Social Context Influences Resting Physiology in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Subjects
2.3. Data Collection
2.3.1. Heart Rate Measurements
2.3.2. Experimental Setup
- “Inactive wakefulness: body touching the ground either with caudal, dorsal, or lateral side. Position of the paws varies, e.g., folded (under body) or stretched out. Head is in an upward position and can be moved around. Eyes are open, but increased blinking can occur.”
- “Resting: body touching the ground either with caudal, dorsal, or lateral side. Position of the paws varies, e.g., folded (under body) or stretched out. Head is in a downward position, either lying on paws, ground, or tucked under the body. Eyes are generally closed, but might be opened and closed again (peeking). Parts of the body occasionally twitching.”
2.4. Procedure
2.5. Data Selection
2.6. Statistical Analyses
3. Results
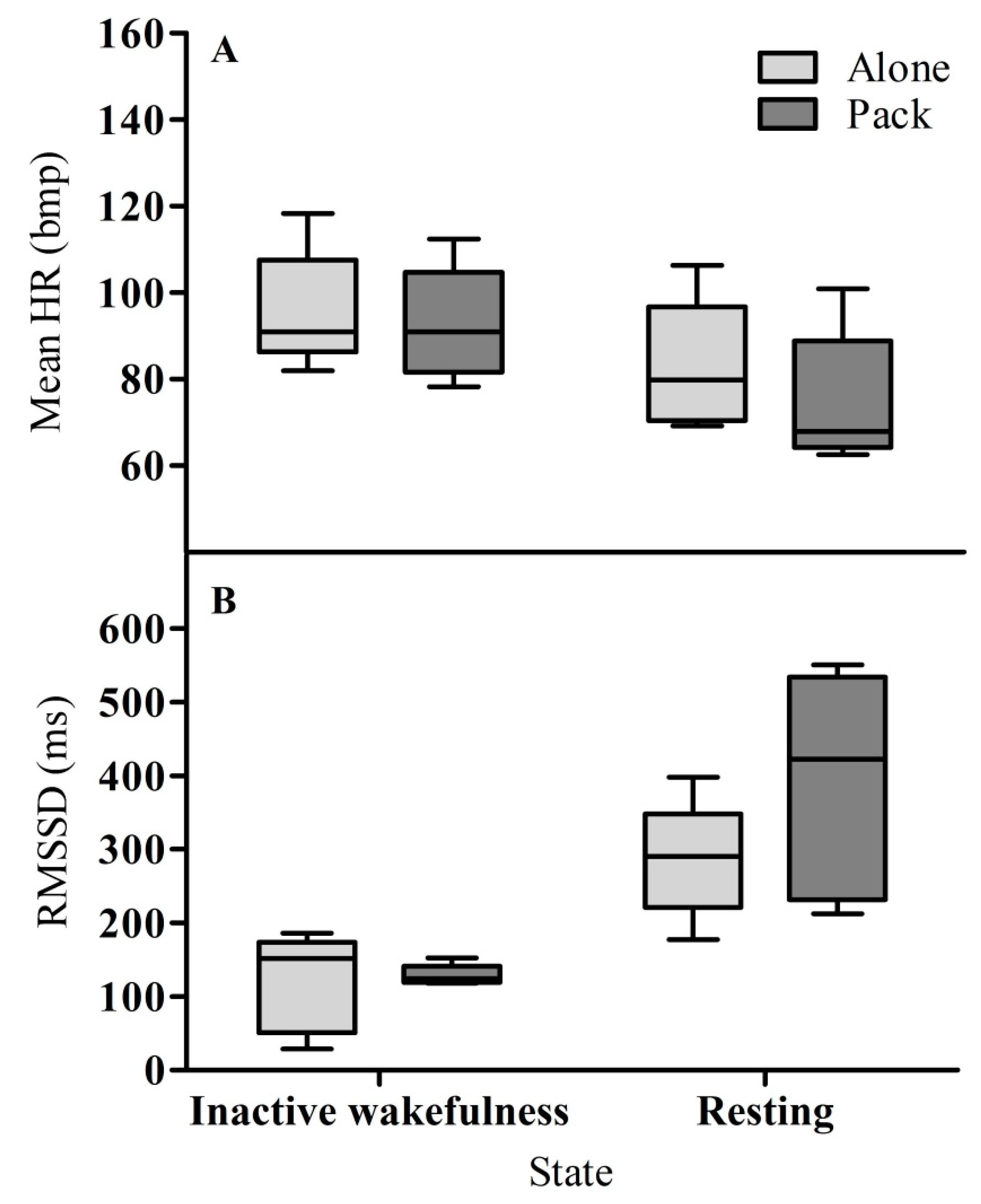
3.1. Heart Rate
3.2. Heart Rate Variability (RMSSD)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Botigué, L.R.; Song, S.; Scheu, A.; Gopalan, S.; Pendleton, A.L.; Oetjens, M.; Taravella, A.M.; Seregély, T.; Zeeb-Lanz, A.; Arbogast, R.M.; et al. Ancient European dog genomes reveal continuity since the Early Neolithic. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Frantz, L.A.F.; Mullin, V.E.; Pionnier-Capitan, M.; Lebrasseur, O.; Ollivier, M.; Perri, A.; Linderholm, A.; Mattiangeli, V.; Teasdale, M.D.; Dimopoulos, E.A.; et al. Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science 2016, 352, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.F.; Kluetsch, C.; Zou, X.J.; Zhang, A.; Luo, L.Y.; Angleby, H.; Ardalan, A.; Ekström, C.; Sköllermo, A.; Lundeberg, J.; et al. mtDNA Data indicate a single origin for dogs south of Yangtze River, less than 16,300 years ago, from numerous wolves. Mol. Biol. Evol. 2009, 26, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Skoglund, P.; Ersmark, E.; Palkopoulou, E.; Dalén, L. Ancient wolf genome reveals an early divergence of domestic dog ancestors and admixture into high-latitude breeds. Curr. Biol. 2015, 25, 1515–1519. [Google Scholar] [CrossRef]
- Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V.J.; Sawyer, S.K.; Greenfield, D.L.; Germonpré, M.B.; Sablin, M.V.; López-Giráldez, F.; Domingo-Roura, X.; et al. Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 2013, 342, 871–874. [Google Scholar] [CrossRef]
- Lindblad-Toh, K.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas, E.J.; Zody, M.C.; et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005, 438, 803–819. [Google Scholar] [CrossRef]
- Axelsson, E.; Ratnakumar, A.; Arendt, M.L.; Maqbool, K.; Webster, M.T.; Perloski, M.; Liberg, O.; Arnemo, J.M.; Hedhammar, Å.; Lindblad-Toh, K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 2013, 495, 360–364. [Google Scholar] [CrossRef]
- Kotrschal, K. How wolves turned into dogs and how dogs are valuable in meeting human social needs. People Anim. Int. J. Res. Pract 2018, 1, 6. [Google Scholar]
- Marshall-Pescini, S.; Cafazzo, S.; Virányi, Z.; Range, F. Integrating social ecology in explanations of wolf–dog behavioral differences. Curr. Opin. Behav. Sci. 2017, 16, 80–86. [Google Scholar] [CrossRef]
