated calves. Am. J. Vet. Res. 2006, 67, 387–391. [Google Scholar] [CrossRef]
- Meirelles, F.V.; Birgel Junior, E.H.; Perecin, F.; Bertolini, M.; Traldi, A.S.; Pimentel, J.R.V.; Komninou, E.R.; Sangali, J.R.; Fantinato Neto, P.; Nunes, M.T.; et al. Delivery of cloned offspring: Experience in Zebu cattle (Bos indicus). Reprod. Fert. Develop. 2010, 22, 88–97. [Google Scholar] [CrossRef]
- Tsunoda, Y. KATO, Y. Recent progress and problems in animal cloning. Differentiation 2002, 69, 158–161. [Google Scholar] [CrossRef]
- Birgel Junior, E.H.; Meirelles, F.V.; Komninou, E.R.; Nunes, M.T.; Pogliani, F.C.; Fantinato Neto, P.; Yasuoka, M.M.; Pimental, J.R.V.; Kubrusly, F.S.; Miglino, M.A. Distúrbios clínicos observados nos primeiros 30 dias de vida de bezerros clonados da raça Nelore. Acta Sci. Vet. 2011, 39, 243–252. [Google Scholar]
- Hill, J.R.; Rousell, A.J.; Cibelli, J.B. Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies). Theriogenology 1999, 51, 1451–1465. [Google Scholar] [CrossRef]
- Fatinato Neto, P. Pulmonary Function Tests to Evaluate Gas Exchange and Pulmonary Capacity in Neonatal Cattle. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2015. [Google Scholar]
- Amory, H.; Desmecht, D.; Linden, A.; McEntee, K.; Rollin, F.; Genicot, B.; Lekeux, P. Growth-induced haemodynamic changes in healthy Friesian calves. Vet. Rec. 1993, 132, 426–434. [Google Scholar] [CrossRef]
- Shellenberger, N.W.; Collinsworth, K.K.; Subbiah, S.; Klein, D.; Neary, J.M. Hypoxia induces an increase in intestinal permeability and pulmonary arterial pressures in neonatal Holstein calves despite feeding the flavonoid rutin. J. Dairy Sci. 2020, 103, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Linden, A.; Desmecht, D.; Amory, H.; Lekeux, P. Cardiovascular response to intravenous administration of 5-hydroxytryptamine