Comparison of the Effects of Nonprotein and Protein Nitrogen on Apoptosis and Autophagy of Rumen Epithelial Cells in Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture and Treatment of Primary Ruminal Epithelial Cells (PRECs)
2.2. Morphological Detection
2.3. Monodansylcadaverine (MDC) Staining Analysis
2.4. Apoptosis Detection by Flow Cytometry
2.5. Intracellular ROS Detection
2.6. Detection of Mitochondrial Membrane Potential (MMP)
2.7. Transmission Electron Microscopy (TEM) Analysis
2.8. Tandem mCherry-EGFP-LC3 Immunofluorescence
2.9. RT-PCR
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
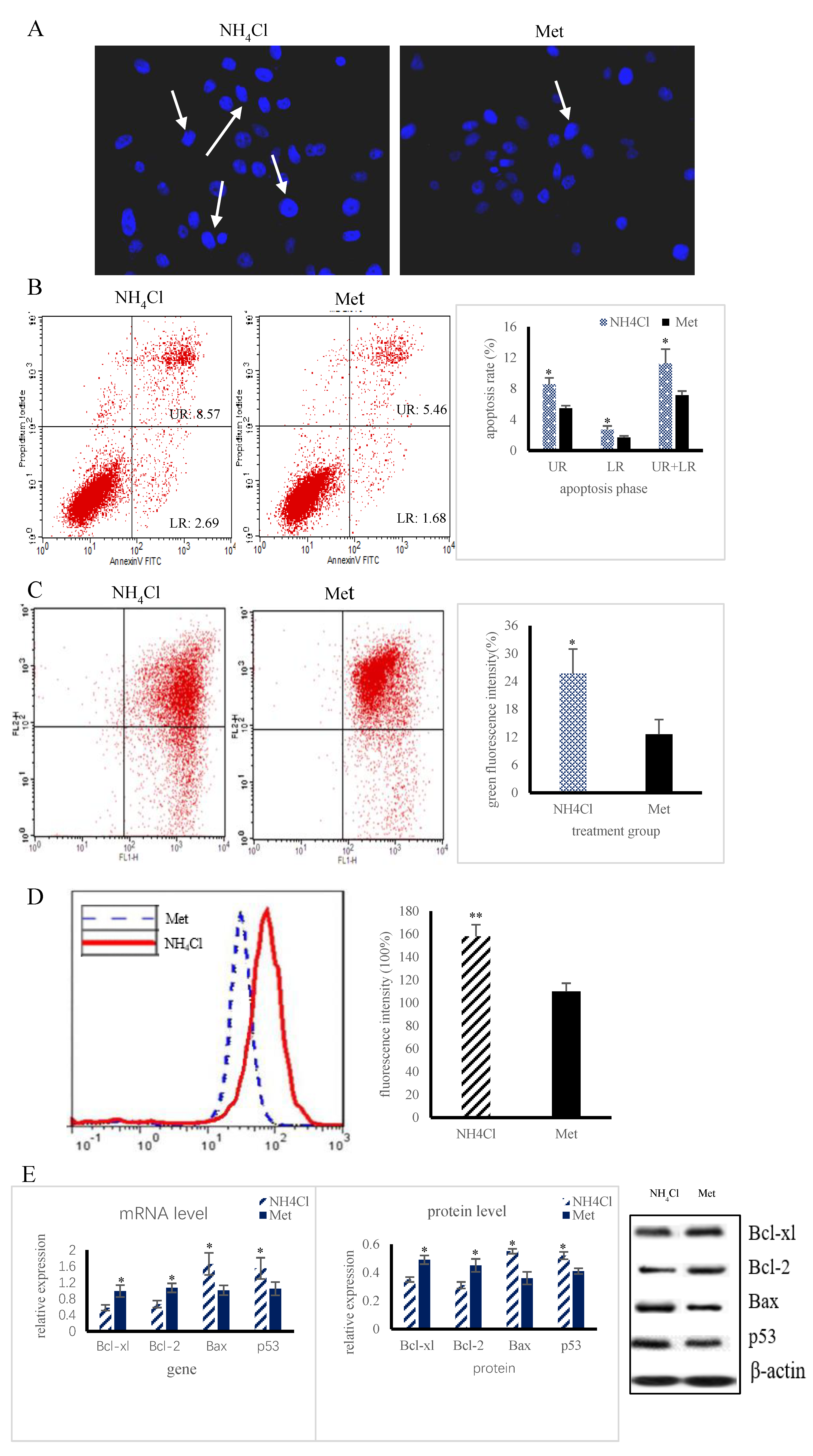
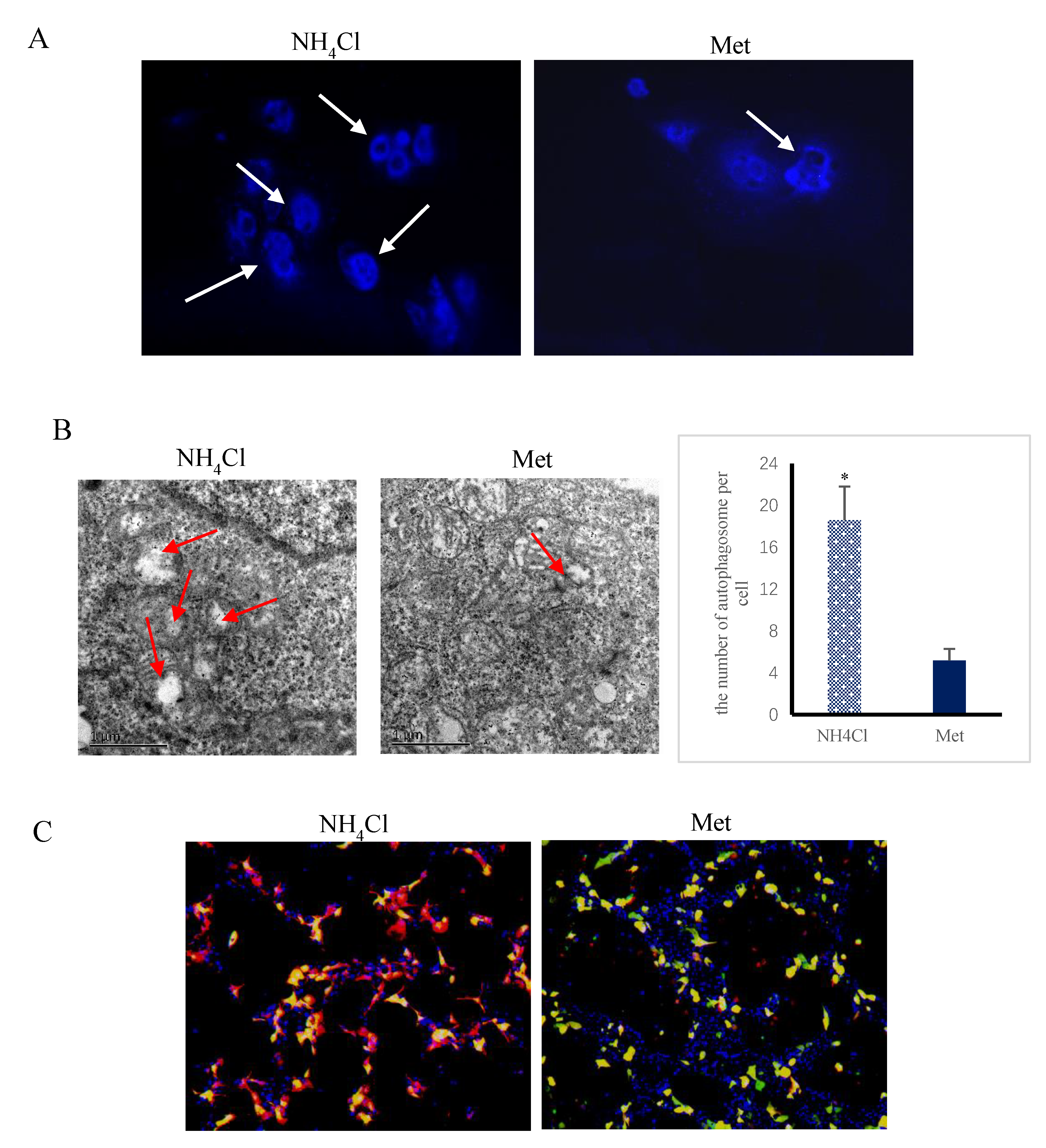
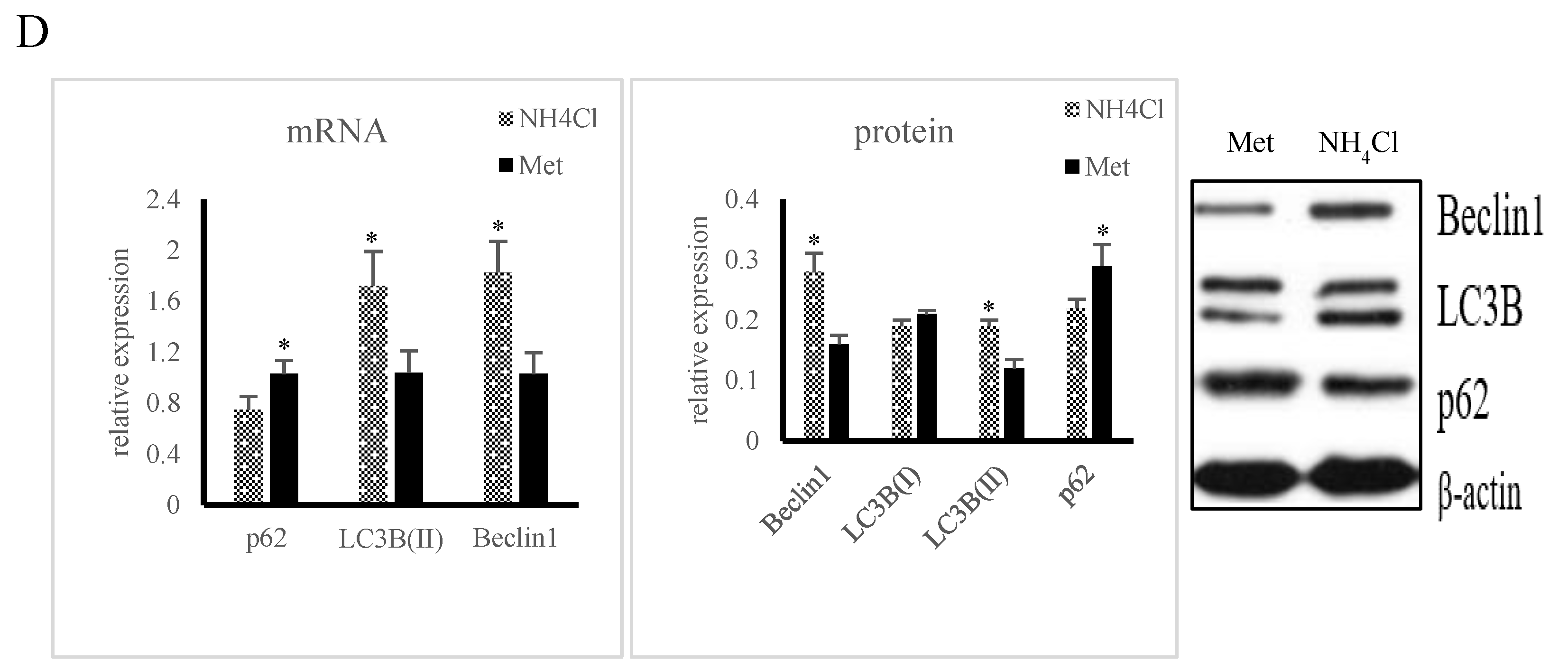
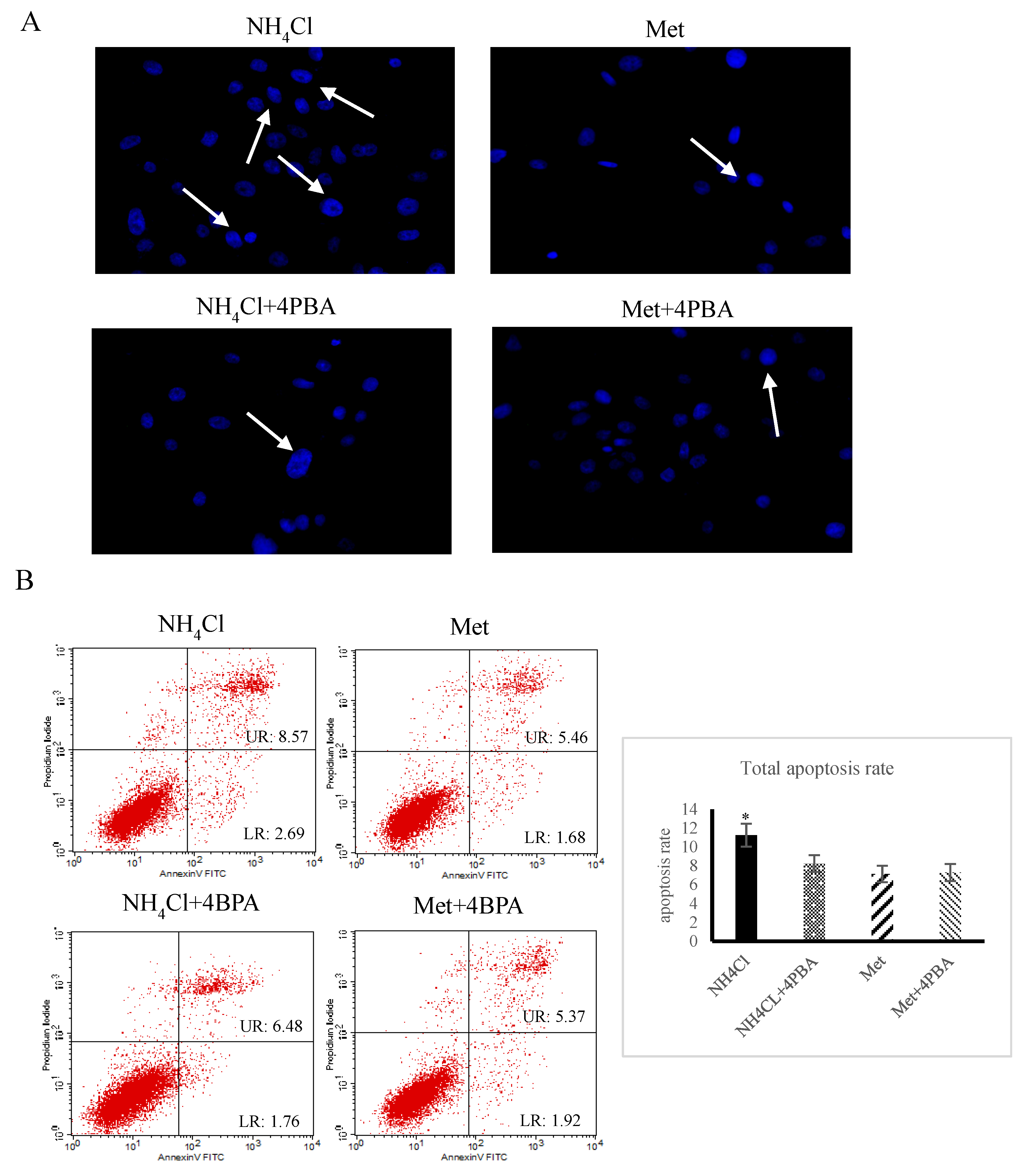
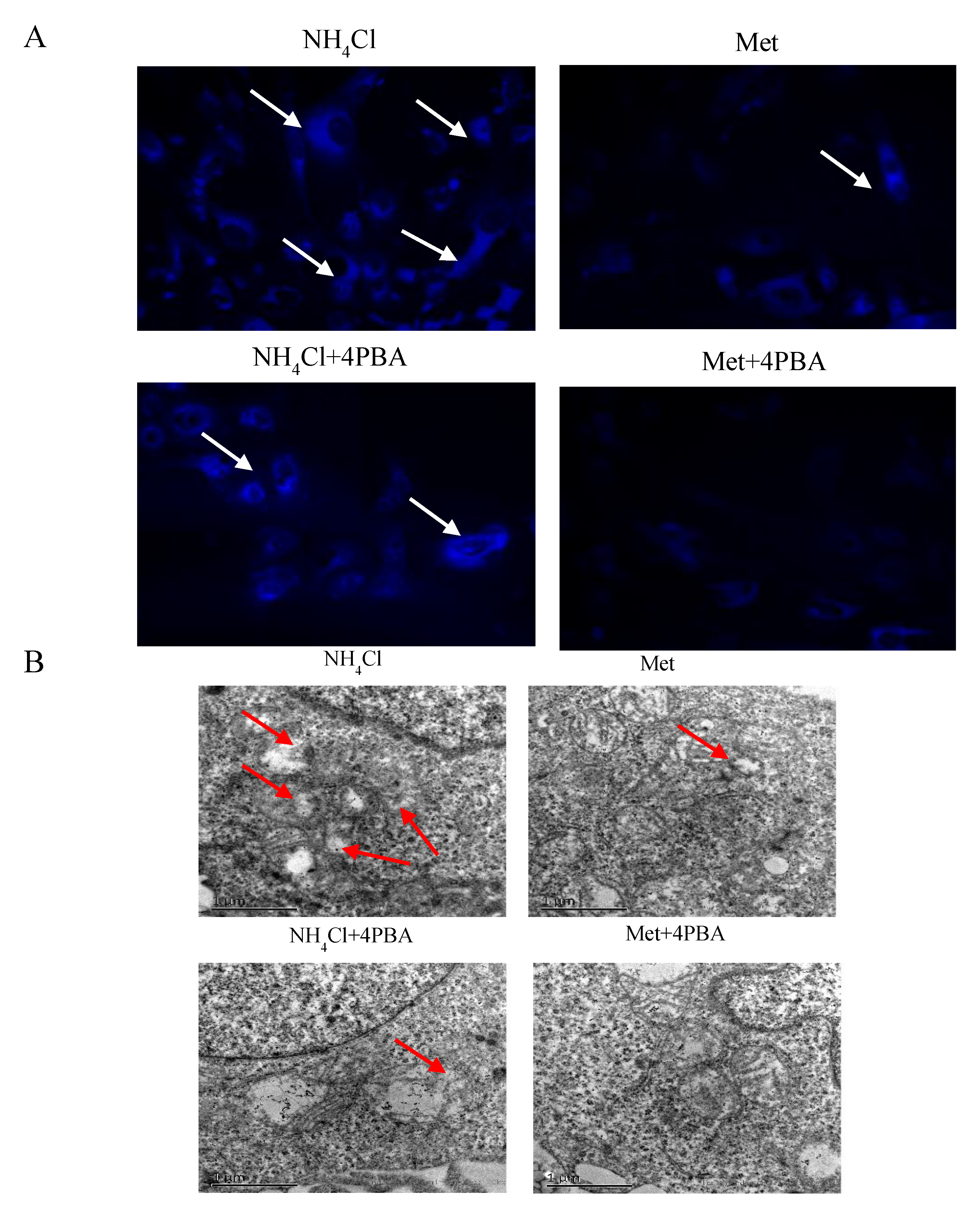
3.1. Apoptosis and Autophagy Induction by NH4Cl and Met in PRECs
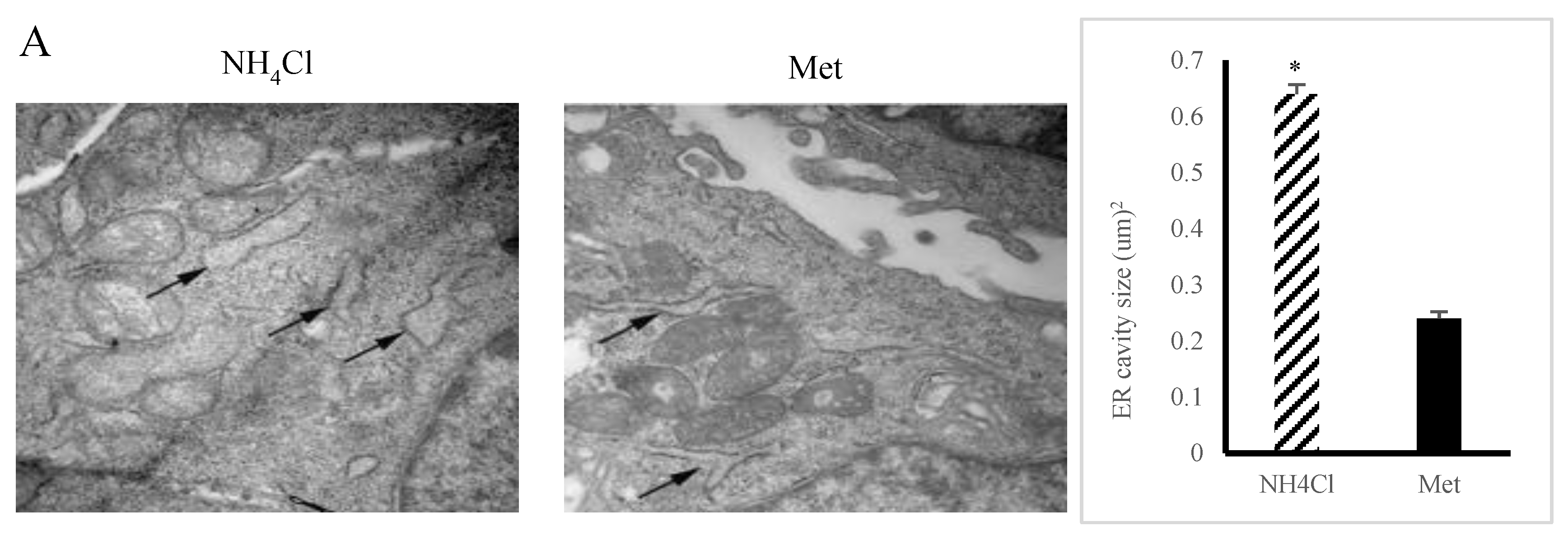
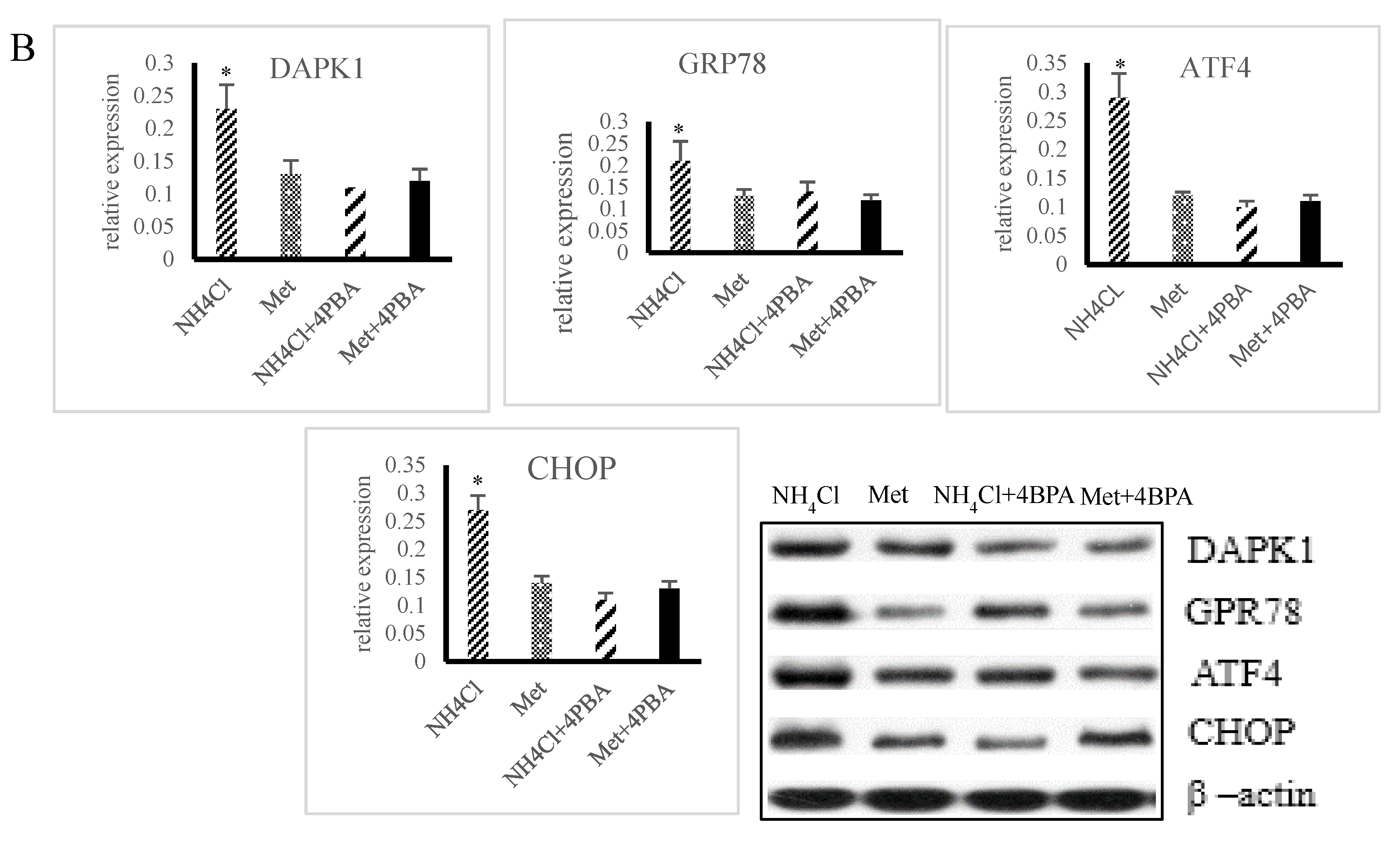
3.2. ER Stress Induction by NH4Cl and Met in PRECs
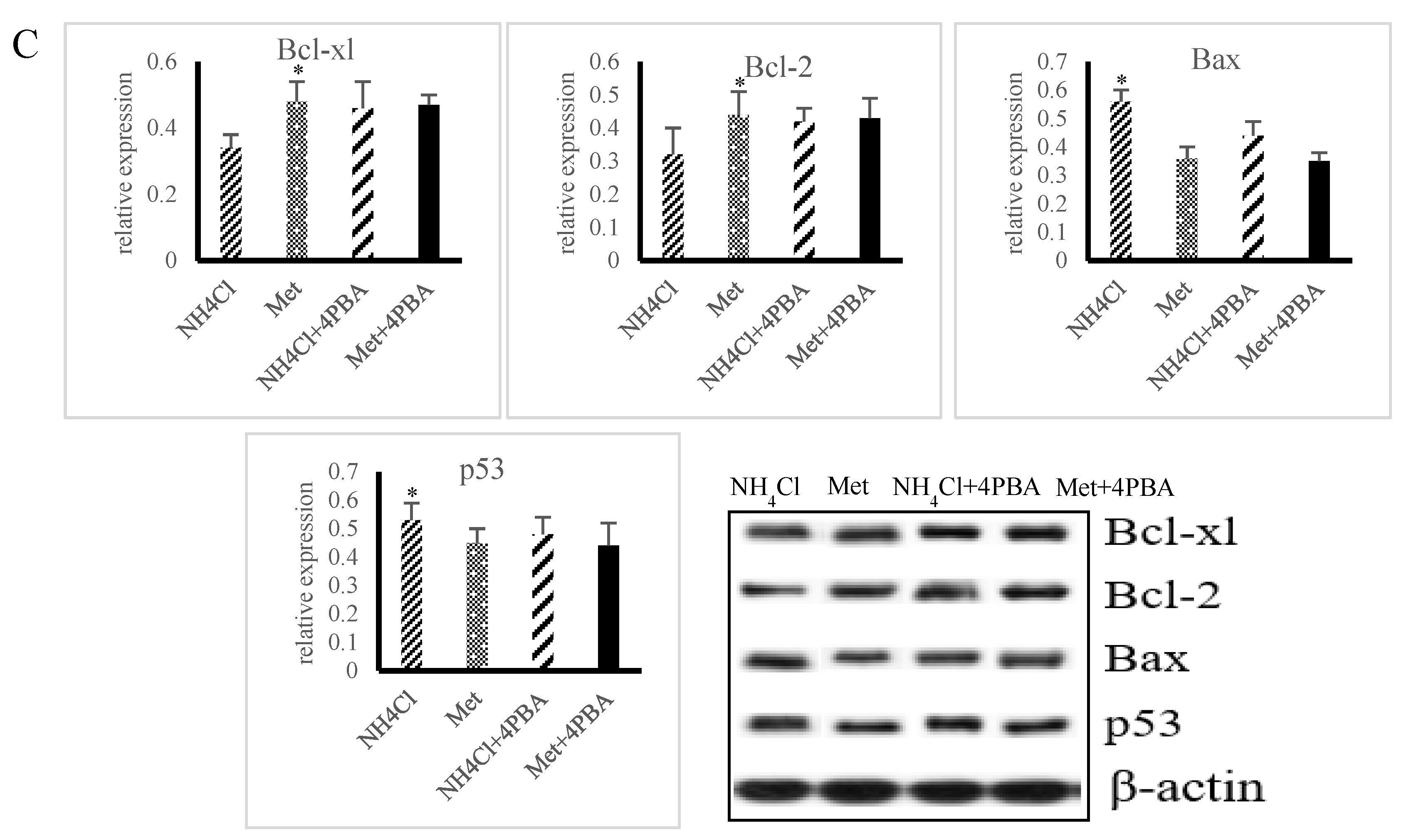
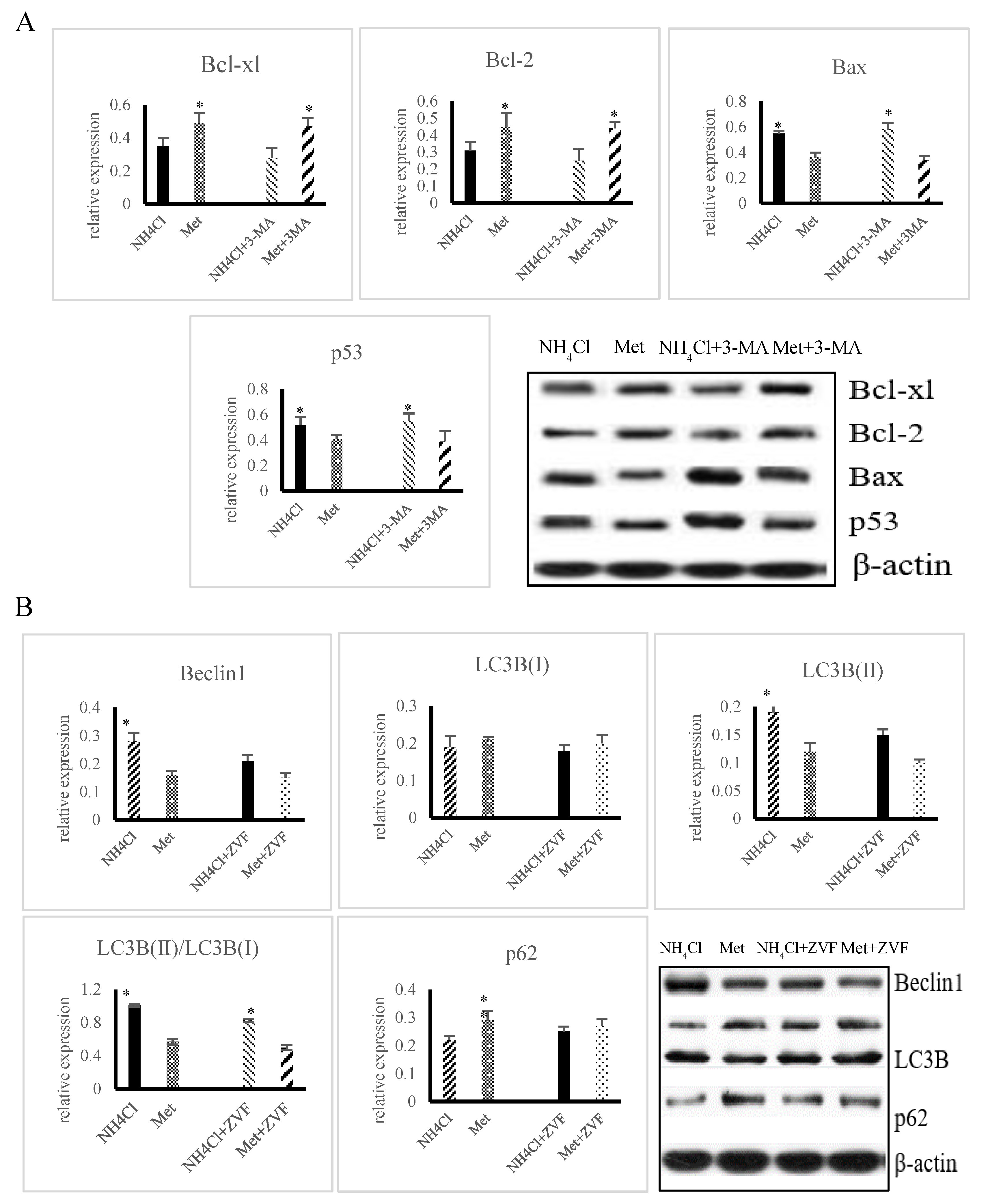
3.3. Effects of ER Stress on Apoptosis Induced by NH4Cl and Met in PRECs
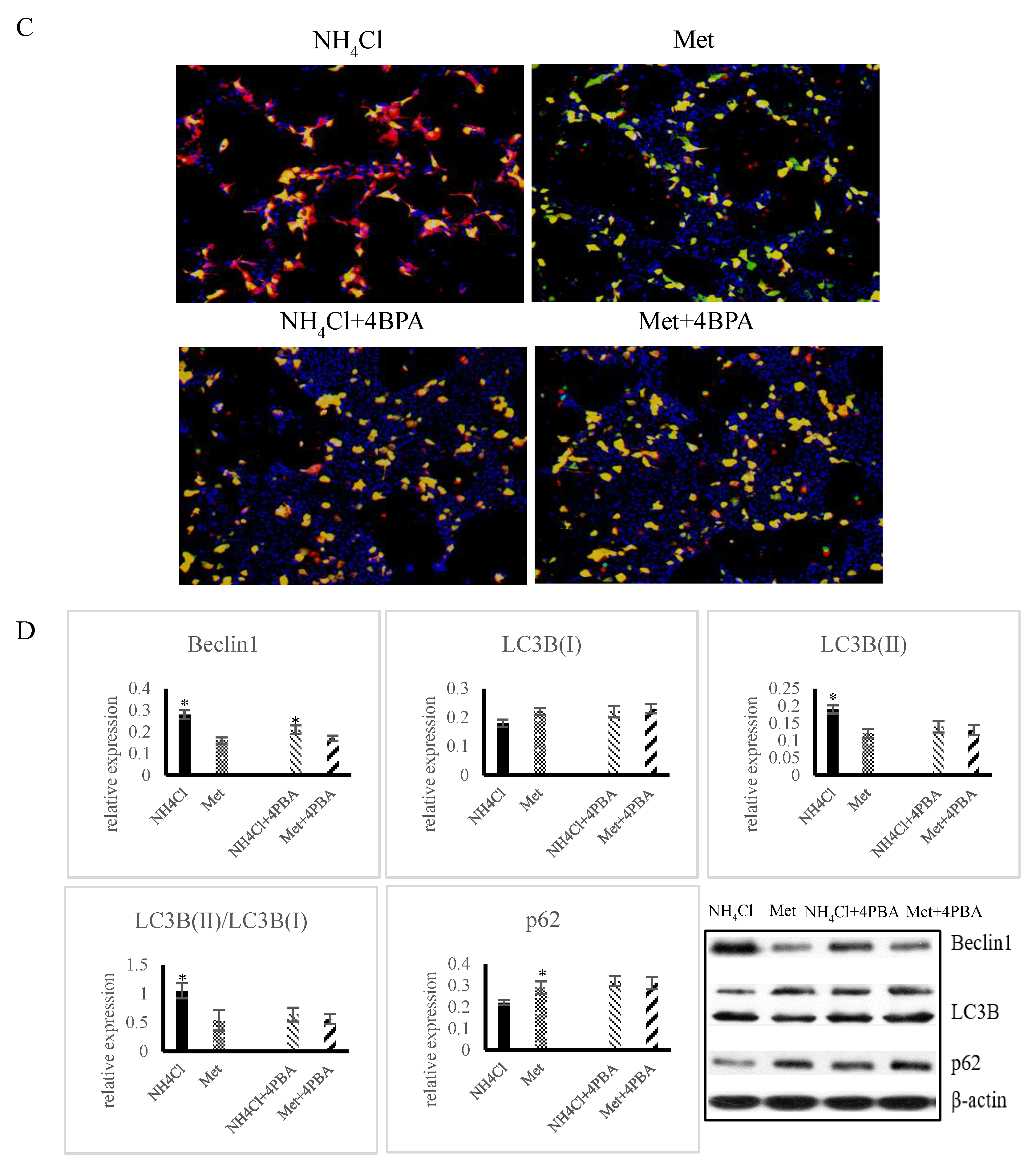
3.4. Effects of ER Stress on Autophagy Induced by NH4Cl and Met in PRECs
3.5. Crosstalk between Apoptosis and Autophagy Induced by NH4Cl and Met in PRECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qi, D.J.; Shi, W.; Black, A.R.; Kuss, M.A.; Pang, X.N.; He, Y.N.; Liu, B.; Duan, B. Repair and regeneration of small intestine: A review of current engineering approaches. Biomaterials 2020, 240, 119832. [Google Scholar] [CrossRef]
