Fatal Pulmonary Hypertension and Right-Sided Congestive Heart Failure in a Kitten Infected with Aelurostrongylus abstrusus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Case
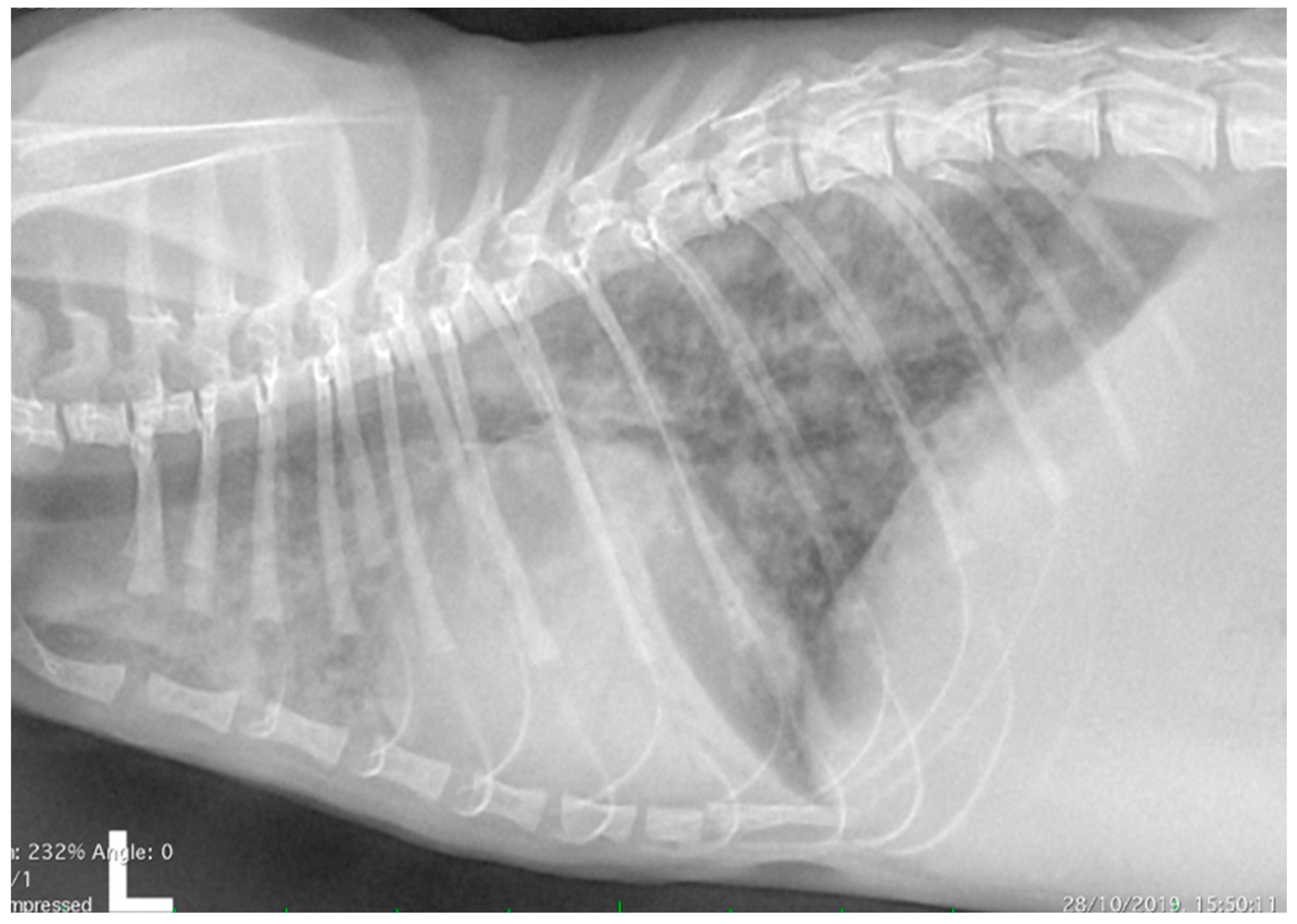
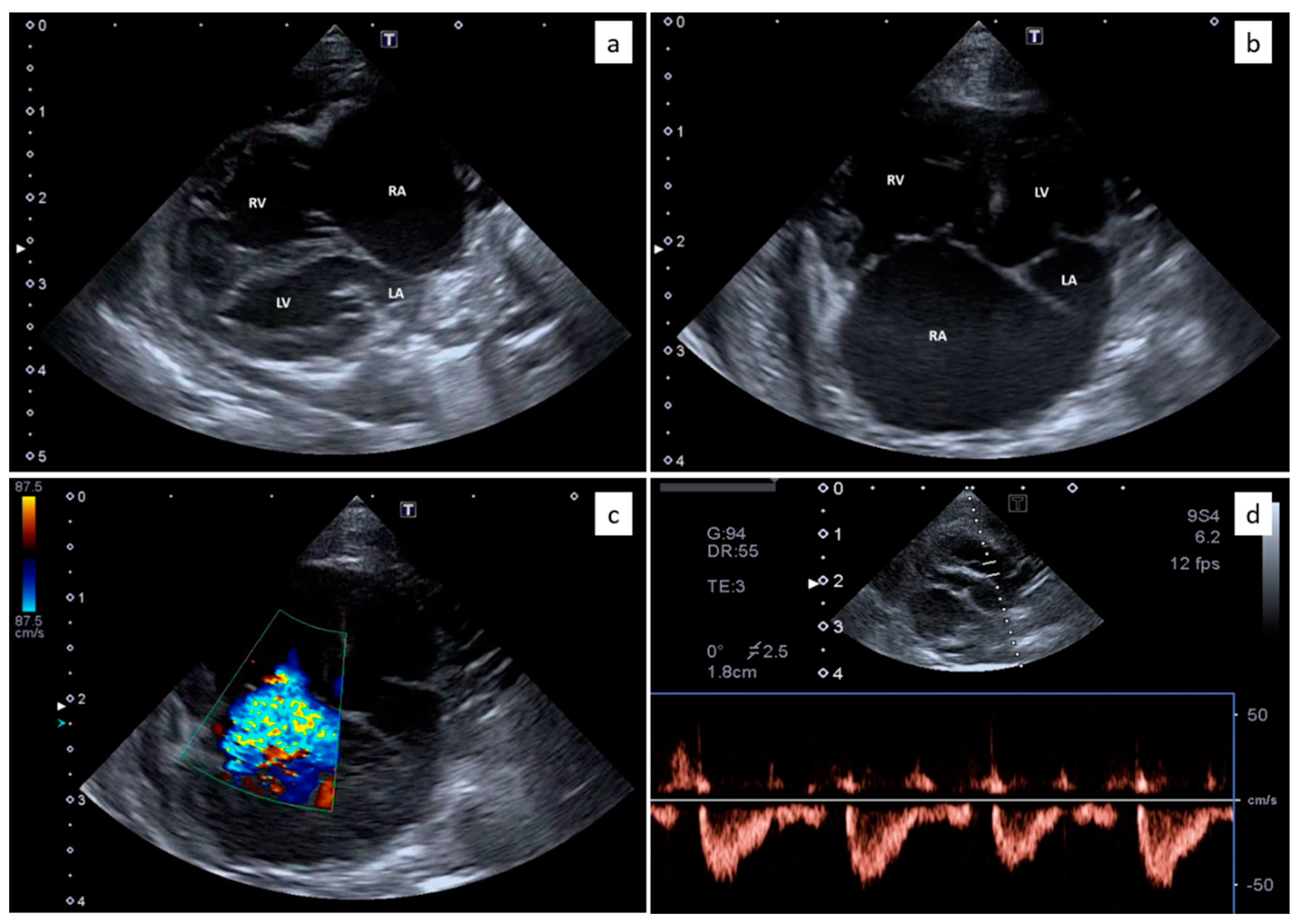
2.2. Thoracic Radiology and Echocardiography
2.3. Parasitological and Molecular Analysis
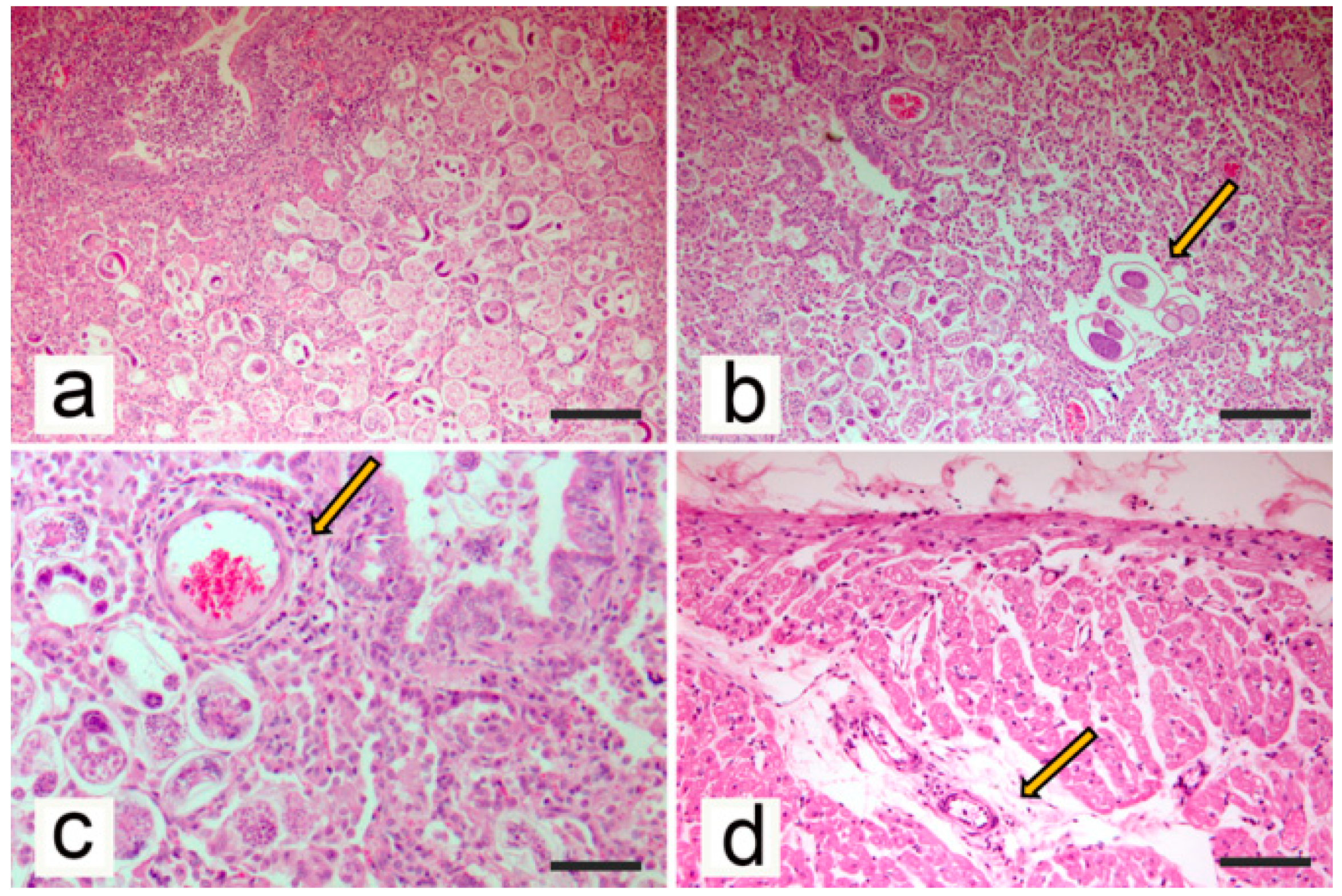
2.4. Histopathological Methods
3. Results
3.1. Clinical Evaluation
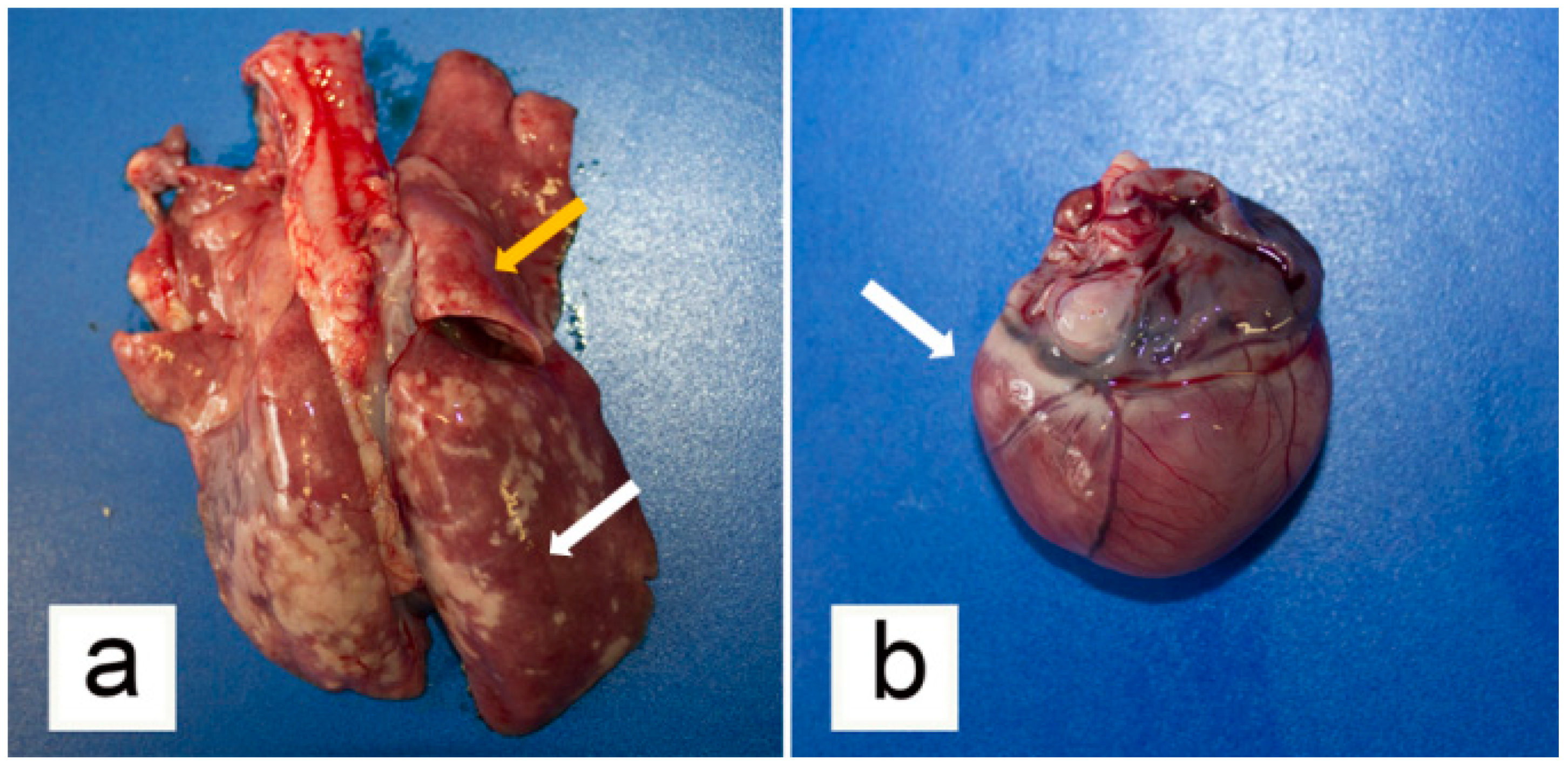
3.2. Post-Mortem Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Traversa, D.; Di Cesare, A. Feline lungworms: What a dilemma. Trends Parasitol. 2013, 29, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Schnyder, M.; Traversa, D.; Di Cesare, A.; Wright, I.; Lacher, D.W. Updates on feline aelurostrongylosis and research priorities for the next decade. Parasites Vectors 2016, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A. Diagnosis and management of lungworm infections in cats: Cornerstones, dilemmas and new avenues. J. Feline Med. Surg. 2016, 18, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, A.; Capelli, G.; Joachim, A.; Hinney, B.; Losson, B.; Kirkova, Z.; René-Martellet, M.; Papadopoulos, E.; Farkas, R.; Napoli, E.; et al. Lungworms and gastrointestinal parasites of domestic cats: A European perspective. Int. J. Parasitol. 2017, 47, 517–528. [Google Scholar] [CrossRef]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef]
- Hoggard, K.R.; Jarriel, D.M.; Bevelock, T.J.; Verocai, G.G. Prevalence survey of gastrointestinal and respiratory parasites of shelter cats in northeastern Georgia, USA. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100270. [Google Scholar] [CrossRef]