after type-2 receptor blockade, by metrenperone, in healthy calves. Vet. J. 1999, 157, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.M.; Garry, F.B.; Holt, T.N.; Knight, A.P.; Gould, D.H.; Dargatz, D.A. Pulmonary arterial pressures, arterial blood-gas tensions, and serum biochemistry of beef calves born and raised at high altitude. Open Access Anim. Physiol. 2013, 5, 1–8. [Google Scholar] [CrossRef][Green Version]
- Shirley, K.L.; Beckman, D.W.; Garrick, D.J. Inheritance of pulmonary arterial pressure in Angus cattle and its correlation with growth. J. Anim. Sci. 2008, 86, 815–819. [Google Scholar] [CrossRef]
- Bleul, U.; Bircher, B.; Jud, R.S.; Kutte, A.P.N. Respiratory and cardiovascular effects of doxapram and theophylline for the treatment of asphyxia in neonatal calves. Theriogenology 2010, 73, 612–619. [Google Scholar] [CrossRef]
- Amory, H.; Linden, A.S.; Desmecht, D.J.M.; Rollin, F.A.; McEntee, K.; Lekeux, P.M. Technical and methodological requirements for reliable haemodynamic measurements in the unsedated calf. Vet. Res. Commun. 1992, 16, 391–401. [Google Scholar] [CrossRef]
- Batchelder, C.A.; Whitcomb, M.B.; Famula, T.R.; Rodriguez-Villamil, P.; Bertolini, M.; Hoffert-Goeres, K.A.; Anderson, G.B. Cardiac adaptations in SCNT newborn cloned calves during the first month of life assessed by echocardiography. Theriogenology 2017, 103, 153–161. [Google Scholar] [CrossRef]
- Davidson, C.; Bonow, R.O. Cardiac Catheterization. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 9th ed.; Bonow, R.O., Mann, D.L., Zipes, D.P., Libby, P., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; p. 382. [Google Scholar]
- Pogliani, F.C. Parâmetros Ecodopplercardiográficos em Bezerros da Raça Nelore Originados Através de Transferência Nuclear de Células Somáticas Adultas—Clonagem. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2010. [Google Scholar]
