Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Cheesemaking
2.3. Physicochemical Analysis of Feed and Cheese
2.4. Analysis of Polyphenols and Their Metabolites in Feed, Milk, and Cheese
2.5. LC-MS/MS Conditions
2.6. Evaluation of Oxidative Status in Cheeses
2.7. Determination of Antioxidant Capacity of Feed and Cheese
2.8. Colorimetric and Rheological Measurements in Cheese
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results
3.1. Milk and Cheese Characteristics and Fatty Acid Profile
3.2. Phenolic Compounds in Feed, Milk and Cheese
3.3. Oxidative Status and Antioxidant Stability in Cheese
3.4. Colorimetric and Rheological Measurements
3.5. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Council. 2017. Available online: www.internationaloliveoil.org (accessed on 17 July 2020).
- Branciari, R.; Ranucci, D.; Ortenzi, R.; Roila, R.; Trabalza-Marinucci, M.; Servili, M.; Papa, P.; Galarini, R.; Valiani, A. Dietary administration of olive millwastewater extract reduces campylobacter spp. prevalence in broiler chickens. Sustainability 2016, 8, 837. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative status and presence of bioactive compounds in meat from chickens fed polyphenols extracted from olive oil industry waste. Sustainability 2017, 1566. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Laudadio, V.; Casalino, E. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ. Sci. Pollut. Res. 2016, 23, 6197–6204. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Sebastiani, B.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Miraglia, D.; Urbani, S.; Esposto, S.; Servili, M. Effect of dietary treatment with olive oil by-product (olive cake) on physico-chemical, sensory and microbial characteristics of beef during storage. Ital. J. Food Saf. 2015, 4. [Google Scholar] [CrossRef]
- Chiofalo, V.; Liotta, L.; Lo Presti, V.; Gresta, F.; Rita, A.; Rosa, D.; Chiofalo, B. Performance, Carcass Characteristics, and Meat Quality of Beef Cattle. Animals 2020, 10, 1176. [Google Scholar] [CrossRef]
- Liotta, L.; Chiofalo, V.; Lo Presti, V.; Chiofalo, B. In Vivo Performances, Carcass Traits, and Meat Quality of Pigs Fed Olive Cake Processing Waste. Animals 2019, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Bartocci, S.; Servili, M.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Meo Zilio, D.; Terramoccia, S. Effect on quanti-quality milk and mozzarella cheese characteristics with further increasing the level of dried stoned olive pomace in diet for lactating buffalo. Asian-Australas. J. Anim. Sci. 2017, 30, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of supplementation of herd diet with olive cake on the composition profile of milk and on the composition, quality and sensory profile of cheeses made therefrom. Animals 2020, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, I.; Branciari, R.; Ortenzi, R.; Ciriaci, M.; Checcarelli, S.; Roila, R.; Capotorti, A.; Spaccini, G.; Valiani, A. Evaluation of the concentration factor of aflatoxin M1 in a semi-hard Pecorino cheese obtained from naturally contaminated milk. Food Control 2018, 85. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2000; ISBN 978-093558467-7. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Branciari, R.; Mughetti, L.; Ranucci, D.; Miraglia, D.; Valiani, A.; Acuti, G.; Selvaggini, R.; Trabalza-Marinucci, M. Influence of manufacturing procedure on the compositional and sensory properties of n-3 fatty acid-enriched pecorino cheese. J. Dairy Res. 2014, 81. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Urbani, E.; Valiani, A.; Trabalza-Marinucci, M.; Dal Bosco, A.; Franceschini, R. Freshwater Fish Burgers Made from Four Di_erent Fish Species as a Valuable Strategy Appreciated by Consumers for Introducing EPA and DHA into a Human Diet. J. Aquat. Food Prod. Technol. 2017, 26, 686–694. [Google Scholar] [CrossRef]
- de la Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Fitó, M.; Covas, M.I.; Muñoz-Aguayo, D.; López-Sabater, M.C. Presence of virgin olive oil phenolic metabolites in human low density lipoprotein fraction: Determination by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2007, 583, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Ranucci, D.; Trabalza-Marinucci, M.; Codini, M.; Orru, M.; Ortenzi, R.; Forte, C.; Ceccarini, M.R.; Valiani, A. Evaluation of the antioxidant properties and oxidative stability of Pecorino cheese made from the raw milk of ewes fed Rosmarinus officinalis L. leaves. Int. J. Food Sci. Technol. 2015, 50. [Google Scholar] [CrossRef]
- Blasi, F.; Urbani, E.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016, 89, 202–207. [Google Scholar] [CrossRef]
- Commission International de l’Eclairage. Colourimetry: Official Recommendations of the International Commission on Illumination; Publication CIE No. 15 (E-1.3.1); Bureau Central de la Commission Internationale del’Eclairage: Paris, France, 1976. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Cheese: Structure, rheology and texture. In Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; pp. 475–532. [Google Scholar]
- ISO. ISO 4120:2004 Sensory Analysis-Methodology-Triangle Test; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. JMP® 9 Basic Analysis and Graphing; SAS Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Martuscelli, M.; Gardini, F.; Torriani, F.; Mastrocola, D.; Serio, A.; Chavez-Lopez, C.; Schirone, M.; Suzzi, G. Production of biogenic amines during the ripening of Pecorino Abruzzese cheese. Int. Dairy J. 2005, 15, 571–578. [Google Scholar] [CrossRef]