- Range, F.; Virányi, Z. Wolves Are Better Imitators of Conspecifics than Dogs. PLoS ONE 2014, 9, e86559. [Google Scholar] [CrossRef]
- Frank, H. Evolution of canine information processing under conditions of natural and artificial selection. Z. Tierpsychol. 1980, 53, 389–399. [Google Scholar] [CrossRef]
- Boitani, L.; Ciucci, P.; Ortolani, A. Behaviour and social ecology of free-ranging dogs. In The Behavioural Biology of Dogs; Jensen, P., Ed.; CABI: Wallingford, UK, 2007; pp. 147–165. ISBN 978-1-84593-187-2. [Google Scholar]
- Cafazzo, S.; Valsecchi, P.; Bonanni, R.; Natoli, E. Dominance in relation to age, sex, and competitive contexts in a group of free-ranging domestic dogs. Behav. Ecol. 2010, 21, 443–455. [Google Scholar] [CrossRef]
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Lord, K.; Feinstein, M.; Smith, B.; Coppinger, R. Variation in reproductive traits of members of the genus Canis with special attention to the domestic dog (Canis familiaris). Behav. Proc. 2013, 92, 131–142. [Google Scholar] [CrossRef]
- Pilot, M.; Malewski, T.; Moura, A.E.; Grzybowski, T.; Oleński, K.; Ruść, A.; Kamiński, S.; Ruiz Fadel, F.; Mills, D.S.; Alagaili, A.N.; et al. On the origin of mongrels: Evolutionary history of free-breeding dogs in Eurasia. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef]
- Mech, L.D.; Boitani, L. Wolf social ecology. In Wolves: Behavior, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 1–34. ISBN 978-0-226-51698-1. [Google Scholar]
- Mech, L.D. Alpha status, dominance, and division of labor in wolf packs. Can. J. Zool. 1999, 77, 1196–1203. [Google Scholar] [CrossRef]
- Mech, L.D. Leadership in wolf, Canis lupus, packs. Can. Field Nat. 2000, 114, 259–263. [Google Scholar]
- MacNulty, D.R.; Smith, D.W.; Vucetich, J.A.; Mech, L.D.; Stahler, D.R.; Packer, C. Predatory senescence in ageing wolves. Ecol. Lett. 2009, 12, 1347–1356. [Google Scholar] [CrossRef]
- MacNulty, D.R.; Smith, D.W.; Mech, L.D.; Vucetich, J.A.; Packer, C. Nonlinear effects of group size on the success of wolves hunting elk. Behav. Ecol. 2012, 23, 75–82. [Google Scholar] [CrossRef]
- Packard, J. Wolf behavior: Reproductive, social and intelligent. In Wolves: Behavior, Ecology, and Conservation; Mech, L., Boitani, L., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 35–65. ISBN 978-0-226-51698-1. [Google Scholar]
- Bonanni, R.; Cafazzo, S.; Valsecchi, P.; Natoli, E. Effect of group size, dominance rank and social bonding on leadership behaviour in free-ranging dogs. Anim. Behav. 2010, 79, 981–991. [Google Scholar] [CrossRef]
- Parker, H.G.; Dreger, D.L.; Rimbault, M.; Davis, B.W.; Mullen, A.B.; Carpintero-Ramirez, G.; Ostrander, E.A. Genomic Analyses Reveal the Influence of Geographic Origin, Migration, and Hybridization on Modern Dog Breed Development. Cell Rep. 2017, 19, 697–708. [Google Scholar] [CrossRef]
- Bonanni, R.; Cafazzo, S. The social organisation of a population of free-ranging dogs in a suburban area of Rome: A reassessment of the effects of domestication on dogs’ behaviour. In The Social Dog; Kaminski, J., Marshall-Pescini, S., Eds.; Academic Press: San Diego, CL, USA, 2014; pp. 65–104. ISBN 978-0-12-407818-5. [Google Scholar]
- Boitani, L.; Francisci, F.; Ciucci, P.; Andreoli, G. Population biology and ecology of feral dogs in central Italy. In The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 217–244. [Google Scholar]
