1. Introduction
Zoos can play important roles in conservation education, and the experiences that visitors have at a zoo can influence visitors’ perceived connections with the animals, as well as visitors’ responses to conservation messages [
1,
2]. Zoo visitors often rate their visits as positive when they can see the animals at close proximity [
3,
4]. Furthermore, visitors often show more interest in animals that are active [
5,
6], and some visitors report having positive emotional responses after having a direct interaction with a zoo animal [
7]. However, popular zoo species can experience greater levels of noise from visitors, as larger crowds can lead to increased ambient sound [
8]. As a result of factors related to zoo visitors (e.g., increased visitor abundance, increased noise), some animals have been shown to exhibit behavioral and physiological changes that can be indicative of stress [
9,
10].
Although studies on the impacts of human visitors on zoo animal behavior have existed for decades [
11,
12], documented responses of zoo animals to visitors have been inconsistent, and such variation is likely due to a range of factors (e.g., species studied, the animals’ individual personalities, characteristics of the zoo visitors, variability in exhibit design [
13,
14]). Furthermore, there is not full agreement on whether certain interactions between the visitor and zoo animal are positive, neutral, or negative [
15]. Studies have examined a range of variables, from visitor abundance, density, and proximity [
16,
17], to visitor noise and visitor activity levels [
8,
18]. Additional studies are needed to understand the extent visitors impact the welfare of the zoo animals [
19], as behavioral changes in an animal do not necessarily indicate a negative effect on animal welfare [
13,
20].
To date, most zoo visitor studies have focused on primates and felids [
21]. Primates sometimes respond to zoo visitors [
12,
17], and at times the primates’ behavioral responses appear to signal that the animals are stressed by the zoo visitors [
22,
23]. Markers of behavioral stress include behavioral changes [
11,
24,
25], differences in visibility while on exhibit [
26], and elevated levels of glucocorticoids [
27,
28]. Nevertheless, it is difficult to make generalizable statements about the impacts of zoo visitors on primates. For example, Kuhar [
26] found that overall western lowland gorillas (
Gorilla gorilla gorilla) did not exhibit much behavioral change as visitor abundance increased; however, when crowd size was large, aggression increased in a bachelor male group, but not in a family group. Carder and Semple [
29] noted a visitor effect in gorillas at one zoo, but not at another. Stoinski et al. [
30] documented that overall gorilla behavior did not appear to be impacted by visitor abundance, but differences existed in the responses by individual gorillas. However, a study of chimpanzees (
Pan troglogytes) and gorillas found that overall the animals’ behaviors and exhibit use did not vary with the number of visitors [
31].
Similar variation also exists in felids. Margulis et al. [
5] did not find evidence that visitor presence impacted six cat species: lion (
Panthera leo), Amur leopard (
P.
pardus orientalis), Amur tiger (
P.
tigris altaica), snow leopard (
P.
uncia), clouded leopard (
Neofelis nebulosi), and fishing cat (
Felis viverrinus). However, Suárez et al. [
32] noted behavioral differences based on the presence or absence of zoo visitors in five felid species: Eurasian lynx (
Lynx lynx), jaguar (
P. onca), bobcat (
L. rufus), ocelot (
Leopardus pardalis), and Asiatic lion (
P. l. persica). A separate study of jaguars found that visitor density and noise level impacted the amount of time the cats spent visible and the male’s level of aggression, while visitor noise levels were associated with increased pacing by the female [
33]. Therefore, even with the most-studied taxa (primates and felids), behavioral responses vary.
Other taxa also show a range of responses. Zoo visitors had little to no impact in studies of flamingos (
Phoenicopterus roseus,
P.
ruber,
P.
chilensis,
P.
andinus, and
P.
minor [
34]), greater rheas (
Rhea americana [
35]), slender-tailed meerkats (
Suricata suricatta [
36]), and African penguins (
Spheniscus demersus [
37]). However, zoo visitors impacted aggressive, huddling, and avoidance behaviors [
38], as well as location within the exhibit area [
39], in little penguins (
Eudyptula minor), aggression and activity levels in female Galápagos giant tortoise (
Chelonoidis nigra [
40]), and vigilance in koalas (
Phascolarctos cinereus [
41]). Furthermore, studies have found variable patterns related to inactive behavior: from resting more when visitor abundance was low [
25] to resting more when visitor abundance was high [
42], to no change in resting behavior [
39].