- Nagamori, Y.; Payton, M.E.; Looper, E.; Apple, H.; Johnson, E.M. Retrospective survey of parasitism identified in feces of client-owned cats in North America from 2007 through 2018. Vet. Parasitol. 2020, 277, 109008. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Chaparro-Gutiérrez, J.J.; Taubert, A.; Hermosilla, C. Angiostrongylus vasorum and Aelurostrongylus abstrusus: Neglected and underestimated parasites in South America. Parasites Vectors 2018, 11, 208. [Google Scholar] [CrossRef]
- Di Cesare, A.; Frangipane di Regalbono, A.; Tessarin, C.; Seghetti, M.; Iorio, R.; Simonato, G.; Traversa, D. Mixed infection by Aelurostrongylus abstrusus and Troglostrongylus brevior in kittens from the same litter in Italy. Parasitol. Res. 2014, 113, 613–618. [Google Scholar] [CrossRef]
- Schnyder, M.; Di Cesare, A.; Basso, W.; Guscetti, F.; Riond, B.; Glaus, T.; Crisi, P.; Deplazes, P. Clinical, laboratory and pathological findings in cats experimentally infected with Aelurostrongylus abstrusus. Parasitol. Res. 2014, 113, 1425–1433. [Google Scholar] [CrossRef]
- Crisi, P.E.; Aste, G.; Traversa, D.; Di Cesare, A.; Febo, E.; Vignoli, M.; Santori, D.; Luciani, A.; Boari, A. Single and mixed feline lungworm infections: Clinical, radiographic and therapeutic features of 26 cases (2013–2015). J. Feline Med. Surg. 2017, 19, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.R.; Hamilton, J.M.; Weatherley, A.J. Changes in the ultrastructure of feline pulmonary arteries following infection with the lungworm Aelurostrongylus abstrusus. Br. Vet. J. 1984, 140, 181–190. [Google Scholar] [CrossRef]