- Sidi, D.; Kuipers, J.R.G.; Heymann, M.A.; Rudolph, A.M. Effects of ambient temperature on oxygen consumption and the circulation in newborn Iambs at rest and during hypoxemia. Pediatr. Res. 1983, 17, 254–258. [Google Scholar] [CrossRef][Green Version]
- Agata, Y.; Hiraishi, S.; Oguchi, K.; Misawa, H.; Horiguchi, Y.; Fujino, N.; Kimio Yashiro, K.; Shimada, N. Changes in left ventricular output from fetal to early neonatal life. J. Pediatr. 1991, 119, 441–445. [Google Scholar] [CrossRef]
- Teitel, D.F.; Iwamoto, H.S.; Rudolph, A.M. Changes in the pulmonary circulation during birth-related events. Pediatr. Res. 1990, 27, 372–378. [Google Scholar] [CrossRef]
- Feitosa, F.L.F.; Perri, S.H.V.; Bovino, F.; Mendes, L.C.N.; Peiró, J.R.; Gasparelli, E.R.F.; Yanaka, R.; Camargo, D.G. Evaluation of the vitality of nelore calves born after normal or dystocic parturitions. Ars. Vet. 2011, 27, 1–7. [Google Scholar]
- Piccione, G.; Casella, S.; Pennisi, P.; Giannetto, C.; Costa, A.; Caola, G. Monitoring of physiological and blood parameters during perinatal and neonatal period in calves. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1–12. [Google Scholar] [CrossRef]
- Bleul, U.; Lejeune, S.; Schwantag, S.; Kahn, W. Blood gas and acid-base analysis of arterial blood in 57 newborn calves. Vet. Rec. 2007, 161, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Mess, A.M.; Ferner, K.J. Evolution and development of gas exchange structures in Mammalia: The placenta and the lung. Respir. Physiol. Neurobiol. 2010, 173, S74–S82. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.P. Effects of Anemia on Periparturient Cows. Master’s Dissertation, University of São Paulo, São Paulo, Brazil, 2018. [Google Scholar]
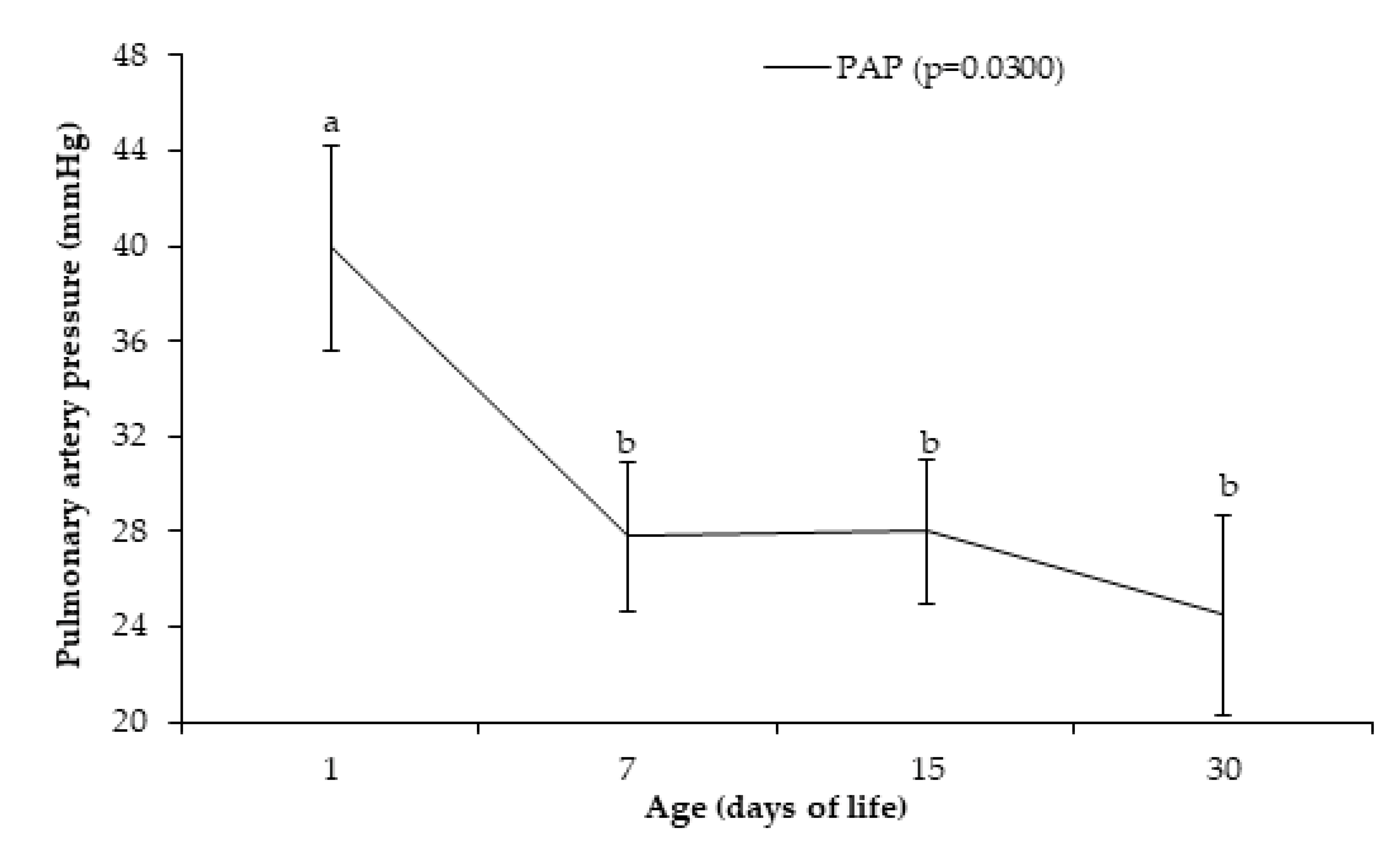
