Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Enzymes: aspartate aminotransferase (AST), alanine aminotransferase (ALP), γ-glutamyl transferase (gGT), lipase (Lip), alpha-amylase, lactate dehydrogenase (LDH), creatine kinase (CK), butyrylcholinesterase (BChE), total esterase (TEA), and adenosine deaminase (ADA).
- Metabolites: creatinine, urea, uric acid, total bilirubin, triglycerides, glucose, and lactate.
- Proteins: total proteins and albumin.
- Minerals: phosphorus and total calcium.
- Hormones: cortisol.
3. Results
3.1. Characteristics of the Cows of the Study
3.2. Differences in the Salivary Chemistry Profile before and after Lameness Treatment
3.3. Changes in the Salivary Analytes between Lameness and Healthy Cows
3.4. Correlations between Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffiths, B.E.; White, D.G.; Oikonomou, G. A cross-sectional study into the prevalence of dairy cattle lameness and associated herd-level risk factors in England and Wales. Front. Vet. Sci. 2018, 5, 65. [Google Scholar] [CrossRef]
- Solano, L.; Barkema, H.W.; Pajor, E.A.; Mason, S.; LeBlanc, S.J.; Zaffino Heyerhoff, J.C.; Nash, C.G.R.; Haley, D.B.; Vasseur, E.; Pellerin, D.; et al. Prevalence of lameness and associated risk factors in Canadian Holstein-Friesian cows housed in free stall barns. J. Dairy Sci. 2015, 98, 6978–6991. [Google Scholar] [CrossRef]
- Weaver, M.D.; Jean, G.S.; Steiner, A. Bovine Surgery and Lameness, 2nd ed.; Blackwell: Oxford, UK, 2005; pp. 198–258. [Google Scholar]
- Cha, E.; Hertl, J.A.; Bar, D.; Gröhn, Y.T. The cost of different types of lameness in dairy cows calculated by dynamic programming. Prev. Vet. Med. 2010, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tadich, N.; Tejeda, C.; Bastias, S.; Rosenfeld, C.; Green, L.E. Nociceptive threshold, blood constituents and physiological values in 213 cows with locomotion scores ranging from normal to severely lame. Vet. J. 2013, 197, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.J.; Waterman, A.E.; Livingston, A. Measurement of mechanical thresholds, plasma cortisol and catecholamines in control and lame cattle: A preliminary study. Res. Vet. Sci. 1996, 61, 172–173. [Google Scholar] [CrossRef]
- Bustamante, H.; Rodríguez, A.; Herzberg, D.; Werner, M. Stress and pain response after oligofructose induced-lameness in dairy heifers. J. Vet. Sci. 2012, 63, 159–166. [Google Scholar] [CrossRef]