- Daniels, T.J. The social organization of free-ranging urban dogs. II. Estrous groups and the mating system. Appl. Anim. Ethol. 1983, 10, 365–373. [Google Scholar] [CrossRef]
- Pal, S.K.; Ghosh, B.; Roy, S. Inter- and intra-sexual behaviour of free-ranging dogs (Canis familiaris). Appl. Anim. Behav. Sci. 1999, 62, 267–278. [Google Scholar] [CrossRef]
- Pal, S.K. Reproductive behaviour of free-ranging rural dogs in West Bengal, India. Acta Theriol. 2003, 48, 271–281. [Google Scholar] [CrossRef]
- Pal, S.K. Parental care in free-ranging dogs, Canis familiaris. Appl. Anim. Behav. Sci 2005, 90, 31–47. [Google Scholar] [CrossRef]
- Paul, M.; Bhadra, A. The great Indian joint families of free-ranging dogs. PLoS ONE 2018, 13, e0197328. [Google Scholar] [CrossRef]
- Daniels, T.J.; Bekoff, M. Spatial and temporal resource use by feral and abandoned dogs. Ethology 1989, 81, 300–312. [Google Scholar] [CrossRef]
- Boitani, L.; Ciucci, P. Comparative social ecology of feral dogs and wolves. Ethol. Ecol. Evol. 1995, 7, 49–72. [Google Scholar] [CrossRef]
- Paul, M.; Majumder, S.S.; Bhadra, A. Grandmotherly care: A case study in Indian free-ranging dogs. J. Ethol. 2014, 32, 75–82. [Google Scholar] [CrossRef]
- Paul, M.; Sau, S.; Nandi, A.K.; Bhadra, A. Clever mothers balance time and effort in parental care: A study on free-ranging dogs. R. Soc. Open Sci. 2017, 4. [Google Scholar] [CrossRef]
- Abis, A. Analisi della Struttura Gerarchica di un Gruppo di Cani Vaganti (Canis familiaris) in una Zona Periferica di Roma in Presenza e in Assenza di Fonti di Competizione. Master’s Thesis, University of Rome La Sapienza, Rome, Italy, 2004. [Google Scholar]
- Bonanni, R.; Cafazzo, S.; Abis, A.; Barillari, E.; Valsecchi, P.; Natoli, E. Age-graded dominance hierarchies and social tolerance in packs of free-ranging dogs. Behav. Ecol. 2017, 28, 1004–1020. [Google Scholar] [CrossRef]
- Boitani, L.; Francisci, F.; Ciucci, P.; Andreoli, G. The ecology and behavior of feral dogs: A case study from central Italy. In The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 342–368. [Google Scholar]
- van Kerkhove, W. A fresh look at the wolf-pack theory of companion-animal dog social behavior. J. Appl. Anim. Welf. Sci. 2004, 7, 279–285, discussion 299–300. [Google Scholar] [CrossRef] [PubMed]
- Barillari, E. Le Relazioni Sociali Tra i Membri di un Gruppo Stabile di Cani Domestici, Ospitati Presso un’oasi Canina, con Particolare Riferimento Alla Gerarchia di Dominanza. Master’s Thesis, University of Rome La Sapienza, Rome, Italy, 2004. [Google Scholar]
- van der Borg, J.A.M.; Schilder, M.B.H.; Vinke, C.M.; de Vries, H. Dominance in domestic dogs: A quantitative analysis of its behavioural measures. PLoS ONE 2015, 10, e0133978. [Google Scholar] [CrossRef]
- Trisko, R.K.; Smuts, B.B. Dominance relationships in a group of domestic dogs (Canis lupus familiaris). Behaviour 2015, 152, 677–704. [Google Scholar] [CrossRef]
- MacNulty, D.R.; Tallian, A.; Stahler, D.R.; Smith, D.W. Influence of group size on the success of wolves hunting bison. PLoS ONE 2014, 9, e112884. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.R.A.; du Toit, J.T.; Bingham, J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: Threats of competition and disease to large wild carnivores. Biol. Conserv. 2004, 115, 369–378. [Google Scholar] [CrossRef]