- Dennler, M.; Bass, D.A.; Gutierrez-Crespo, B.; Schnyder, M.; Guscetti, F.; Di Cesare, A.; Deplazes, P.; Kircher, P.R.; Glaus, T.M. Thoracic computed tomography, angiographic computed tomography, and pathology findings in six cats experimentally infected with Aelurostrongylus abstrusus. Vet. Radiol. Ultrasound 2013, 54, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.J.; Lamb, C.R.; Boswood, A. Right-to-left shunting patent ductus arteriosus with pulmonary hypertension in a cat. J. Small Anim. Pract. 2003, 44, 184–188. [Google Scholar] [CrossRef]
- Campbell, F.E.; Thomas, W.P. Congenital supravalvular mitral stenosis in 14 cats. J. Vet. Cardiol. 2012, 14, 281–292. [Google Scholar] [CrossRef]
- Aoki, T.; Sugimoto, K.; Sunahara, H.; Fujii, Y. Patent ductus arteriosus ligation in two young cats with pulmonary hypertension. J. Vet. Med. Sci. 2013, 75, 199–202. [Google Scholar] [CrossRef]
- Toaldo, M.B.; Guglielmini, C.; Diana, A.; Giunti, M.; Dondi, F.; Cipone, M. Reversible pulmonary hypertension in a cat. J. Small Anim. Pract. 2011, 52, 271–277. [Google Scholar] [CrossRef]
- Evola, M.G.; Edmondson, E.F.; Reichle, J.K.; Biller, D.S.; Mitchell, C.W.; Valdés-Martínez, A. Radiographic and histopathologic characteristics of pulmonary fibrosis in nine cats. Vet. Radiol. Ultrasound 2014, 55, 133–140. [Google Scholar] [CrossRef]
- MacPhail, C.M.; Innocenti, C.M.; Kudnig, S.T.; Veir, J.K.; Lappin, M.R. Atypical manifestations of feline inflammatory polyps in three cats. J. Feline Med. Surg. 2007, 9, 219–225. [Google Scholar] [CrossRef]
- Vezzosi, T.; Schober, K.E. Doppler-derived echocardiographic evidence of pulmonary hypertension in cats with left-sided congestive heart failure. J. Vet. Cardiol. 2019, 23, 58–68. [Google Scholar] [CrossRef]