- Abbeddou, S.; Rischkowsky, B.; Hilali, M.E.D.; Hess, H.D.; Kreuzer, M. Influence of feeding Mediterranean food industry by-products and forages to Awassi sheep on physicochemical properties of milk, yoghurt and cheese. J. Dairy Res. 2011, 78, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Innosa, D.; Bennato, F.; Ianni, A.; Martino, C.; Grotta, L.; Pomilio, F.; Martino, G. Influence of olive leaves feeding on chemical-nutritional quality of goat ricotta cheese. Eur. Food Res. Technol. 2020, 246, 923–930. [Google Scholar] [CrossRef]
- Salvatore, E.; Pes, M.; Falchi, G.; Pagnozzi, D.; Furesi, S.; Fiori, M.; Roggio, T.; Addis, M.F.; Pirisi, A. Effect of whey concentration on protein recovery in fresh ovine ricotta cheese. J. Dairy Sci. 2014, 97, 4686–4694. [Google Scholar] [CrossRef]
- Jayanegara, A.; Kreuzer, M.; Wina, E.; Leiber, F. Significance of phenolic compounds in tropical forages for the ruminal bypass of polyunsaturated fatty acids and the appearance of biohydrogenation intermediates as examined in vitro. Anim. Prod. Sci. 2011, 51, 1127–1136. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Pallara, G.; Buccioni, A.; Pastorelli, R.; Minieri, S.; Mele, M.; Rapaccini, S.; Messini, A.; Pauselli, M.; Servili, M.; Giovannetti, L.; et al. Effect of stoned olive pomace on rumen microbial communities and polyunsaturated fatty acid biohydrogenation: An in vitro study. BMC Vet. Res. 2014, 10, 1–15. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.P.; Motilva, M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Rubió, L.; Macià, A.; Castell-Auví, A.; Pinent, M.; Blay, M.T.; Ardévol, A.; Romero, M.P.; Motilva, M.J. Effect of the co-occurring olive oil and thyme extracts on the phenolic bioaccesibility and bioavailability assessed by in vitro digestion and cell models. Food Chem. 2014, 149, 277–284. [Google Scholar] [CrossRef]
- Corona, G.; Spencer, J.; Dessì, M. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mañá, C.; Farré, M.; Pujadas, M.; Mustata, C.; Menoyo, E.; Pastor, A.; Langohr, K.; De La Torre, R. Ethanol induces hydroxytyrosol formation in humans. Pharmacol. Res. 2015, 95–96, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef]
- Pastor, A.; Rodríguez-Morató, J.; Olesti, E.; Pujadas, M.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Covas, M.I.; Solá, R.; Motilva, M.J.; et al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubió, L.; Hernaéz, Á.; Tormos, C.; Motilva, M.J.; Fitó, M.; et al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef]
- Luciano, G.; Pauselli, M.; Servili, M.; Mourvaki, E.; Serra, A.; Monahan, F.J.; Lanza, M.; Priolo, A.; Zinnai, A.; Mele, M. Dietary olive cake reduces the oxidation of lipids, including cholesterol, in lamb meat enriched in polyunsaturated fatty acids. Meat Sci. 2013, 93, 703–714. [Google Scholar] [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Mannelli, F.; Cappucci, A.; Pini, F.; Pastorelli, R.; Decorosi, F.; Giovannetti, L.; Mele, M.; Minieri, S.; Conte, G.; Pauselli, M.; et al. Effect of different types of olive oil pomace dietary supplementation on the rumen microbial community profile in Comisana ewes. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit Rev Food Sci Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, M.; Mohamed, S.H.S.; Seleet, F.L. Production and evaluation of soft cheese fortified with ginger extract as a functional dairy food. Polish J. Food Nutr. Sci. 2012, 62, 77–83. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef] [PubMed]
| Item | Concentrates | |
|---|---|---|
| C | SDP | |
| Ingredients | ||
| Maize flour | 24.58 | 23.58 |
| Wheat bran | 22.50 | 21.50 |
| Wheat flour middlings | 13.50 | 13.00 |
| Soybean meal | 13.50 | 13.50 |
| Barley flour | 9.00 | 9.00 |
| Laminated linseed | 9.00 | 9.00 |
| Soybean oil | 4.50 | 4.50 |
| Calcium carbonate | 1.44 | 1.44 |
| Magnesium oxide | 0.36 | 0.36 |
| Sodium bicarbonate | 0.90 | 0.90 |
| Sodium chloride | 0.45 | 0.45 |
| Vitamin–mineral supplement a | 0.27 | 0.27 |
| Spray-dried olive mill wastewater phenolics | 0.00 | 2.50 |
| Chemical composition | ||
| Dry matter | 88.40 | 88.53 |
| Crude protein | 15.93 | 15.48 |
| Crude fat | 10.21 | 10.49 |
| Ash | 6.30 | 6.97 |
| NDF | 21.56 | 21.57 |
| ADF | 5.74 | 5.66 |
| ADL | 1.55 | 1.42 |
| Ca | 1.30 | 1.45 |
| P | 0.81 | 0.77 |
| Fatty acids | ||
| C14:0 | 0.16 | 0.10 |
| C16:0 | 14.57 | 13.74 |
| C18:0 | 5.68 | 4.88 |
| C18:1n9c | 22.01 | 22.48 |
| C18:2n6c | 42.63 | 43.34 |
| C18:3n3 | 13.79 | 14.53 |
| C20:0 | 0.42 | 0.34 |
| C20:1n9 | 0.34 | 0.29 |
| C22:0 | 0.40 | 0.32 |
| Polyphenols | ||
| Hydroxytyrosol (HT) | 0.07 | 272 |
| Tyrosol (T) | 0.32 | 37.1 |
| Verbascoside | 0.04 | 243 |
| Pinoresinol | 0.14 | 0.29 |
| Cheese Composition | Dietary Treatment | SEM | p-Value | |
|---|---|---|---|---|
| C | SDP | |||
| Moisture (g/100 g) * | 42.58 | 42.28 | 0.18 | 0.574 |
| Fat (g/100 g) | 26.21 | 26.10 | 0.26 | 0.876 |
| Protein (g/100 g) | 26.73 | 27.10 | 0.31 | 0.672 |
| Ash (g/100 g) | 4.47 | 4.52 | 0.05 | 0.739 |
| NaCl (g/100 g) | 2.16 | 2.22 | 0.08 | 0.815 |
| pH | 5.28 | 5.31 | 0.01 | 0.325 |
| Fatty Acids | Dietary Treatment | SEM | p-Value | |
|---|---|---|---|---|
| C | SDP | |||
| C4:0 | 4.15 | 3.92 | 0.04 | 0.079 |
| C6:0 | 2.10 | 1.98 | 0.08 | 0.602 |
| C8:0 | 1.59 | 1.49 | 0.05 | 0.475 |
| C10:0 | 4.50 | 4.06 | 0.10 | 0.162 |
| C12:0 | 2.66 | 2.46 | 0.06 | 0.284 |
| C14:0 | 9.04 | 8.93 | 0.11 | 0.725 |
| C15:0 | 0.94 b | 0.85 a | 0.01 | 0.002 |
| C16:0 | 24.76 | 24.89 | 0.16 | 0.785 |
| C17:0 | 0.56 b | 0.53 a | 0.00 | 0.001 |
| C18:0 | 9.06 | 8.81 | 0.06 | 0.204 |
| Total SFA | 59.35 | 57.92 | 0.25 | 0.070 |
| C14:1 | 0.17 | 0.17 | 0.00 | 0.567 |
| C16:1 | 0.82 a | 0.86 b | 0.00 | 0.024 |
| C18:1n9t | 1.48 | 1.55 | 0.12 | 0.835 |
| C18:1n7t | 6.54 | 7.21 | 0.13 | 0.092 |
| C18:1n9c | 20.75 | 20.96 | 0.16 | 0.641 |