Additionally, some zoo exhibits encourage direct physical interactions between the visitor and animals [
43,
44]. In visitor-feeding experiences with crowned lemurs (
Eulemur coronatus [
44]) and giraffe (
Giraffa camelopardalis [
45,
46]), zoo animals did not appear to be negatively impacted by the increased visitor contact. Although petting-zoo goats (
Capra hircus), llamas (
Llama glama), and Vietnamese pot-bellied pigs (
Sus scrofa) did not respond to zoo visitors in the same manner, Farrand et al. [
47] concluded that the animals’ welfare was not negatively impacted by visitor interactions. Petting-zoo African pygmy goats (
Capra hircus) and Romanov sheep (
Ovis aries) showed a reduction in undesirable behaviors when a retreat area was available, underlying the importance of exhibit design in animal welfare [
48]. When multiple exhibit types were studied, Bennett’s wallabies (
Macropus rufogriseus) fed more and exhibited more interactive behaviors in the no-interaction exhibits; however, resting, locomotion, and vigilance did not differ between exhibit types [
49]. In addition, quokka (
Setonix brachyurus) were less likely to be visible when visitors were present in walk-though exhibits, but the effect of visitor presence was not considered to be great [
50].
Individual animals can vary in their responses to zoo visitors [
21,
51,
52]. A study of three polar bears (
Ursus maritimus) found one animal increased and two animals decreased stereotypical behavior during periods of higher visitor density [
53]. A recent review [
21] highlighted that individuals may not perceive their environment in the same manner, even when these individuals are in the same exhibit; thus, individual traits and temperament may greatly impact behavioral responses. For example, the social rank of a Japanese macaque (
Macaca fuscata) predicted probability of aggression towards zoo visitors [
54]. Therefore, it is important to also consider individual differences when assessing zoo animal behavior [
26] and welfare [
55].
Although the behavior of some zoo animals may change when visitors are present, such behavioral changes are not necessarily negative: in a study of 12 primate species, the primates spent more time in the front of the exhibit and attempted to interact with visitors when large, active visitor groups were present [
12]. When orangutans were given a preference test, they did not show aversion to the public viewing area [
56]. When treatments were applied that varied in the extent of interactions that servals (
Leptailurus serval) had with zoo visitors, the overall decrease in stereotypic pacing led to the conclusion that some visitor-encounter programs may have a short-term positive benefit for the animals [
57]. Furthermore, the relationship between variables (e.g., visitor abundance, zoo animal behavior) is not necessarily causal: an increase in certain behaviors may attract zoo visitors [
23]. Therefore, visitors and zoo animals may mutually impact the other’s behavior [
5].
Here we present findings from 10 independent studies on 16 species that all focused on the impact of human visitors on zoo animals. Each of these 10 studies examined the extent to which one or more independent variable (e.g., abundance, noise, proximity, solicitation of interactions with zoo animals) impacted zoo animal behavior. Because individual animals may have different behavioral responses, whenever possible, we focused our analyses on individual animals, so that animals that demonstrated a change in behavior could be immediately identified, which is important for managing individual animals in a zoo [
58,
59]. Based on the findings from previous studies (summarized above), we predicted that zoo visitors would be associated with changes in interactive, vigilance, social, aggression, stereotypic, rest, and visibility behaviors of the zoo animals.
Our work contributes new species to the body of literature on the zoo visitor effect: 10 of the 16 species studied were not represented in a recent review of the literature on zoo visitor impacts on animal behavior [
21]. Four of these newly studied species were fish, on which there have never been published studies documenting their responses to zoo visitors [
21], even though fish (e.g., Chondrichthyes) are often part of animal–visitor interactive exhibits [
60]. Findings from research on how visitors affect zoo animals can be integrated into plans for exhibit designs, and hopefully create experiences that are positive for both the visitor and the zoo animal [
61]. However, without published data for a wider representation of taxa at zoological parks, analyzing taxonomic-wide patterns of the impacts of zoo visitors on zoo animals is not fully possible. Based on findings from our studies, we make recommendations for zoo animal management, specifically in the context of the responses of zoo animals to humans.