- Crisi, P.E.; Traversa, D.; Di Cesare, A.; Luciani, A.; Civitella, C.; Santori, D.; Boari, A. Irreversible pulmonary hypertension associated with Troglostrongylus brevior infection in a kitten. Res. Vet. Sci. 2015, 102, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Dirven, M.; Szatmári, V.; van den Ingh, T.; Nijsse, R. Reversible pulmonary hypertension associated with lungworm infection in a young cat. J. Vet. Cardiol. 2012, 14, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Domenech, O.; Iacona, M.; Marchesotti, F.; Zini, E.; Venco, L.; Tognetti, R. Echocardiographic evaluation of the right atrial area index in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2018, 32, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Domenech, O.; Costa, G.; Marchesotti, F.; Venco, L.; Zini, E.; Del Palacio, M.; Tognetti, R. Echocardiographic evaluation of the right ventricular dimension and systolic function in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2018, 32, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Sloss, M.W.; Kemp, R.L.; Zajac, A.M. Fecal examination: Dogs and cats. In Veterinary Clinical Parasitology, 6th ed.; Iowa State University Press: Ames, IA, USA, 1994; p. 198. [Google Scholar]
- Brianti, E.; Giannetto, S.; Dantas-Torres, F.; Otranto, D. Lungworms of the genus Troglostrongylus (Strongylida: Crenosomatidae): Neglected parasites for domestic cats. Vet. Parasitol. 2014, 202, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Veronesi, F.; di Regalbono, A.F.; Iorio, R.; Traversa, D. Novel Molecular Assay for Simultaneous Identification of Neglected Lungworms and Heartworms Affecting Cats. J. Clin. Microbiol. 2015, 53, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Philbey, A.W.; Krause, S.; Jeffries, R. Verminous pneumonia and enteritis due to hyperinfection with Aelurostrongylusabstrusus in a kitten. J. Comp. Pathol. 2014, 150, 357–360. [Google Scholar] [CrossRef]