- Kennedy, P.M.; Milligan, L.P. Input of endogenous protein into the fore stomachs of sheep. Can. J. Anim. Sci. 1980, 60, 1029–1038. [Google Scholar] [CrossRef]
- Wang, K.L.; Chu, D.X.; Wu, J.; Zhao, M.L.; Zhang, M.M.; Li, B.J.; Du, W.J.; Du, J.M.; Guo, R.X. Cinobufagin induced cell apoptosis and protective autophagy through the ROS/MAPK signaling pathway. Life Sci. 2019, 116642. [Google Scholar] [CrossRef]
- Liu, Z.J.; Hu, Y.L.; Feng, Y.; Guo, Y.B.; Liu, Y.F.; Yang, J.L.; Mao, Q.S.; Xue, W.J. Rafoxanide promotes apoptosis and autophagy of gastric cancer cells by suppressing PI3K /Akt/mTOR pathway. Exp. Cell Res. 2019, 385, 111691. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, S.X.; Shi, J.; Gong, G.Y.; Yu, J.W.; Niu, Y.; Yang, C.M.; Ma, X.C.; Fang, M.Y. Polyphyllin I induces apoptosis and autophagy via modulating JNK and mTOR pathways in human acute myeloid leukemia cells. Chem. Biol. Interact. 2019, 311, 108793. [Google Scholar] [CrossRef]
- Huynh, T.Y.L.; Zareba, I.; Baszanowska, W.; Lewoniewska, S.; Palka, J. Understanding the role of key amino acids in regulation of proline dehydrogenase/proline oxidase (prodh/pox)-dependent apoptosis/autophagy as an approach to targeted cancer therapy. Mol. Cell Biochem. 2020, 466, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.Y.; Zhou, G.B.; Jin, Z.H.; Wang, G.L.; Li, H.X. Effects of rumen protected methionine on performance, apoptosis of lymphocytes and related genes in dairy cows under heat stress. Chin. J. Anim. Nutr. 2009, 21, 665–672. [Google Scholar]