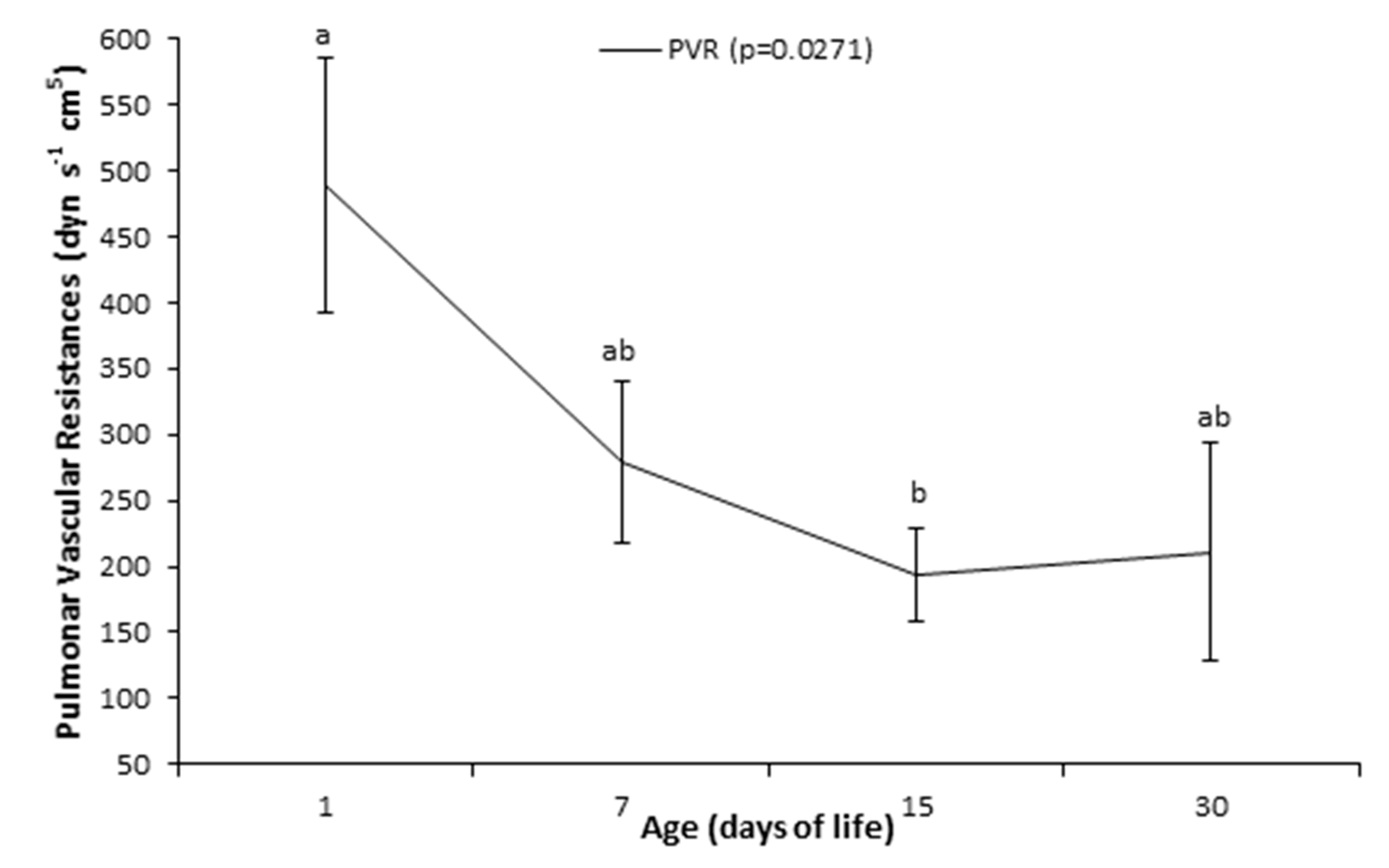

| Variable | Age (Days of Life) | |||
|---|---|---|---|---|
| 1st Day | 7th Day | 15th Day | 30th Day | |
| Right ventricle pressure (mmHg) | 29.20 ± 2.44 a | 22.50 ± 1.41 b | 20.90 ± 1.40 b | 18.50 ± 1.55 b |
| Right atrium capillary wedge pressure (mmHg) | 4.80 ± 0.96 | 6.00 ± 0.99 | 5.10 ± 0.57 | 5.10 ± 0.81 |
| Pulmonary capillary wedge pressure (mmHg) | 7.20 ± 1.02 b | 10.10 ± 0.95 a | 12.10 ± 0.80 a | 9.30 ± 1.19 ab |
| Cardiac output (L/min) | 5.86 ± 0.55 | 5.97 ± 0.69 | 7.01 ± 0.66 | 6.73 ± 0.47 |
| Heart rate (bpm) | 126.10 ± 5.44 a | 110.30 ± 4.87 b | 98.60 ± 2.20 bc | 99.10 ± 4.67 c |
| Variable 1 | Sample Source | Age (Days of Life) | |||
|---|---|---|---|---|---|
| 1st Day | 7th Day | 15th Day | 30th Day | ||
| pH | Vena cava | 7.351 ± 0.01 b | 7.358 ± 0.02 ab | 7.374 ± 0.01 ab | 7.403 ± 0.01 a |
| Right atrium | 7.353 ± 0.01 b | 7.356 ± 0.01 b | 7.362 ± 0.01 b | 7.414 ± 0.01 a | |
| Right ventricle | 7.359 ± 0.01 b | 7.365 ± 0.01 b | 7.372 ± 0.01 ab | 7.408 ± 0.01 a | |
| Pulmonary artery | 7.362 ± 0.01 b | 7.369 ± 0.01 b | 7.383 ± 0.01 ab | 7.406 ± 0.01 a | |
| pO2 (mmHg) | Vena cava | 23.20 ± 1.67 b | 25.60 ± 1.87 ab | 29.70 ± 1.48 a | 30.60 ± 2.14 a |
| Right atrium | 25.10 ± 2.10 c | 28.60 ± 1.19 bc | 30.60 ± 1.75 b | 35.40 ± 0.90 a | |
| Right ventricle | 27.20 ± 2.00 | 29.00 ± 0.89 | 28.50 ± 1.93 | 29.90 ± 1.86 | |
| Pulmonary artery | 30.40 ± 2.37 | 28.50 ± 0.970 | 32.66 ± 1.76 | 33.00 ± 0.96 | |
| pCO2 (mmHg) | Vena cava | 52.64 ± 1.95 | 48.76 ± 1.89 | 52.60 ± 1.91 | 52.26 ± 1.00 |
| Right atrium | 49.71 ± 2.75 | 48.30 ± 1.50 | 47.43 ± 3.17 | 49.68 ± 0.74 | |
| Right ventricle | 47.89 ± 2.19 | 47.27 ± 1.67 | 48.88 ± 2.40 | 52.33 ± 1.30 | |
| Pulmonary artery | 50.32 ± 0.80 ab | 46.85 ± 1.64 b | 49.78 ±1.56 ab | 51.23 ± 0.94 a | |