4. Discussion
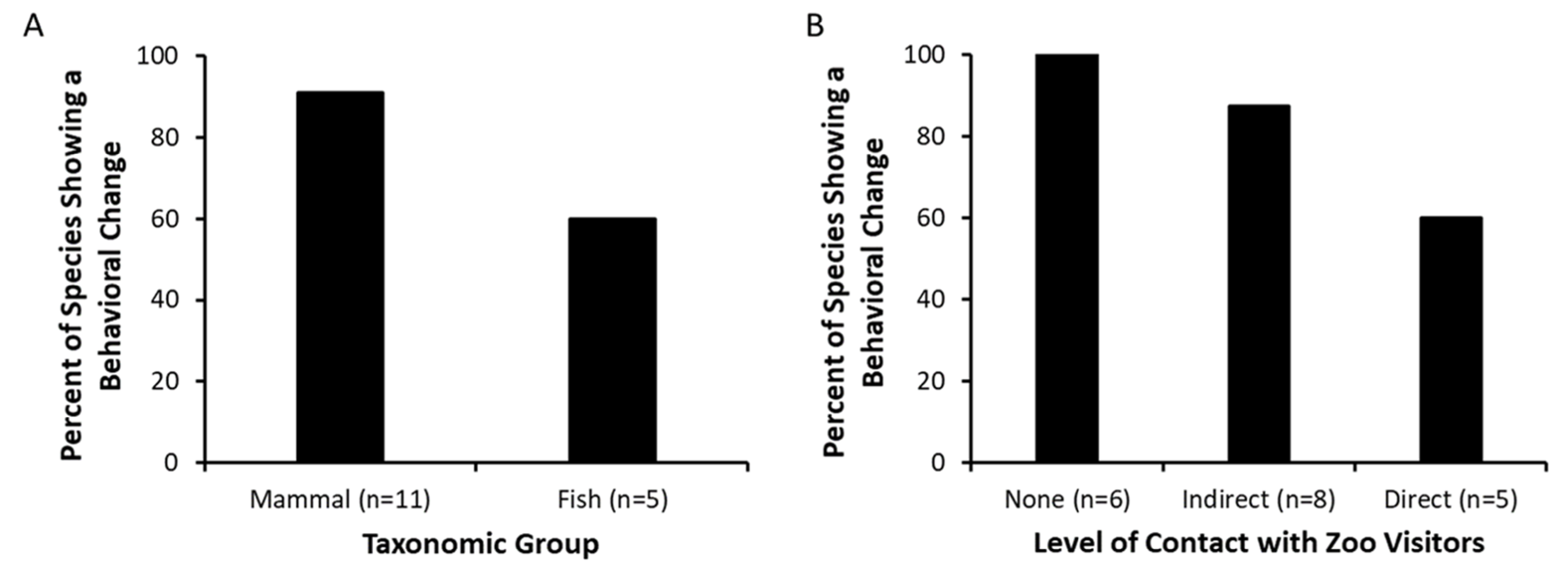
In 13 of the 16 species studied, we found evidence that visitors impact the behavior of zoo animals. Behavioral changes were noted across taxonomic groups, with all but one mammal species demonstrating a behavioral change associated with zoo visitors. Furthermore, we noted behavioral changes in animals housed in exhibits with no contact allowed between zoo visitors and zoo animals, exhibits allowing for indirect contact (e.g., glass window), and exhibits allowing for direct contact between zoo visitors and animals. We acknowledge that our 10 studies were independent studies that did not focus on the same independent variables for all studies, and that direct comparisons cannot be made across all 16 species as to what primarily influences behavioral changes in zoo animals. However, such comparisons were not the intent of our analysis. Instead, our goal was to document the extent to which a range of variables associated with zoo visitors were related to behavioral changes in zoo animals.