- Traversa, D.; Lia, R.P.; Iorio, R.; Boari, A.; Paradies, P.; Capelli, G.; Avolio, S.; Otranto, D. Diagnosis and risk factors of Aelurostrongylus abstrusus (Nematoda, Strongylida) infection in cats from Italy. Vet. Parasitol. 2008, 153, 182–186. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Milillo, P.; Iorio, R.; Otranto, D. Aelurostrongylus abstrusus in a feline colony from central Italy: Clinical features, diagnostic procedures and molecular characterization. Parasitol. Res. 2008, 103, 1191–1196. [Google Scholar] [CrossRef]
- Hawley, M.M.; Johnson, L.R.; Traversa, D.; Bucy, D.; Vernau, K.M.; Vernau, W. Respiratory distress associated with lungworm infection in a kitten. J. Feline Med. Surg. Open Rep. 2016, 2, 2055116916675801. [Google Scholar] [CrossRef]
- Lacava, G.; Zini, E.; Marchesotti, F.; Domenech, O.; Romano, F.; Manzocchi, S.; Venco, L.; Auriemma, E. Computed tomography, radiology and echocardiography in cats naturally infected with Aelurostrongylus abstrusus. J. Feline Med. Surg. 2017, 19, 446–453. [Google Scholar] [CrossRef]
- Winter, R.L.; Dillon, A.R.; Cattley, R.C.; Blagburn, B.L.; Tillson, D.M.; Johnson, C.M.; Brawner, W.R.; Welles, E.G.; Barney, S. Effect of heartworm disease and heartworm-associated respiratory disease (HARD) on the right ventricle of cats. Parasites Vectors 2017, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Fossum, T.W.; Miller, M.W.; Rogers, K.S.; Bonagura, J.D.; Meurs, K.M. Chylothorax associated with right-sided heart failure in five cats. J. Am. Vet. Med. Assoc. 1994, 204, 84–89. [Google Scholar] [PubMed]
- Koffas, H.; Fuentes, V.L.; Boswood, A.; Connolly, D.J.; Brockman, D.J.; Bonagura, J.D.; Meurs, K.M.; Koplitz, S.; Baumwart, R. Double chambered right ventricle in 9 cats. J. Vet. Intern. Med. 2007, 21, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Novo-Matos, J.; Hurter, K.; Bektas, R.; Grest, P.; Glaus, T. Patent ductus arteriosus in an adult cat with pulmonary hypertension and right-sided congestive heart failure: Hemodynamic evaluation and clinical outcome following ductal closure. J. Vet. Cardiol. 2014, 16, 197–203. [Google Scholar] [CrossRef]