| Bicarbonate (mmol/L) | Vena cava | 28.97 ± 1.18 b | 27.53 ± 1.59 b | 30.47 ±1.04 ab | 32.33 ± 0.60 a |
| Right atrium | 28.49 ± 1.13 ab | 27.01 ±1.38 ab | 27.31 ± 2.30 b | 31.52 ± 0.75 a | |
| Right ventricle | 27.92 ± 0.85 b | 26.96 ± 1.41 b | 29.62 ± 1.35 b | 32.82 ± 0.77 a | |
| Pulmonary artery | 28.53 ± 1.00 b | 26.93 ± 1.45 b | 30.50 ±1.23 ab | 35.04 ± 2.94 a | |
| BE (mmol/L) | Vena cava | 3.90 ± 1.34 b | 2.60 ± 1.92 b | 5.80 ± 1.26 a | 8.10 ± 0.75 a |
| Right atrium | 3.30 ± 1.31 ab | 2.00 ± 1.67 ab | 2.40 ± 2.55 b | 7.20 ± 0.95 a | |
| Right ventricle | 2.80 ± 1.08 b | 2.20 ± 1.57 b | 5.70 ± 1.66 ab | 8.60 ± 0.90 a | |
| Pulmonary artery | 3.40 ± 1.24 b | 2.30 ± 1.71 b | 5.88 ± 1.44 ab | 7.70 ± 0.92 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuoka, M.M.; Monteiro, B.M.; Fantinato-Neto, P.; Paiano, R.B.; Fantoni, D.T.; Otsuki, D.A.; Birgel Junior, E.H. Transient Pulmonary Artery Hypertension in Holstein Neonate Calves. Animals 2020, 10, 2277. https://doi.org/10.3390/ani10122277
Yasuoka MM, Monteiro BM, Fantinato-Neto P, Paiano RB, Fantoni DT, Otsuki DA, Birgel Junior EH. Transient Pulmonary Artery Hypertension in Holstein Neonate Calves. Animals. 2020; 10(12):2277. https://doi.org/10.3390/ani10122277
Chicago/Turabian StyleYasuoka, Melina Marie, Bruno Moura Monteiro, Paulo Fantinato-Neto, Renan Braga Paiano, Denise Tabacchi Fantoni, Denise Aya Otsuki, and Eduardo Harry Birgel Junior. 2020. "Transient Pulmonary Artery Hypertension in Holstein Neonate Calves" Animals 10, no. 12: 2277. https://doi.org/10.3390/ani10122277
APA StyleYasuoka, M. M., Monteiro, B. M., Fantinato-Neto, P., Paiano, R. B., Fantoni, D. T., Otsuki, D. A., & Birgel Junior, E. H. (2020). Transient Pulmonary Artery Hypertension in Holstein Neonate Calves. Animals, 10(12), 2277. https://doi.org/10.3390/ani10122277






