Incidence of Bacteremia Consequent to Different Endoscopic Procedures in Dogs: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
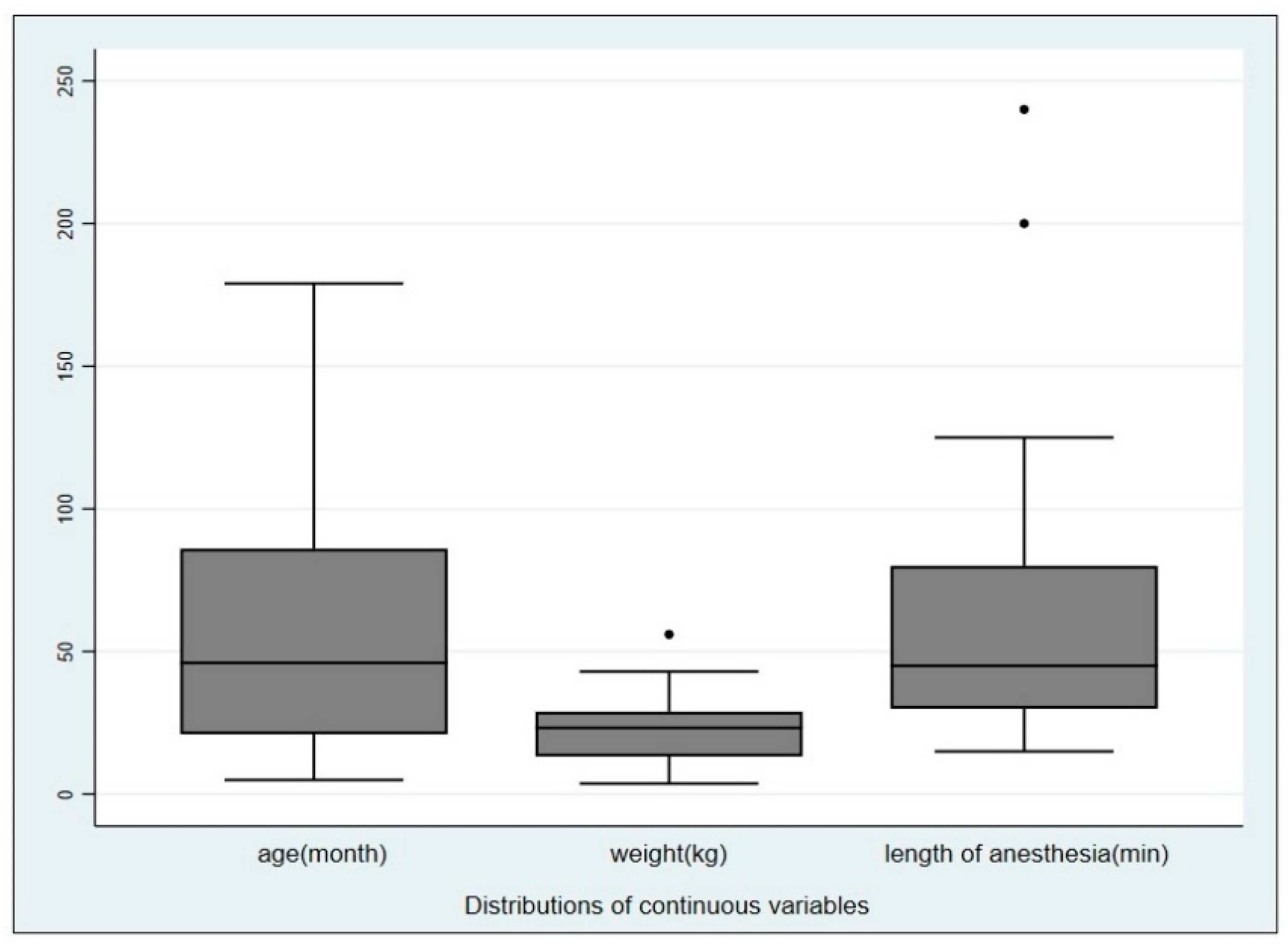
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bignone, I.; Tomassone, L.; Lotti, U.; Gianella, P. Chronic enteropathy food-and immunosuppressant-responsive: Comparison of clinicopathological findings and follow-up. Veterinaria 2017, 31, 219–226. [Google Scholar]
- Bongard, A.B.; Furrow, E.; Granick, J.L. Retrospective evaluation of factors associated with degree of esophagitis, treatment, and outcomes in dogs presenting with esophageal foreign bodies (2004–2014): 114 cases. J. Veter Emerg. Crit. Care 2019, 29, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Dollo, V.; Chambers, G.; Carothers, M. Endoscopic retrieval of gastric and oesophageal foreign bodies in 52 cats. J. Small Anim. Pr. 2019, 61, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara-Igarashi, A.; Yu, Y.; Hamamoto, Y.; Hasegawa, D.; Fujita, M. Dynamic pharyngeal collapse in three cats with different pharyngeal pathology. J. Veter Med. Sci. 2019, 81, 1012–1016. [Google Scholar] [CrossRef]
- Gianella, P.; Pietra, M.; Crisi, P.E.; Famigli Bergamini, P.; Fracassi, F.; Morini, M.; Boari, A. Evaluation of clinicopathological features in cats with chronic gastrointestinal signs. Pol. J. Vet. Sci. 2017, 20, 403–410. [Google Scholar] [CrossRef]
- Marchesi, M.C.; Caivano, D.; Conti, M.B.; Beccati, F.; Valli, L.; Busechian, S.; Rueca, F. A specific laryngeal finding in dogs with bronchial vegetal foreign bodies: A retrospective study of 63 cases. J. Veter Med. Sci. 2019, 81, 213–216. [Google Scholar] [CrossRef]
- Nelson, D.B. Infectious disease complications of Gl endoscopy: Part I, endogenous infections. Gastrointest. Endosc. 2003, 57, 546–556. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Khashab, M.A.; Chithadi, K.V.; Acosta, R.D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Fonkalsrud, L.; et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 2015, 81, 81–89. [Google Scholar] [CrossRef]
- Karam, G.G.; Chastre, J.; Wilcox, M.H.M.; Vincent, J.-L. Antibiotic strategies in the era of multidrug resistance. Crit. Care 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Woolhouse, M.; Waugh, C.; Perry, M.R.; Nair, H. Global disease burden due to antibiotic resistance—State of the evidence. J. Glob. Heal. 2016, 6, 010306. [Google Scholar] [CrossRef]
- Coughlin, G.P.; Butler, R.N.; Alp, M.H.; Kerr Grant, A. Colonoscopy and bacteraemia. Gut 1977, 18, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Kashani, B.; Shahabi, P.; Behzadnia, N.; Taheri, Z.M.; Mansouri, D.; Masjedi, M.R.; Zargari, L.; Negad, L.S. Incidence of fever and bacteriemia following flexible fiberoptic bronchoscopy: A prospective study. Acta Med. Iran. 2011, 48, 385–388. [Google Scholar]
- Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.; Fukami, N.; Hwang, J.H.; Ikenberry, S.O.; et al. Adverse events of upper GI endoscopy. Gastrointest. Endosc. 2012, 76, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qi, H.; Liu, Z.; Ge, N.; Guo, J.; Wang, G.; Liu, X.; Wang, S.; Sun, S. Effect of Povidone-iodine Washing of Gastrointestinal Mucosa or Taking Proton Pump Inhibitors on Bacteremia after Endoscopic Ultrasonography-guided Fine Needle Aspiration. Endosc. Ultrasound 2012, 1, 90–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, K.R.; Maddox, C.W.; Ridgway, M.D.; Clark-Price, S.C.; Dossin, O. Incidence of bacteremia following upper gastrointestinal endoscopy and biopsy in healthy dogs before, during, and after treatment with omeprazole. Am. J. Veter Res. 2013, 74, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.; Fickenscher, Y.; Meyer, K.; Failing, K.; Weiss, D.J. Canine and feline hematology reference values for the ADVIA 120 hematology system. Veter Clin. Pathol. 2004, 33, 32–38. [Google Scholar] [CrossRef]
- Couto, C.G.; Iazbik, M.C. Effects of blood donation on arterial blood pressure in retired racing Greyhounds. J. Vet. Intern. Med. 2005, 19, 845–848. [Google Scholar] [CrossRef]
- Boucher, C.; Henton, M.M.; Becker, P.J.; Kirberger, R.M.; Hartman, M.J. Comparative efficacy of three antiseptics as surgical skin preparations in dogs. Veter Surg. 2018, 47, 792–801. [Google Scholar] [CrossRef]
- Garcia, R.A.; Spitzer, E.D.; Beaudry, J.; Beck, C.; Diblasi, R.; Gilleeny-Blabac, M.; Haugaard, C.; Heuschneider, S.; Kranz, B.P.; McLean, K.; et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am. J. Infect. Control. 2015, 43, 1222–1237. [Google Scholar] [CrossRef]
- Brosius, J.; Palmer, M.L.; Kennedy, P.J.; Noller, H.F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 1978, 75, 4801–4805. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, P.J.; Eykyn, S.J.; Cotton, P.B. Gastrointestinal instrumentation, bacteraemia, and endocarditis. Gut 1983, 24, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Pien, B.C.; Sundaram, P.; Raoof, N.; Costa, S.F.; Mirrett, S.; Woods, C.W.; Reller, L.B.; Weinstein, M.P. The Clinical and Prognostic Importance of Positive Blood Cultures in Adults. Am. J. Med. 2010, 123, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.; Skoglund, E.; Muldrew, K.L.; Garey, K.W. Economic health care costs of blood culture contamination: A systematic review. Am. J. Infect. Control. 2019, 47, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, V.; Miglio, A.; Cappelli, K.; Capomaccio, S.; Sgariglia, E.; Marenzoni, M.L.; Antognoni, M.T.; Coletti, M.; Mangili, V.; Passamonti, F. Detection of bacterial contamination and DNA quantification in stored blood units in 2 veterinary hospital blood banks. Veter Clin. Pathol. 2016, 45, 406–410. [Google Scholar] [CrossRef]
- Miglio, A.; Stefanetti, V.; Antognoni, M.; Cappelli, K.; Capomaccio, S.; Coletti, M.; Passamonti, F. Stored Canine Whole Blood Units: What is the Real Risk of Bacterial Contamination? J. Veter Intern. Med. 2016, 30, 1830–1837. [Google Scholar] [CrossRef]
- Cerquetella, M.; Rossi, G.; Suchodolski, J.S.; Schmitz, S.S.; Allenspach, K.; Rodríguez-Franco, F.; Furlanello, T.; Gavazza, A.; Marchegiani, A.; Unterer, S.; et al. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pr. 2020, 61, 211–215. [Google Scholar] [CrossRef]