- Marti, S.; Janzen, E.; Pajor, E.; Orsel, K.; Jelinski, M.; Dorin, C.; Thomson, D.; Coetzee, J.; Shearer, J.; Millman, S.; et al. Relationship between biomarkers of stress, inflammation and pain, and lameness in feedlot cattle. J. Anim. Sci. 2018, 96, 9. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Mau, M. Saliva proteomics as an emerging, non-invasive tool to study livestock physiology, nutrition and diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Giri, U.; Nagaraj, V.; Sankaran, A.; Ramesh, R.; Rajaram, S.; Muthanandam, S.; Arumugam, S.; Santhanam, V. Sialochemical profile in depressive individuals under antidepressant therapy: An observational study. J. Clin. Diagn. Res. 2018, 12, VC06–VC09. [Google Scholar] [CrossRef]
- Miller, B.E.; Deutsch, O.; Redlich, M.; Konttinen, Y.T.; Benoliel, R.; Zaks, B.; Davidovich, E.; Palmon, A.; Aframian, D.J. Sialochemistry and cortisol levels in patients with Sjogren’s syndrome. Oral Dis. 2012, 18, 255–259. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M..; Escribano, D.; Martínez-Subiela, S.; Martín-Cuervo, M.; Lamy, E.; Tecles, F.; Cerón, J.J. Changes in saliva analytes in equine acute abdominal disease: A sialochemistry approach. BMC Vet. Res. 2019, 15, 187. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Monkeviciene, I.; Ceron, J.J.; Silinskas, I.; Vallejo-Mateo, P.J.; Tecles, F.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Zelvyte, R. Biochemical changes in saliva of cows with inflammation: A pilot study. Res. Vet. Sci. 2019, 124, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Flower, F.C.; Weary, D.M. Effect of hoof pathologies on subjective assessments of dairy cow gait. J. Dairy Sci. 2006, 89, 139–146. [Google Scholar] [CrossRef]
- Escribano, D.; Fuentes-Rubio, M.; Ceron, J.J. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Investig. 2012, 24, 918–923. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder; Lang, A.; Buchner, A. G*Power: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Boston, Mass: Houghton Mifflin: London, UK, 2003; ISBN 0618124055. [Google Scholar]
- Van Os, J.M.C.; Weary, D.M.; Costa, J.H.C.; Hötzel, M.J.; von Keyserlingk, M.A.G. Sampling strategies for assessing lameness, injuries, and body condition score on dairy farms. J. Dairy Sci. 2019, 102, 8290–8304. [Google Scholar] [CrossRef]
- Brush, M.G. The effect of ACTH injections on plasma corticosteroid levels and milk yield in the cow. J. Endocrinol. 1960, 21, 155–160. [Google Scholar] [CrossRef]
- Van der Kolk, J.H.; Breukink, H.J.; Wensing, T.; Mol, J.A. Adrenocortical function testing in dairy cows and its effect on milk yield. Vet. Q. 1991, 13, 144–147. [Google Scholar] [CrossRef]
- Jurkovich, V.; Bakony, M.; Laky, E.; Ruff, F.; Kézér, F.L.; Bende, A.; Kovács, L. Cardiac vagal tone, plasma cortisol, and dehydroepiandrosterone response to an ACTH challenge in lame and nonlame dairy cows. Domest. Anim. Endocrinol. 2020, 71, 106388. [Google Scholar] [CrossRef]