- Tan, H.; Yang, K.; Li, Y.; Shaw, T.I.; Wang, Y.; Blanco, D.B.; Wang, X.; Cho, J.H.; Wang, H.; Rankin, S.; et al. Integrative Proteomics and Phosphoproteomics Profiling Reveals Dynamic Signaling Networks and Bioenergetics Pathways Underlying T Cell Activation. Immunity 2017, 246, 488–503. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.Z.; Ying, Y.; Liu, Y.; Sun, C.B.; Dai, C.; Zhao, S.; Tian, S.Z.; Peng, J.; Han, N.P.; Yuan, J.L.; et al. Antifibrotic action of Yifei Sanjie formula enhanced autophagy via PI3K-AKT-mTOR signaling pathway in mouse model of pulmonary fibrosis. Biomed. Pharmacother. 2019, 118, 109293. [Google Scholar] [CrossRef]
- Liu, J.; Tan, H.; Sun, Y.; Zhou, S.; Cao, J.; Wang, F. The preventive effects of heparin-superoxide dismutase on carbon tetrachlofide-induced acute liver failure and hepatic fibrosis in mice. Mol. Cell Biochem. 2009, 327, 219–228. [Google Scholar] [CrossRef]
- Hayashi, H.; Mizuno, T.; Horikawa, R.; Nagasaka, H.; Yabuki, T.; Takikawa, H.; Sugiyama, Y. 4-Phenylbutyrate modulates ubiquitination of hepatocanalicular MRP2 and reduces serum total bilirubin concentration. J. Hepatol. 2012, 56, 1136–1144. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Li, M.; Wang, R.X.; Qian, Y.X. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish. Immun. 2018, 79, 313–320. [Google Scholar] [CrossRef]