- Harvey, A.M.; Battersby, I.A.; Faena, M.; Fews, D.; Darke, P.G.; Ferasin, L. Arrhythmogenic right ventricular cardiomyopathy in two cats. J. Small Anim. Pract. 2005, 46, 151–156. [Google Scholar] [CrossRef]
- Chetboul, V.; Passavin, P.; Trehiou-Sechi, E.; Gouni, V.; Poissonnier, C.; Pouchelon, J.L.; Desquilbet, L. Clinical, epidemiological and echocardiographic features and prognostic factors in cats with restrictive cardiomyopathy: A retrospective study of 92 cases (2001–2015). J. Vet. Intern. Med. 2019, 33, 1222–1231. [Google Scholar] [CrossRef]
- Hamilton, J.M. The influence of infestation by Aelurostrongylus abstrusus on the pulmonary vasculature of the cat. Br. Vet. J. 1970, 126, 202–209. [Google Scholar] [CrossRef]
- Losonsky, J.M.; Thrall, D.E.; Prestwood, A.K. Radiographic evaluation of pulmonary abnormalities after Aelurostrogylus abstrusus inoculation in cats. Am. J. Vet. Res. 1983, 44, 478–482. [Google Scholar]
- Genchi, M.; Ferrari, N.; Fonti, P.; De Francesco, I.; Piazza, C.; Viglietti, A. Relation between Aelurostrongylus abstrusus larvae excretion, respiratory and radiographic signs in naturally infected cats. Vet. Parasitol. 2014, 206, 182–187. [Google Scholar] [CrossRef]
- Gerdin, J.A.; Slater, M.R.; Makolinski, K.V.; Looney, A.L.; Appel, L.D.; Martin, N.M.; McDonough, S.P. Post-mortem findings in 54 cases of anesthetic associated death in cats from two spay-neuter programs in New York State. J. Feline Med. Surg. 2011, 13, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, M.B. Radiographic-pathologic findings in experimental Aelurostrongylus abstrusus infection in cats. J. Am. Vet. Rad. Soc. 1979, 20, 81. [Google Scholar] [CrossRef]