Overall, we found that as visitor intensity increased in abundance, noise, and/or proximity, a majority of the animals studied demonstrated an increase in alert, vigilance, or visitor-interactive behaviors, and a decrease in social and rest behaviors. However, patterns were not consistent across all species, and behavioral differences sometimes existed between individuals living within the same social group. Furthermore, zoo visitor abundance was not consistent across exhibits: both exhibits of white-cheeked gibbons attracted relatively large numbers of visitors (
Appendix A), while the northern night monkeys never had more than seven visitors at one time, suggesting that that the visitor effect can potentially impact individuals in particular exhibits more frequently and for longer durations than individuals in exhibits that are not frequented by visitors as often and to the same extent.
The results from our studies support previous findings that the responses to zoo visitors can be variable [
13,
14]. Zoo visitors had an impact on the behaviors for 81.3% of the species we studied, but the lack of a behavioral change noted for three (18.7%) of the species we studied does not lead us to conclude that these three species were not impacted by zoo visitors. First, in some of the studies, the researchers did not identify individual animals; therefore, potential differences in individual animals’ behavioral responses were not detectable in those studies. Second, the three species for which we did not detect behavioral changes (tamandua, brownbanded bamboo shark, and white-spotted bamboo shark) are primarily nocturnal. While one species, tamandua, was housed in an exhibit set for nocturnal conditions, the two species of bamboo sharks were in a diurnal exhibit. These bamboo sharks spent most of the study resting in one location that was not in close proximity to where human visitors stood. Third, our 10 studies rarely measured more than two zoo visitor variables at a time. It is possible that for studies where no behavioral change was detected, had the study examined additional variables, the results may have differed. For example, Choo [
16] found that overall orangutans were impacted by the proximity of zoo visitors, but not by visitor abundance or activity. In our study, the wolves’ behaviors often changed as visitor abundance and visitor noise changed, but there were no differences based on visitor presence versus absence. Therefore, future studies that record both visitor abundance as well as visitor noise and proximity could help tease apart the factors that most prominently impact the zoo animals. Such information can then be used directly by the zoo in their communication with visitors. For example, Sherwen et al. [
36] found that the use of signs and the positioning of zoo employees reduced visitor noise around a meerkat exhibit.
The goal of our study was to determine to what extent the zoo animals demonstrated behavioral changes associated with changes in zoo visitor abundance, presence, noise, proximity, or food provisioning. We acknowledge that individual animals may vary drastically in their previous experiences with zoo visitors (e.g., frequency, intensity, and overall nature of interactions with visitors). Further, individual animals can vary in how they cope with changing environments and stressful situations [
21,
54]. That said, given the dearth of information published on some of the species we studied, documenting the behavioral changes that occur is a first step in gaining a better understanding of the visitor effect, and understanding how individual animals respond. Our studies examined behavioral changes at the individual level, whenever possible, because of the importance of understanding individual animals’ responses [
58,
59].
Although some behaviors (e.g., stereotypic behavior, aggression) are often associated with negative animal welfare, and other behaviors (e.g., play, affiliative social interactions) are sometimes associated with positive welfare [
40,
67], Sherwen and Hemsworth [
21] stressed that many behaviors may not have clear associations with welfare status, and behavioral changes do not always indicate that the change is negative [
68]. We acknowledge that an animal’s behavioral response (for example, decreasing rest behavior) does not necessarily indicate a particular emotional response. Therefore, we did not categorize the behaviors based on what the welfare implications were. We present our findings to add to the body of scientific literature on the responses of animals to zoo visitors, to help form a better understanding of zoo animal responses.
Based on our findings from studies of 16 species, we discuss below our recommendations for the management of zoo animals. These recommendations address (1) the importance of short-term studies that allow for the assessment of behavioral responses by individual animals, (2) the extent to which exhibit design may impact individual animals, and (3) considerations for future research studies.
4.1. Study Design: Short-Term Assessments on Individual Animals
We recommend that when it is not feasible to conduct long-term projects on many species in multiple exhibits at a zoo, short-term monitoring programs that are based on well-established, species-appropriate ethograms can provide a great deal of information on a range of species at a particular zoo, and across multiple institutions. The benefits of individual-based monitoring are known [
58,
59]. Such monitoring programs could involve animal keepers and zoo educational staff, as well as members of the public (e.g., trained zoo volunteers, students taking a behavior course). These short-term assessments can quickly highlight if there are species or individuals that may be of concern.