- Bhatia, V.; Singh, R.; Acharya, S.K. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut 2006, 55, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Kawai, A.; Uchiyama, H.; Takano, S.; Nakamura, N.; Ohkuma, S. Autophagosome lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy 2007, 3, 154–157. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, M.; Janjetovic, K.; Paunovic, V.; Ciric, D.; Kravic-Stevovic, T.; Trajkovic, V. Comparative analysis of cell death mechanisms induced by lysosomal autophagy inhibitors. Eur. J. Pharmacol. 2019, 859, 172540. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Yang, Y.F. β-Glucan from Saccharomyces cerevisiae induces SBD-1 production in ovine ruminal epithelial cells via the Dectin-1–Syk–NF-κB signaling pathway. Cell Signal 2019, 53, 304–315. [Google Scholar] [CrossRef]
- Ge, H.; Li, Z.G.; Jiang, L.P.; Li, Q.J.; Geng, C.Y.; Yao, X.F.; Shi, X.X.; Liu, Y.; Cao, J. Cr (VI) induces crosstalk between apoptosis and autophagy through endoplasmic reticulum stress in A549 cells. Chem. Biol. Interact. 2019, 298, 35–42. [Google Scholar] [CrossRef]
- Dixit, R.; Agrawal, L.; Singh, S.P.; Prateeksha; Singh, P.C.; Prasad, V.; Chauhan, P.S. Paenibacillus lentimorbus induces autophagy for protecting tomato from Sclerotium rolfsii infection. Microbiol. Res. 2018, 215, 164–174. [Google Scholar] [CrossRef]