- Schmidt, P.A.; Mech, L.D. Wolf pack size and food acquisition. Am. Nat. 1997, 150, 513–517. [Google Scholar] [CrossRef]
- Majumder, S.S.; Bhadra, A.; Ghosh, A.; Mitra, S.; Bhattacharjee, D.; Chatterjee, J.; Nandi, A.K.; Bhadra, A. To be or not to be social: Foraging associations of free-ranging dogs in an urban ecosystem. Acta Ethol. 2014, 17, 1–8. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Carr, G.M. Variation in dog society: Between resource dispersion and social flux. In The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 199–216. [Google Scholar]
- Pal, S.K.; Ghosh, B.; Roy, S. Agonistic behaviour of free-ranging dogs (Canis familiaris) in relation to season, sex and age. Appl. Anim. Behav. Sci. 1998, 59, 331–348. [Google Scholar] [CrossRef]
- Cafazzo, S.; Marshall-Pescini, S.; Lazzaroni, M.; Virányi, Z.; Range, F. The effect of domestication on post-conflict management: Wolves reconcile while dogs avoid each other. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef]
- Dale, R.; Range, F.; Stott, L.; Kotrschal, K.; Marshall-Pescini, S. The influence of social relationship on food tolerance in wolves and dogs. Behav. Ecol. Sociobiol. 2017, 71. [Google Scholar] [CrossRef] [PubMed]
- Range, F.; Ritter, C.; Virányi, Z. Testing the myth: Tolerant dogs and aggressive wolves. Proc. Royal Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Valsecchi, P.; Natoli, E. Pattern of individual participation and cheating in conflicts between groups of free-ranging dogs. Anim. Behav. 2010, 79, 957–968. [Google Scholar] [CrossRef]
- Bonanni, R.; Natoli, E.; Cafazzo, S.; Valsecchi, P. Free-ranging dogs assess the quantity of opponents in intergroup conflicts. Anim. Cogn. 2011, 14, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Sen Majumder, S.; Sau, S.; Nandi, A.K.; Bhadra, A. High early life mortality in free-ranging dogs is largely influenced by humans. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Kortekaas, K.; Kotrschal, K. Does socio-ecology drive differences in alertness between wolves and dogs when resting? Behav. Process. 2019, 166. [Google Scholar] [CrossRef]
- Adams, G.J.; Johnson, K.G. Sleep-wake cycles and other night-time behaviours of the domestic dog Canis familiaris. Appl. Anim. Behav. Sci. 1993, 36, 233–248. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the advantages of flocking. J. Theor. Biol. 1973, 38, 419–422. [Google Scholar] [CrossRef]
- Razmjou, S. Mental workload in heat: Toward a framework for analyses of stress states. Aviat. Space Environ. Med. 1996, 67, 530–538. [Google Scholar]
- Oken, B.S.; Salinsky, M.C.; Elsas, S.M. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clin. Neurophysiol. 2006, 117, 1885–1901. [Google Scholar] [CrossRef]
- Creel, S. Social dominance and stress hormones. Trends Ecol. Evol. 2001, 16, 491–497. [Google Scholar] [CrossRef]
- Künzl, C.; Sachser, N. The behavioral endocrinology of domestication: A comparison between the domestic guinea pig (Cavia aperea f. porcellus) and its wild ancestor, the cavy (Cavia aperea). Horm. Behav. 1999, 35, 28–37. [Google Scholar] [CrossRef]