- Rawlings, C.A.; Losonsky, J.M.; Lewis, R.E.; Hubble, J.J.; Prestwood, A.K. Response of the feline heart to Aelurostrongylus abstrusus. J. Am. Anim. Hosp. Assoc. 1980, 16, 573–578. [Google Scholar]
- Di Cesare, A.; Veronesi, F.; Traversa, D. Felid Lungworms and Heartworms in Italy: More Questions than Answers? Trends Parasitol. 2015, 31, 665–675. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezzosi, T.; Perrucci, S.; Parisi, F.; Morelli, S.; Maestrini, M.; Mennuni, G.; Traversa, D.; Poli, A. Fatal Pulmonary Hypertension and Right-Sided Congestive Heart Failure in a Kitten Infected with Aelurostrongylus abstrusus. Animals 2020, 10, 2263. https://doi.org/10.3390/ani10122263
Vezzosi T, Perrucci S, Parisi F, Morelli S, Maestrini M, Mennuni G, Traversa D, Poli A. Fatal Pulmonary Hypertension and Right-Sided Congestive Heart Failure in a Kitten Infected with Aelurostrongylus abstrusus. Animals. 2020; 10(12):2263. https://doi.org/10.3390/ani10122263
Chicago/Turabian StyleVezzosi, Tommaso, Stefania Perrucci, Francesca Parisi, Simone Morelli, Michela Maestrini, Giulia Mennuni, Donato Traversa, and Alessandro Poli. 2020. "Fatal Pulmonary Hypertension and Right-Sided Congestive Heart Failure in a Kitten Infected with Aelurostrongylus abstrusus" Animals 10, no. 12: 2263. https://doi.org/10.3390/ani10122263
APA StyleVezzosi, T., Perrucci, S., Parisi, F., Morelli, S., Maestrini, M., Mennuni, G., Traversa, D., & Poli, A. (2020). Fatal Pulmonary Hypertension and Right-Sided Congestive Heart Failure in a Kitten Infected with Aelurostrongylus abstrusus. Animals, 10(12), 2263. https://doi.org/10.3390/ani10122263