- Ran, T.; Li, H.Z.; Liu, Y.; Zhou, C.S.; Tang, S.X.; Han, X.F.; Wang, M.; He, Z.X.; Kang, J.H.; Yan, Q.X.; et al. Cloning, phylogenetic analysis, and distribution of free fatty acid receptor GPR120 expression along the gastrointestinal tract of housing versus grazing kid goats. J. Agric. Food Chem. 2016, 64, 2333–2341. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yan, Q.X.; Tang, S.X.; Tan, Z.L.; Han, X.F.; Zhou, C.S.; Kang, J.H.; Wang, M. Proteomic Analysis of Isolated Plasma Membrane Fractions from the Mammary Gland in Lactating Cows. J. Agric. Food Chem. 2015, 63, 7388–7398. [Google Scholar] [CrossRef]
- Karaki, S.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef]
- Tesseraud, S.; Everaert, N.; Ezzine, S.B.; Collin, A.; Berri, S.M.C. Manipulating tissue metabolism by amino acids. World Poult. Sci. 2011, 67, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Li, G.W.; Burkhardt, D.; Gross, C.; Weissman, J.S. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 2014, 157, 624–635. [Google Scholar] [CrossRef] [Green Version]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Westphal, D.; Dewson, G.; Menard, M.; Frederick, P.; Iyer, S.; Bartolo, R.; Gibson, L.; Czabota, R.P.E.; Smith, B.J.; Adams, J.M.; et al. Apoptotic pore formation is associated with in-plane insertion of Bak or Bax central helices into the mitochondrial outer membrane. Proc. Natl. Acad. Sci. USA 2014, 111, 4076–4085. [Google Scholar] [CrossRef] [Green Version]