Previous studies have demonstrated that individuals can vary in their behavioral responses [
21,
51,
52]. We found further evidence that individual animals of the same species in the same exhibit do not always respond in the same manner to humans. Therefore, we also recommend that studies are designed so that behavioral responses by individual animals may be detected. Although it may be necessary at times to collect data on the entire group, such as when it is not possible to accurately identify individuals, we suggest that data on individual animals are collected whenever possible. Single-subject experimental designs (SSDs) can also be important [
59], especially if there is an individual animal of concern. While it is important to gain a general understanding of the impact of visitors on all species at a zoo, individuals within a species may react in different ways to visitor presence and other variables associated with visitors [
8,
30]. Therefore, it may be worth doing these initial assessments, and then consider SSDs or more involved, long-term studies to examine if changes in exhibit design, visitor behavior, or management practices lead to changes in the impact on the zoo animals (in the case that the impact was originally deemed to be negative).
4.2. Exhibit Design
Zoo visitors are often interested in seeing zoo animals in close proximity [
4], but proximity to humans can be a source of stress for zoo animals [
69]. Sometimes modifications to enclosures can minimize the visitor effect: when netting in front of an enclosure modified the visibility between gorillas and zoo visitors, the gorillas’ behaviors changed (reduced aggression and stereotypical behavior), but the visitors’ perceptions also changed [
70]. In a separate study, when visual contact between zoo visitors and capuchins (
Sapajus apella) was reduced, the capuchins exhibited a decrease in aggression and in fecal glucocorticoid metabolite concentration, but the number of visitors also decreased [
28]. Therefore, having zoos monitor the visibility of the animals [
71] can address the zoo visitors’ experiences as well as identify potential issues relating to animal stress.
We found widespread behavioral responses of animals to zoo visitors, and these responses occurred in animals in traditional exhibits to animals in exhibits that were designed to encourage interactions between zoo animals and visitors. All species (100%) that had more traditional exhibits (e.g., zoo animals and visitors were physically distanced from each other due to a water body, elevation difference, and/or vegetation) demonstrated some level of behavioral change associated with zoo visitors. Behavioral changes also occurred for the majority of species in indirect-contact and direct-contact exhibits (87.5% and 60% of species, respectively). These findings illustrate the widespread extent to which zoo animals respond to visitors.
An effective exhibit design can help protect the animals from potential negative consequences of large numbers of visitors [
16,
31]. In some exhibits, animals rotate on and off exhibit; it is possible that having restricted access to on-exhibit areas (which are often outdoors) could impact the animal’s behavior, as could being off exhibit where zoo visitors are not present. Based on our findings, we recommend that attention is paid to how individuals use their exhibit, and we recommend that all exhibits offer areas of refuge that are adequate in size for all individuals to enter at one time.
Although zoo animals may seek refuge at times, visitors can potentially be a source of environmental enrichment for the zoo animals [
20,
56]. In our study, when visitors were present at a window, the gibbons spent more time interacting with the humans than they did with each other, and the gibbons spent more time at the window when visitors were present. However, when visitors were present at the window, there was no difference in gibbon behavior when the visitors solicited interactions with the gibbons compared to when the visitors did not initiate contact. These findings suggest that the visitors attracted the attention of the gibbons, but whether or not it was the visitor who initiated contact did not influence the behavior of the gibbons. Because the gibbons were able to access the entire exhibit, but the interactive window was only available on one side of the exhibit, the gibbons had to approach the window to interact with the humans. Our findings appeared to indicate that the gibbons sought out interactions with the humans. The gibbons did not rest or engage in social behavior as often as they did when no visitors were present, but additional study is needed to determine to what extent visitors could potentially impact short- and long-term social bonds between conspecifics in the same social group.
We found that some individuals appeared to initiate interactions with humans (e.g., the gibbons in the indirect-contact exhibit), not all animals did so. For example, the wolves’ behavioral changes (e.g., increased alert behavior and decreased rest behavior in some individuals) appeared to be a response to the visitor abundance and visitor noise. For both tamanduas we studied, no differences in behaviors were detected based on human proximity, but the sustained stereotypic pacing of the male tamandua and the frequency that both animals were on exhibit yet hidden from view are important findings to address overall in regards to the housing and husbandry of these individuals. These differences in responses could be due to species differences or each individual animal’s history with humans; a more extensive data set is needed to draw such conclusions.