- Porges, S.W. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233. [Google Scholar] [CrossRef]
- Tobaldini, E.; Nobili, L.; Strada, S.; Casali, K.R.; Braghiroli, A.; Montano, N. Heart rate variability in normal and pathological sleep. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, R.; Cerutti, S.; Viardot, G.; Werner, S.; Bianchi, A.M. Modulation of the sympatho-vagal balance during sleep: Frequency domain study of heart rate variability and respiration. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef]
- Chouchou, F.; Desseilles, M. Heart rate variability: A tool to explore the sleeping brain? Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Viola, A.U.; Tobaldini, E.; Chellappa, S.L.; Casali, K.R.; Porta, A.; Montano, N. Short-term complexity of cardiac autonomic control during sleep: REM as a potential risk factor for cardiovascular system in aging. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Koella, W.P. A modern neurobiological concept of vigilance. Experientia 1982, 38, 1426–1437. [Google Scholar] [CrossRef]
- Siegel, J.M. Sleep viewed as a state of adaptive inactivity. Nat. Rev. Neurosci. 2009, 10, 747–753. [Google Scholar] [CrossRef]
- Gauthier-Clerc, M.; Tamisier, A.; Cezilly, F. Sleep-vigilance trade-off in Green-winged Teals (Anas crecca crecca). Can. J. Zool. 1998, 76, 2214–2218. [Google Scholar] [CrossRef]
- Wang, M.Y.; Ruckstuhl, K.E.; Xu, W.X.; Blank, D.; Yang, W.K. Human activity dampens the benefits of group size on vigilance in khulan (Equus hemionus) in Western China. PLoS ONE 2016, 11, e0146725. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Gergely, A.; Galambos, Á.; Kis, A. Heart rate and heart rate variability during sleep in family dogs (Canis familiaris). Moderate effect of pre-sleep emotions. Animals 2018, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Bálint, A.; Eleőd, H.; Körmendi, J.; Bódizs, R.; Reicher, V.; Gácsi, M. Potential Physiological Parameters to Indicate Inner States in Dogs: The analysis of ECG, and respiratory signal during different sleep phases. Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, R.; Smith, C.; Smetzer, D. Sinus arrhythmia in the dog. Am. J. Physiol. 1966, 210, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jonckheer-Sheehy, V.S.M.; Vinke, C.M.; Ortolani, A. Validation of a Polar® human heart rate monitor for measuring heart rate and heart rate variability in adult dogs under stationary conditions. J. Vet. Behav. 2012, 7, 205–212. [Google Scholar] [CrossRef]
- Brucks, D.; Essler, J.L.; Marshall-Pescini, S.; Range, F. Inequity aversion negatively affects tolerance and contact-seeking behaviours towards partner and experimenter. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- D’Aniello, B.; Semin, G.R.; Alterisio, A.; Aria, M.; Scandurra, A. Interspecies transmission of emotional information via chemosignals: From humans to dogs (Canis lupus familiaris). Anim. Cogn. 2018, 21, 67–78. [Google Scholar] [CrossRef]