- Banerjee, S.; Shen, B.; Baron, T.H.; Nelson, D.B.; Anderson, M.A.; Cash, B.D.; Dominitz, J.A.; Gan, S.I.; Harrison, M.E.; Ikenberry, S.O.; et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 2008, 67, 791–798. [Google Scholar] [CrossRef]
- El Batrawy, S.; Elassal, G.; Moustafa, A.; Hafez, H. Bacteremia associated with bronchoscopy. Egypt. J. Chest Dis. Tuberc. 2014, 63, 701–704. [Google Scholar] [CrossRef]
- Rello, J.; Ochagavia, A.; Sabanes, E.; Roque, M.; Mariscal, D.; Reynaga, E.; Vallés, J. Evaluation of Outcome of Intravenous Catheter-related Infections in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2000, 162, 1027–1030. [Google Scholar] [CrossRef]
- Silverstein, D.; Greene, C.E. Infectious Diseases of the Dog and Cat, 4th ed.; Elsevier: St. Louis, MS, USA, 2012; pp. 359–369. [Google Scholar]
- Serrano, M.R.G.; Escartín, N.L.; Arriaza, M.M.; Díaz, J.C.R. Microbiological diagnosis of bacteraemia and fungaemia: Blood cultures and molecular methods. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2019, 37, 335–340. [Google Scholar] [CrossRef]
| Animals | Pre-Procedure Result | Post-Procedure Result | Procedure Attributable Result |
|---|---|---|---|
| n = 53 | − | − | − |
| n = 8 | + | + | − |
| n = 5 | + | − | − |
| n = 8 | − | + | + |
| Categorical Variables | Categories | # of Patients | % | Pearson χ2 | Fischer’s Exact p-Value | Degrees of Freedom |
|---|---|---|---|---|---|---|
| Sex | Male | 38 | 51.3 | 0.446 | 0.387 | 1 |
| Female | 36 | 48.7 | ||||
| Age | ≤12 months | 9 | 12.2 | 4.2351 | 0.273 * | 3 |
| >12 ≤57 | 34 | 45.9 | ||||
| >57 ≤84 | 12 | 16.2 | ||||
| >84 | 19 | 25.7 | ||||
| Weight | ≥3 kg ≤10.5 kg, | 12 | 16.0 | 0.946 | 0.614 * | 2 |
| ≥10.6 kg <25 kg, | 33 | 45.0 | ||||
| ≥25 kg | 29 | 39.0 | ||||
| Endoscopy procedures (dichotomous) | airways | 37 | 50.0 | 2.242 | 0.261 | 1 |
| digestive tract | 37 | 50.0 | ||||
| Endoscopy procedures (categorical) | gastroscopy | 20 | 27.0 | 3.724 | 0.284 * | 4 |
| gastro-duodeno-colonoscopy | 17 | 23.0 | ||||
| rhinoscopy | 18 | 24.3 | ||||
| bronchoscopy | 11 | 14.9 | ||||
| rhino+broncho | 8 | 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspardo, A.; Sabetti, M.C.; Zanoni, R.G.; Morandi, B.; Galiazzo, G.; Mion, D.; Pietra, M. Incidence of Bacteremia Consequent to Different Endoscopic Procedures in Dogs: A Preliminary Study. Animals 2020, 10, 2265. https://doi.org/10.3390/ani10122265
Gaspardo A, Sabetti MC, Zanoni RG, Morandi B, Galiazzo G, Mion D, Pietra M. Incidence of Bacteremia Consequent to Different Endoscopic Procedures in Dogs: A Preliminary Study. Animals. 2020; 10(12):2265. https://doi.org/10.3390/ani10122265
Chicago/Turabian StyleGaspardo, Alba, Maria Chiara Sabetti, Renato Giulio Zanoni, Benedetto Morandi, Giorgia Galiazzo, Domenico Mion, and Marco Pietra. 2020. "Incidence of Bacteremia Consequent to Different Endoscopic Procedures in Dogs: A Preliminary Study" Animals 10, no. 12: 2265. https://doi.org/10.3390/ani10122265
APA StyleGaspardo, A., Sabetti, M. C., Zanoni, R. G., Morandi, B., Galiazzo, G., Mion, D., & Pietra, M. (2020). Incidence of Bacteremia Consequent to Different Endoscopic Procedures in Dogs: A Preliminary Study. Animals, 10(12), 2265. https://doi.org/10.3390/ani10122265