In our study of the orangutans, the location of the human observer appeared to impact the physical location of one of the female orangutans. Furthermore, for both female orangutans, they rested less and interacted with humans (e.g., direct eye contact, reaching toward a human) more when they were in closest proximity with a human observer. Although this study did not focus on quantifying zoo visitor abundance, it did provide novel findings about the potential impacts of human observers on zoo animals. We found the orangutans interacted with humans through gazing and gesturing behaviors, both of which are ways to communicate information between two individuals [
72,
73,
74]. Whereas Kaplan and Rogers [
73] found that captive orangutans avoided direct stares and exhibited less direct gazes in comparison to wild orangutans, the two female orangutans at the Memphis Zoo did directly look at humans. However, the responses of the two female orangutans were not identical, as one of the females spent much less time in close proximity with humans, and the male, when on exhibit, spent all of the time in one spot, elevated and at a distance from humans. Such varied responses to exhibit use and humans highlight the importance of assessing individual animals’ behaviors, and determining to what extent the exhibit’s features allow each individual to choose the extent to which they are in view of (or proximity to) humans.
The only exhibit that allowed for direct contact between zoo visitors and zoo animals was the interactive fish exhibit. Behavioral changes were noted in the cownose ray, southern stingray, and bonnethead shark. Although cownose rays increased solitary swimming behavior (and decreased social swimming) when more visitors were present, food provisioning impacted neither the time spent swimming versus resting, nor the physical use of the pool (periphery versus inside). These findings suggest that there may be variability between the individual cownose rays (as the groups were not the same from year to year), or that the rays had different responses to visitor variables. For example, the abundance of visitors may impact the rays in a different manner than whether or not the visitors are engaged in feeding the rays at a particular time, as the rays may anticipate receiving food from the visitors.
4.3. Recommendations for Future Studies
Visitors tend to show a greater interest in mammals, as well as animals with larger bodies and higher activity levels [
6]. Furthermore, much of the published literature also focuses on mammals, with emphases on primates and felids [
21,
75]. Our study includes findings on understudied (or never-studied) species, which are first steps in adding to the general understanding of how zoo animals respond to humans. However, many unknowns still exist regarding the impacts that zoo visitors have on captive species. Ideally, we would be able to identify the primary variables associated with species (or individuals) that demonstrate behavioral changes associated with the visitor effect. However, determining these predictive variables is a difficult task, as the responses by the animals may be based on a variety of factors. Multiple variables (e.g., enclosure design, interactions with zoo public, proximity to potential predators, interactions with animal keepers) can impact the behavioral and physiological responses of zoo animals [
11,
76,
77,
78]. As the number of studies increase, and the number of species expands, identifying predictive variables may be more possible. We recommend that studies of the impacts of zoo visitors expand to include species that are underrepresented in the literature, and, when possible, take note of behaviors associated with individual animals.
Recently there has been an increase in research addressing the welfare of zoo animals [
79], specifically research that measures behavior and physiology [
80]. There is no single strategy for assessing welfare that is most appropriate for all zoo animals [
81], as the needs and responses of these animals vary by species [
82], as well as on an individual basis [
59,
83]. Furthermore, it may be that factors other than (or in addition to) zoo visitors are primarily impacting an animal’s behavior. For example, in our study the male tamandua spent 61.8% of the scans exhibiting stereotypic pacing, and such pacing was consistent throughout the study, at different levels of visitor presence. Our finding suggests that additional factors may have been contributing to the stereotypic behavior of this individual animal, but we cannot rule out that zoo visitors did not contribute to the pacing behavior. In such situations, we recommend a holistic approach to animal management to examine multiple factors that may be impacting a particular individual animal.
Noise and disruptions may lead to stress in some zoo animals, resulting in physiological changes as well as behavioral changes [
9,
10,
27,
69]. In addition, minimizing such disruptions could be critical for targeted breeding programs of threatened or endangered species. Further research on the hormone profiles of zoo animals could provide a better understanding of both behavioral and physiological factors related to zoo visitor presence. Such information taken together would be helpful in then assessing whether any changes indicate an animal welfare concern [
19].