- Tecles, F.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Escribano, D.; Cerón, J.J. Total esterase measurement in saliva of pigs: Validation of an automated assay, characterization and changes in stress and disease conditions. Res. Vet. Sci. 2017, 114, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Miró, S.; López-Arjona, M.; Rubio, C.P.; Martínez-Subiela, S.; Cerón, J.J.; Tecles, F. Application of a score for evaluation of pain, distress and discomfort in pigs with lameness and prolapses: Correlation with saliva biomarkers and severity of the disease. Res. Vet. Sci. 2019, 126, 155–163. [Google Scholar] [CrossRef]
- Lee, P.C.; Purcell, E.S.; Borysewicz, R.; Klein, R.M.; Werlin, S.L. Developmental delay of lingual lipase expression after guanethidine-induced sympathectomy. Proc. Soc. Exp. Biol. Med. 1992, 199, 192–198. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Escribano, D.; Quiles, A.; López-Arjona, M.; Cerón, J.; Martínez-Subiela, S.; Hevia, M.L.; Tecles, F. Evaluation of new biomarkers of stress in saliva of sheep. Animal 2019, 13, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha-Amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef]
- de Sousa-Pereira, P.; Cova, M.; Abrantes, J.; Ferreira, R.; Trindade, F.; Barros, A.; Gomes, P.; Colaço, B.; Amado, F.; Esteves, P.J.; et al. Cross-species comparison of mammalian saliva using an LC-MALDI based proteomic approach. Proteomics 2015, 15, 1598–1607. [Google Scholar] [CrossRef]
- Kivelä, J.; Parkkila, S.; Parkkila, A.K.; Leinonen, J.; Rajaniemi, H. Salivary carbonic anhydrase isoenzyme VI. J. Physiol. 1999, 520, 315–320. [Google Scholar] [CrossRef]
- Belge, F.; Bildik, A.; Belge, A.; Kiliçalp, D.; Atasoy, N. Possible association between chronic laminitis and some biochemical parameters in dairy cattle. Aust. Vet. J. 2004, 82, 556–557. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Escribano, D.; Martín-Cuervo, M.; Tecles, F.; Cerón, J.J. Salivary alpha-amylase activity and cortisol in horses with acute abdominal disease: A pilot study. BMC Vet. Res. 2018, 14, 156. [Google Scholar] [CrossRef]
- Herzberg, D.; Strobel, P.; Ramirez-Reveco, A.; Werner, M. Chronic inflammatory lameness increases Cytokine concentration in the spinal cord of dairy cows. Front. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef] [PubMed]
| Productive Parameters | Descriptive Statistics | L Group | H Group |
|---|---|---|---|
| Parity | Mean ± SD 1 | 4.1 ± 1.04 | 3.5 ± 0.80 |
| 95% CI 2 | 3.0–5.0 | 3.0–4.0 | |
| DIM 3 (days) | Mean ± SD | 115 ± 65.7 | 106 ± 51.5 |
| 95% CI | 18–207 | 73–135 | |
| BCS 4 (score) | Mean ± SD | 2.73–0.39 | 3.07 ± 0.31 |
| 95% CI | 2.50–3.00 | 2.75–3.50 | |