| C18:1n7c | 1.07 | 1.03 | 0.01 | 0.104 |
| Total MUFA | 30.84 | 31.79 | 0.16 | 0.180 |
| C18:2n6t | 1.45 | 1.35 | 0.03 | 0.358 |
| C18:2n6c | 4.39 | 4.68 | 0.04 | 0.052 |
| C18:3n3 | 1.19 | 1.28 | 0.02 | 0.190 |
| C18:2 9c11t | 2.56 | 2.77 | 0.10 | 0.458 |
| C20:4n6 | 0.07 | 0.07 | 0.00 | 0.647 |
| C20:5n3 | 0.04 | 0.05 | 0.00 | 0.203 |
| C22:5n3 | 0.07 | 0.08 | 0.00 | 0.286 |
| C22:6n3 | 0.03 a | 0.04 b | 0.00 | 0.017 |
| Total PUFA | 9.81 | 10.29 | 0.08 | 0.060 |
| Phenolic Compounds | Dietary Treatment | SEM | p-Value | |
|---|---|---|---|---|
| C (µg/kg dw a) | SDP (µg/kg dw) | |||
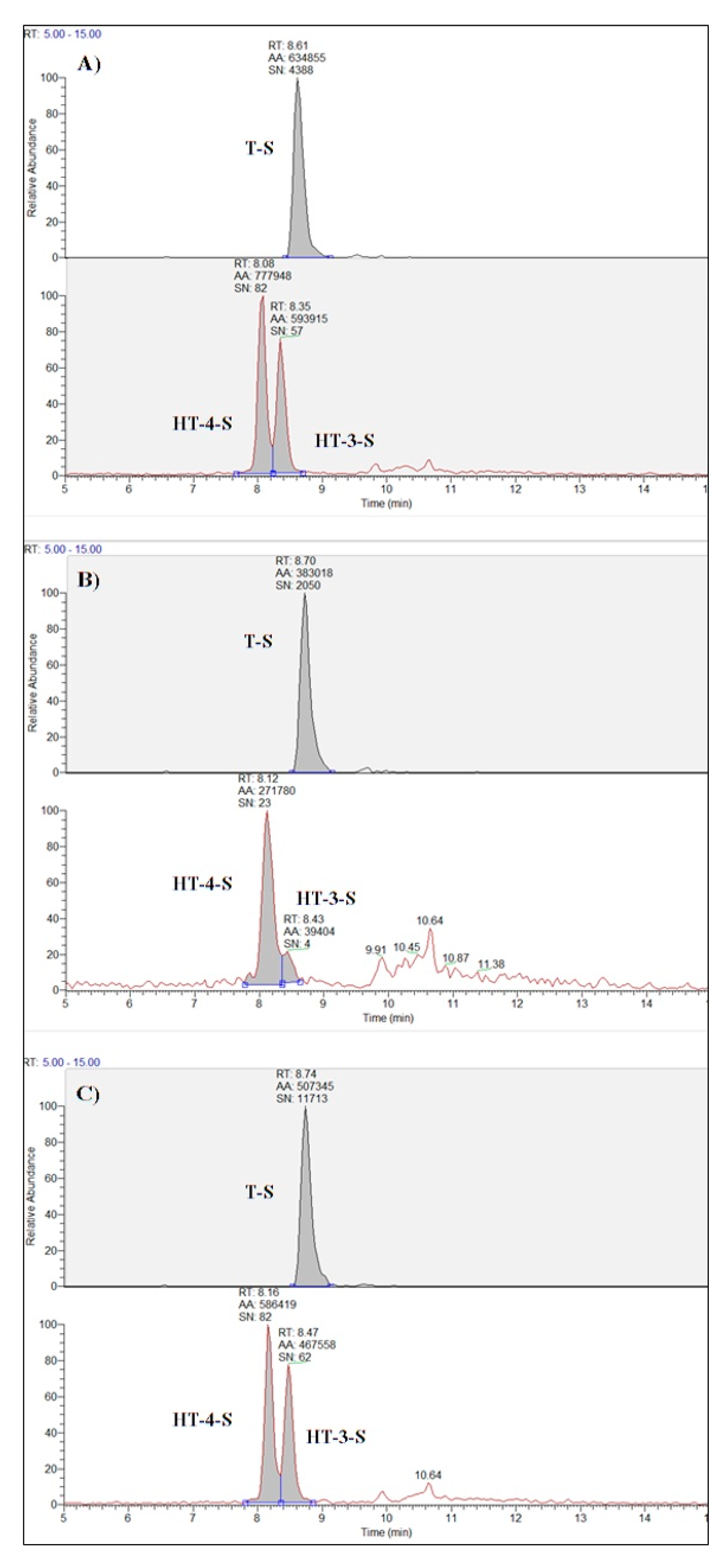
| Hydroxytyrosol-4-O-sulphate (HT-4-S) | 22 a | 773 b | 7.30 | <0.001 |
| Hydroxytyrosol-3-O-sulphate (HT-3-S) | 14 a | 549 b | 9.19 | <0.001 |
| Tyrosol sulphate (T-S) | 70 a | 176 b | 6.71 | 0.002 |
| Hydroxytyrosol (HT) | 29 | 33 | 4.12 | 0.772 |
| Tyrosol (T) | 613 | 808 | 46.42 | 0.232 |
| Dietary Treatment | SEM | p-Value | ||
|---|---|---|---|---|
| C | SDP | |||
| DPPH (µmol TE/100 g) | ||||
| Concentrates | 127.41 a | 245.73 b | 0.48 | <0.001 |
| Cheese | 405.02 a | 430.50 b | 1.59 | <0.001 |
| ABTS (µmol TE/100 g) | ||||
| Concentrates | 179.12 a | 533.47 b | 3.26 | <0.001 |
| Cheese | 22.39 a | 49.64 b | 1.29 | <0.001 |
| TBARS (mg MDA/kg) | ||||
| Cheese | 0.16 b | 0.10 a | 0.01 | <0.001 |
| Item | Dietary Treatment | SEM | p-Value | |
|---|---|---|---|---|
| C | SDP | |||
| L* | 78.59 | 77.83 | 0.75 | 0.323 |
| a* | −3.93 | −3.80 | 0.08 | 0.235 |
| b* | 15.71 | 15.22 | 0.25 | 0.227 |
| Attribute | C | SDP | SEM | p-Value |
|---|---|---|---|---|
| Hardness (g) | 4118.23 | 3746.54 | 162.39 | 0.139 |
| Springiness | 0.76 | 0.76 | 0.00 | 0.299 |
| Cohesiveness | 0.70 | 0.67 | 0.01 | 0.089 |
| Adhesiveness (J) | 48.87 | 42.57 | 5.42 | 0.501 |
| Gumminess (g) | 2873.00 | 2524.85 | 125.70 | 0.084 |
| Chewiness (g) | 2179.46 | 1927.77 | 99.52 | 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. https://doi.org/10.3390/ani10111941
Branciari R, Galarini R, Miraglia D, Ranucci D, Valiani A, Giusepponi D, Servili M, Acuti G, Pauselli M, Trabalza-Marinucci M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals. 2020; 10(11):1941. https://doi.org/10.3390/ani10111941
Chicago/Turabian StyleBranciari, Raffaella, Roberta Galarini, Dino Miraglia, David Ranucci, Andrea Valiani, Danilo Giusepponi, Maurizio Servili, Gabriele Acuti, Mariano Pauselli, and Massimo Trabalza-Marinucci. 2020. "Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules" Animals 10, no. 11: 1941. https://doi.org/10.3390/ani10111941
APA StyleBranciari, R., Galarini, R., Miraglia, D., Ranucci, D., Valiani, A., Giusepponi, D., Servili, M., Acuti, G., Pauselli, M., & Trabalza-Marinucci, M. (2020). Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals, 10(11), 1941. https://doi.org/10.3390/ani10111941