- Klee, M.; Pallauf, K.; Alcalá, S.; Fleischer, A.; Pimentel-Muiños, F.X. Mitochondrial apoptosis induced by bh3-only molecules in the exclusive presence of endoplasmic reticular bak. EMBO J. 2014, 28, 1757–1768. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Wang, K.; Wang, X.; Yin, F.; Li, C.; Wang, C.; Zhao, B.; Zhong, C.; Zhang, J.; et al. Methionine and cystine double deprivation stress suppresses glioma proliferation via inducing ROS/autophagy. Toxicol. Lett. 2015, 232, 349–355. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhu, H.T.; Chen, K.L.; Dong, X.; Wei, J.; Qiu, C.; Xue, J.H. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2013, 12, 158–173. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Li, Z.; Jiang, Y.; Si, J.; Chang, Q.; Liu, X.; Pan, R. Cajaninstilbene acid protects corticosterone-induced injury in PC12 cells by inhibiting oxidative and endoplasmic reticulum stress-mediated apoptosis. Neurochem. Int. 2014, 78, 43–52. [Google Scholar] [CrossRef]
- Guo, M.L.; Liao, K.; Periyasamy, P.; Yang, L.; Cai, Y.; Callen, S.E.; Buch, S. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 2015, 11, 995–1009. [Google Scholar] [CrossRef] [Green Version]
- Periyasamy, P.; Guo, M.L.; Buch, S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy 2016, 12, 1310–1329. [Google Scholar] [CrossRef] [Green Version]
- Murthy, C.R.; Rama Rao, K.V.; Bai, G.; Norenberg, M.D. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J. Neurosci. Res. 2001, 66, 282–288. [Google Scholar] [CrossRef]