- Romero, T.; Konno, A.; Hasegawa, T. Familiarity bias and physiological responses in contagious yawning by dogs support link to empathy. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Grandi, L.C.; Heinzl, E.; Pelosi, A.; Prato Previde, E.; Valsecchi, P. How good is this food? A study on dogs’ emotional responses to a potentially pleasant event using infrared thermography. Physiol. Behav. 2016, 159, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wormald, D.; Lawrence, A.J.; Carter, G.; Fisher, A.D. Reduced heart rate variability in pet dogs affected by anxiety-related behaviour problems. Physiol. Behav. 2017, 168, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Essner, A.; Sjöström, R.; Ahlgren, E.; Lindmark, B. Validity and reliability of Polar® RS800CX heart rate monitor, measuring heart rate in dogs during standing position and at trot on a treadmill. Physiol. Behav. 2013, 114–115, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Essner, A.; Sjöström, R.; Ahlgren, E.; Gustås, P.; Edge-Hughes, L.; Zetterberg, L.; Hellström, K. Comparison of Polar® RS800CX heart rate monitor and electrocardiogram for measuring inter-beat intervals in healthy dogs. Physiol. Behav. 2015, 138, 247–253. [Google Scholar] [CrossRef] [PubMed]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Wacker, K. Do Equally Raised Wolves and Dogs Differ in Their Circadian and Circannual Time Budgets and Their Response to Humans? Master’s Thesis, Ludwig-Maximilian-Universität, München, Germany, 2020. [Google Scholar]
- Cobb, M.L.; Iskandarani, K.; Chinchilli, V.M.; Dreschel, N.A. A systematic review and meta-analysis of salivary cortisol measurement in domestic canines. Domest. Anim. Endocrinol. 2016, 57, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Colussi, A.; Perrotta, M.G.; Stefanon, B. Salivary cortisol concentration in healthy dogs is affected by size, sex, and housing context. J. Vet. Behav. 2015, 10, 302–306. [Google Scholar] [CrossRef]
- Schöberl, I.; Kortekaas, K.; Schöberl, F.F.; Kotrschal, K. Algorithm-supported visual error correction (AVEC) of heart rate measurements in dogs, Canis lupus familiaris. Behav. Res. 2015, 47, 1356–1364. [Google Scholar] [CrossRef]
- Baayen, R.H. Analyzing Linguistic Data: A Practical Introduction to Statistics Using R.; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-70918-7. [Google Scholar]
- Schielzeth, H. Simple means to improve the interpretability of regression coefficients: Interpretation of regression coefficients. Methods Ecol. Evol. 2010, 1, 103–113. [Google Scholar] [CrossRef]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 255–278. [Google Scholar] [CrossRef]
- Schielzeth, H.; Forstmeier, W. Conclusions beyond support: Overconfident estimates in mixed models. Behav. Ecol. 2009, 20, 416–420. [Google Scholar] [CrossRef]
- Matuschek, H.; Kliegl, R.; Vasishth, S.; Baayen, H.; Bates, D. Balancing Type I error and power in linear mixed models. J. Mem. Lang. 2017, 94, 305–315. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2011; ISBN 978-1-4129-7514-8. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 2nd ed.; Sage Publications, Inc.: London, UK, 2005; ISBN 978-0-7619-4452-2. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists, 1st ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Nieuwenhuis, R.; te Grotenhuis, H.F.; Pelzer, B.J. influence.ME: Tools for detecting influential data in mixed effects models. R J. 2012, 4, 38–47. [Google Scholar] [CrossRef]
- Dobson, A.J. An Introduction to Generalized Linear Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2002; ISBN 978-1-58488-165-0. [Google Scholar]