| Milk yield (kg/day) | Mean ± SD | 31.3 ± 11.91 | 40.8 ± 6.97 |
| 95% CI | 20.7–42.5 | 36.0–45.0 |
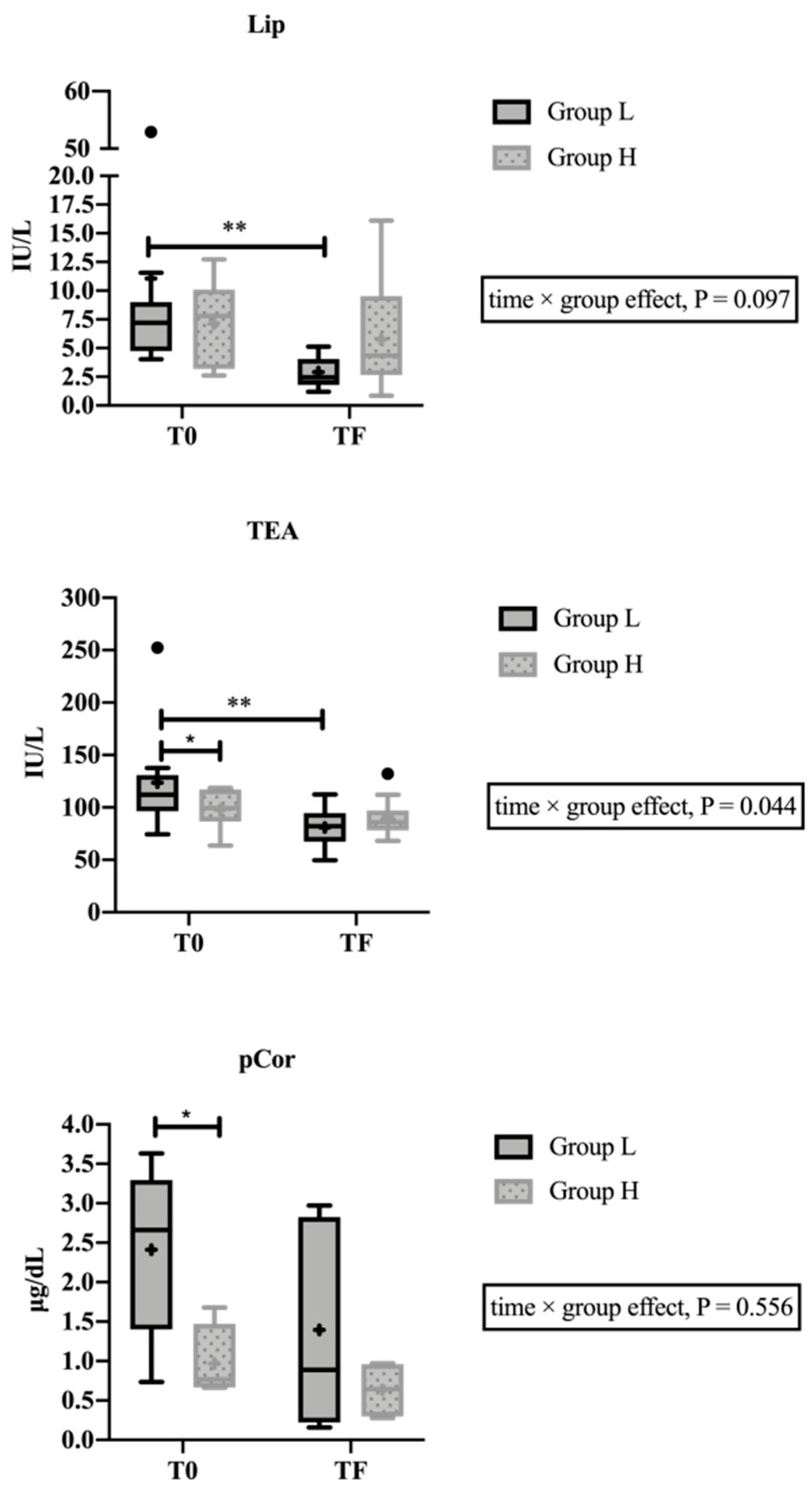
| Salivary Analytes | T0 | TF | p-Value |
|---|---|---|---|
| AST 1 (IU/L) | 9.5 [7.80–13.60] | 12.6 [6.18–16.83] | 0.172 |
| ALP 2 (IU/L) | 12.5 [7.93–27.80] | 13.4 [6.63–19.65] | 0.682 |
| gGT 3 (IU/L) | 41.2 (22.33) | 28.4 (11.72) | 0.532 |
| Lip 4 (IU/L) | 6.7 (1.56) | 2.6 (1.22) | <0.001 |
| sAA 5 (IU/L) | 5.3 [2.25–3.95] | 3.9 [3.25–7.95] | 0.984 |
| LDH 6 (IU/L) | 48.2 [40.05–69.35] | 36.5 [26.03–136.50] | 0.973 |
| CK 7 (IU/L) | 2.9 (0.89) | 4.1 (2.60) | 0.403 |
| BChE 8 (nmol/mL/min) | 12.7 (6.34) | 7.8 (3.68) | 0.154 |
| TEA 9 (IU/L) | 111.4 (18.20) | 74.0 (20.99) | 0.034 |
| ADA 10 (IU/L) | 7.6 (4.23) | 6.5 (4.66) | 0.600 |
| Creatinine (μmol/L) | 22.1 (6.80) | 25.6 (9.64) | 0.367 |
| Urea (mmol/L) | 2.4 [2.15–2.67] | 2.7 [1.86–3.29] | 0.817 |
| Uric acid (μmol/L) | 10.1 (5.17) | 7.7 (6.48) | 0.521 |
| Triglycerides (mmol/L) | 0.14 [0.097–0.223] | 0.08 [0.068–0.152] | 0.113 |
| Glucose (mmol/L) | 0.01 [0.007–0.012] | 0.02 [0.007–0.084] | 0.226 |
| Lactate (mmol/L) | 0.50 [0.295–0.763] | 0.25 [0.143–0.948] | 0.765 |
| Total protein (g/L) | 0.78 [0.504–1.541] | 0.82 [0.500–1.301] | 0.839 |
| Albumin (g/L) | 0.3 (0.08) | 0.3 (0.07) | 0.695 |
| Phosphorus (mmol/L) | 6.4 (2.60) | 6.5 (3.68) | 0.937 |
| Total calcium (mmol/L) | 0.18 [0.127–0.407] | 0.33 [0.249–0.893] | 0.076 |
| Cortisol (μg/dL) | 0.190 (0.148) | 0.172 (0.137) | 0.836 |
| Variable | Lip (IU/L) | TEA (IU/L) | PCor (µg/dL) |
|---|---|---|---|
| Lip 1 (IU/L) | 0.58 *** | −0.02 | |
| TEA 2 (IU/L) | 0.58 *** | 0.09 | |
| pCor 3 (µg/dL) | −0.02 | 0.09 | |
| NRS 4 (score) | 0.33 * | 0.43 ** | 0.53 * |
| LSS 5 (score) | 0.28 | 0.35 * | 0.48 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Želvytė, R.; Tecles, F.; Rubio, C.P. Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study. Animals 2020, 10, 2078. https://doi.org/10.3390/ani10112078
Contreras-Aguilar MD, Vallejo-Mateo PJ, Želvytė R, Tecles F, Rubio CP. Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study. Animals. 2020; 10(11):2078. https://doi.org/10.3390/ani10112078
Chicago/Turabian StyleContreras-Aguilar, María D., Pedro Javier Vallejo-Mateo, Rasa Želvytė, Fernando Tecles, and Camila Peres Rubio. 2020. "Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study" Animals 10, no. 11: 2078. https://doi.org/10.3390/ani10112078
APA StyleContreras-Aguilar, M. D., Vallejo-Mateo, P. J., Želvytė, R., Tecles, F., & Rubio, C. P. (2020). Changes in Saliva Analytes Associated with Lameness in Cows: A Pilot Study. Animals, 10(11), 2078. https://doi.org/10.3390/ani10112078