- Boya, P.; Gonzalez-Polo, R.A.; Casares, N.; Perfettini, J.L.; Dessen, P.; Larochette, N.; Metivier, D.; Meley, D.; Souquere, S.; Yoshimori, T.; et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell Biol. 2005, 25, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xia, C.; Lv, Y.; Li, C.; Mei, Q.; Li, H.; Wang, H.; Li, S. Crosstalk influence between P38MAPK and autophagy on mitochondria-mediated apoptosis induced by anti-fas antibody/actinomycin D in human hepatoma bel-7402 cells. Molecules 2017, 22, 1705. [Google Scholar] [CrossRef] [Green Version]
| Gene Name | GeneBank No. | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|---|
| CTGAGGGATGGAAGGGTC | |||
| Beclin1 | XM_017025264 | 159 | |
| TGGGCTGTGGTAAGTAATG | |||
| CGGGTTGAGGAGACACACAA | |||
| LC3B | XM_011529083 | 141 | |
| ATGAG CCGGACATCTTCCAC | |||
| CCATGCAGGTGAGCTTCGT | |||
| p62 | XM_018051607 | 155 | |
| GAATCTGCGAGAGACACCATC | |||
| GCTACGACACGGAGTTCCA | |||
| Bcl-2 | XM_005696234 | 113 | |
| CCCAGTTGATGCCGCTCT | |||
| GAGCTGGTTGACTTTCTC | |||
| BCL-XL | XM_006498612 | ||
| TCCATCTCCGATTCAGTCCCT | 142 | ||
| TGAAGCGCATTGGAGATG | |||
| Bax | XM_013971446.2 | 185 | |
| GGCCTTGAGCACCAGTTT | |||
| GAATGTCCGAATGAAGCG | |||
| p53 | XM_005693530 | 121 | |
| CGTAGTTGCCAGGGTAGG | |||
| ATGGCTACTGCTGCGTCGT | |||
| β-actin | AF481159·1 | 161 | |
| TTGAAGGTGGTCTCGTGGAT |
| Name | No. | Host Species | Dilution | Supplier |
|---|---|---|---|---|
| Caspase-3 | 9665s | Rabbit | 1:1000 | CST |
| BCL-2 | ab59348 | Rabbit | 1:1000 | Abcam |
| BAX | 50599-2-AP | Rabbit | 1:2000 | Proteintech |