- Forstmeier, W.; Schielzeth, H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 2011, 65, 47–55. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: www.R-project.org (accessed on 11 October 2019).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lima, S.L.; Rattenborg, N.C.; Lesku, J.A.; Amlaner, C.J. Sleeping under the risk of predation. Anim. Behav. 2005, 70, 723–736. [Google Scholar] [CrossRef]
- Grigg, E.K.; Nibblett, B.M.; Robinson, J.Q.; Smits, J.E. Evaluating pair versus solitary housing in kennelled domestic dogs (Canis familiaris) using behaviour and hair cortisol: A pilot study. Vet. Rec. Open 2017, 4. [Google Scholar] [CrossRef]
- Tuber, D.S.; Hennessy, M.B.; Sanders, S.; Miller, J.A. Behavioral and glucocorticoid responses of adult domestic dogs (Canis familiaris) to companionship and social separation. J. Comp. Psychol. 1996, 110. [Google Scholar] [CrossRef]
- Grippo, A.J.; Lamb, D.G.; Carter, C.S.; Porges, S.W. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry 2007, 62, 1162–1170. [Google Scholar] [CrossRef]
- Späni, D.; Arras, M.; König, B.; Rülicke, T. Higher heart rate of laboratory mice housed individually vs in pairs. Lab. Anim. 2003, 37, 54–62. [Google Scholar] [CrossRef]
- Dreschel, N.A.; Granger, D.A. Physiological and behavioral reactivity to stress in thunderstorm-phobic dogs and their caregivers. Appl. Anim. Behav. Sci. 2005, 95, 153–168. [Google Scholar] [CrossRef]
- Grimm, M.S.; Emerman, J.T.; Weinberg, J. Effects of social housing condition and behavior on growth of the Shionogi mouse mammary carcinoma. Physiol. Behav. 1996, 59, 633–642. [Google Scholar] [CrossRef]
- Adams, G.J.; Johnson, K.G. Sleep, work, and the effects of shift work in drug detector dogs Canis familiaris. Appl. Anim. Behav. Sci. 1994, 41, 115–126. [Google Scholar] [CrossRef]
- Bódizs, R.; Kis, A.; Gácsi, M.; Topál, J. Sleep in the dog: Comparative, behavioral and translational relevance. Curr. Opin. Behav. Sci. 2020, 33, 25–33. [Google Scholar] [CrossRef]
- Hawking, F.; Lobban, M.C.; Gammage, K.; Worms, M.J. Circadian rhythms (activity, temperature, urine and microfilariae) in dog, cat, hen, duck, thamnomys and gerbillus. J. Interdiscip. Cycle Res. 1971, 2, 455–473. [Google Scholar] [CrossRef]
- Creel, S. Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal. 2005, 86, 255–264. [Google Scholar] [CrossRef]
- DeVries, A.C.; Glasper, E.R.; Detillion, C.E. Social modulation of stress responses. Physiol. Behav. 2003, 79, 399–407. [Google Scholar] [CrossRef]
- Kotrschal, K.; Hirschenhauser, K.; Möstl, E. The relationship between social stress and dominance is seasonal in greylag geese. Anim. Behav. 1998, 55, 171–176. [Google Scholar] [CrossRef]
- da Silva Vasconcellos, A.; Ades, C.; Kotrschal, K. Social stress in wolves. In Wolves: Biology, Behavior and Conservation; Maia, A.P., Crussi, H.F., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 157–176. ISBN 978-1-62100-916-0. [Google Scholar]
- Hezzell, M.J.; Humm, K.; Dennis, S.G.; Agee, L.; Boswood, A. Relationships between heart rate and age, bodyweight and breed in 10,849 dogs. J. Small Anim. Pract. 2013, 54, 318–324. [Google Scholar] [CrossRef]
- Cruz Aleixo, A.S.; Alfonso, A.; Oba, E.; Ferreira de Souza, F.; Salgueiro Cruz, R.K.; Fillippi, M.G.; Chiacchio, S.B.; Tsunemi, M.; Gomes Lourenço, M.L. Scaling relationships among heart rate, electrocardiography parameters, and body weight. Top. Companion Anim. Med. 2017, 32, 66–71. [Google Scholar] [CrossRef]
- Ferasin, L.; Ferasin, H.; Little, C.J.L. Lack of correlation between canine heart rate and body size in veterinary clinical practice. J. Small Anim. Pract. 2010, 51, 412–418. [Google Scholar] [CrossRef]
- Lamb, A.P.; Meurs, K.M.; Hamlin, R.L. Correlation of heart rate to body weight in apparently normal dogs. J. Vet. Cardiol. 2010, 12, 107–110. [Google Scholar] [CrossRef]
- Nganvongpanit, K.; Kongsawasdi, S.; Chuatrakoon, B.; Yano, T. Heart rate change during aquatic exercise in small, medium and large healthy dogs. Thai J. Vet. Med. 2011, 41, 455–461. [Google Scholar]
| Dog | Sex | Pack # | NPM | Rank 1 | Body Weight (kg) | Age (Year) |
|---|---|---|---|---|---|---|
| Asali | ♂ | 1 | 1 | High | 31.04 | 2.9 |
| Hakima | ♂ | - | - | - | 15.45 | 2.8 |
| Kilio | ♂ | 2 | 1 | High | 26.95 | 3.7 2 |
| Maisha | ♂ | 3 | 2 | Low | 24.44 | 3.7 2 |
| Meru | ♂ | 4 (5) | 1 | Low | 34.60 | 2.8 |
| Rafiki | ♂ | 3 | 2 | High | 19.64 | 3.7 |
| Term | Est | SE | Lower CI | Upper CI | χ2 | df | p | Min | Max |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 96.228 | 4.772 | 87.128 | 105.321 | 1 | 89.688 | 102.114 | ||
| State 2 | −11.461 | 2.538 | −16.255 | −6.749 | 1 | −13.294 | −9.760 | ||
| Condition 3 | −5.262 | 6.254 | −17.070 | 7.297 | 1 | −11.595 | −0.604 | ||
| Temperature 4 | −0.481 | 1.642 | −3.638 | 2.560 | 0.081 | 1 | 0.776 | −1.317 | −0.122 |
| Weight 5 | 3.469 | 3.651 | −4.214 | 11.553 | 0.884 | 1 | 0.347 | −1.805 | 7.959 |
| NPM 6 | 1.943 | 3.904 | −5.655 | 9.472 | 0.243 | 1 | 0.622 | −3.399 | 7.434 |
| State:Condition | −5.307 | 2.624 | −10.533 | −0.360 | 3.856 | 1 | 0.050 7 | −7.622 | −3.715 |
| Term | Est | SE | Lower CI | Upper CI | χ2 | df | p | Min | Max |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 132.082 | 24.989 | 85.355 | 175.707 | 1 | 107.514 | 139.409 | ||
| State 2 | 142.074 | 40.145 | 62.199 | 216.346 | 1 | 128.355 | 166.965 | ||
| Condition 3 | 6.677 | 36.688 | −65.434 | 78.870 | 1 | −14.192 | 27.809 | ||
| Temperature 4 | −26.511 | 15.030 | −56.345 | 1.782 | 2.274 | 1 | 0.132 | −36.163 | −16.589 |
| Weight 5 | −31.192 | 15.403 | −63.686 | −3.041 | 3.799 | 1 | 0.051 | −43.397 | 15.742 |
| NPM 6 | 26.870 | 15.578 | −5.351 | 57.150 | 2.827 | 1 | 0.093 | 18.702 | 87.269 |
| State:Condition | 94.399 | 44.382 | 8.527 | 185.034 | 3.930 | 1 | 0.047 7 | 47.253 | 133.593 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kortekaas, K.; Kotrschal, K. Social Context Influences Resting Physiology in Dogs. Animals 2020, 10, 2214. https://doi.org/10.3390/ani10122214
Kortekaas K, Kotrschal K. Social Context Influences Resting Physiology in Dogs. Animals. 2020; 10(12):2214. https://doi.org/10.3390/ani10122214
Chicago/Turabian StyleKortekaas, Kim, and Kurt Kotrschal. 2020. "Social Context Influences Resting Physiology in Dogs" Animals 10, no. 12: 2214. https://doi.org/10.3390/ani10122214
APA StyleKortekaas, K., & Kotrschal, K. (2020). Social Context Influences Resting Physiology in Dogs. Animals, 10(12), 2214. https://doi.org/10.3390/ani10122214