| P53 | 10442-1-AP | Rabbit | 1:1200 | Proteintech |
| BCL-XL | 10783-1-AP | Rabbit | 1:1200 | Proteintech |
| LC3 | ab192890 | Rabbit | 1:2000 | Abcam |
| Beclin1 | ab62557 | Rabbit | 1:1500 | Abcam |
| p62 | ab155686 | Rabbit | 1:2000 | Abcam |
| DAPK1 | 25136-1-AP | Rabbit | 1:1000 | Proteintech |
| GPR78 | ab198787 | Rabbit | 1:1000 | Abcam |
| ATF4 | ab184909 | Rabbit | 1:1000 | Abcam |
| CHOP | ab179823 | Rabbit | 1:2000 | Abcam |
| β-actin | ab179467 | Rabbit | 1:5000 | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Z.; Zhou, C.; Kang, J.; Tan, Z. Comparison of the Effects of Nonprotein and Protein Nitrogen on Apoptosis and Autophagy of Rumen Epithelial Cells in Goats. Animals 2020, 10, 2079. https://doi.org/10.3390/ani10112079
Kong Z, Zhou C, Kang J, Tan Z. Comparison of the Effects of Nonprotein and Protein Nitrogen on Apoptosis and Autophagy of Rumen Epithelial Cells in Goats. Animals. 2020; 10(11):2079. https://doi.org/10.3390/ani10112079
Chicago/Turabian StyleKong, Zhiwei, Chuanshe Zhou, Jinhe Kang, and Zhiliang Tan. 2020. "Comparison of the Effects of Nonprotein and Protein Nitrogen on Apoptosis and Autophagy of Rumen Epithelial Cells in Goats" Animals 10, no. 11: 2079. https://doi.org/10.3390/ani10112079
APA StyleKong, Z., Zhou, C., Kang, J., & Tan, Z. (2020). Comparison of the Effects of Nonprotein and Protein Nitrogen on Apoptosis and Autophagy of Rumen Epithelial Cells in Goats. Animals, 10(11), 2079. https://doi.org/10.3390/ani10112079





