Influence of Particle Size and Xylanase in Corn-Soybean Pelleted Diets on Performance, Nutrient Utilization, Microbiota and Short-Chain Fatty Acid Production in Young Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds and Housing
2.3. Experimental Diets
2.4. Experimental Procedures
2.5. Samples Analyses
2.6. Pellet Quality
2.7. Dry Matter Solubility and Water Retention Capacity
2.8. Short-Chain Fatty Acids
2.9. Microbial Diversity Analysis
2.10. Statistical Analysis
3. Results
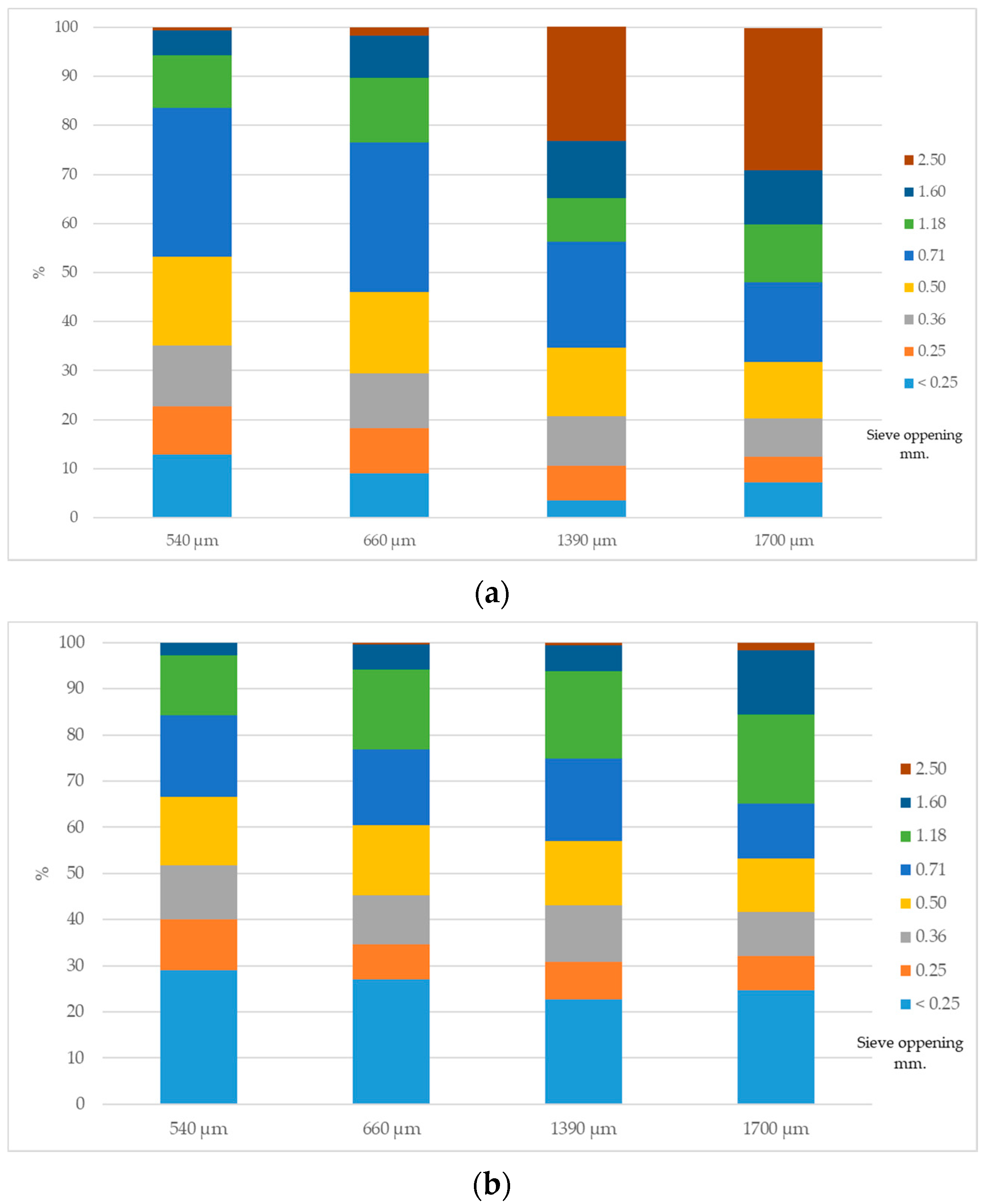
3.1. Feed Analyses and Diet Particle Size Analysis
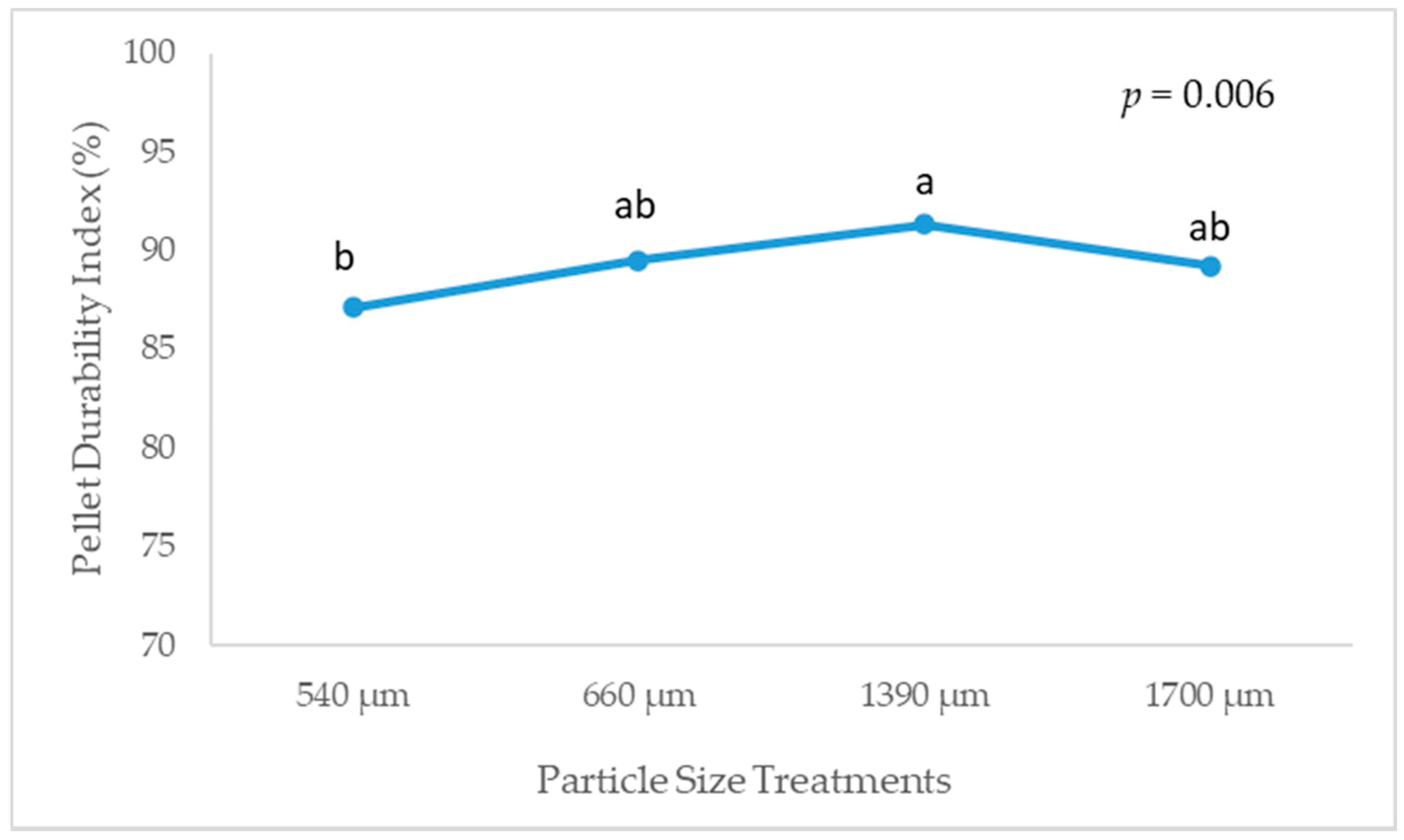
3.2. Pellet Quality, Solubilization of Dry Matter and Water Retention Capacity
3.3. Growth Performance
3.4. Apparent Ileal and Total Tract Digestibility
3.5. Relative Organ Weights
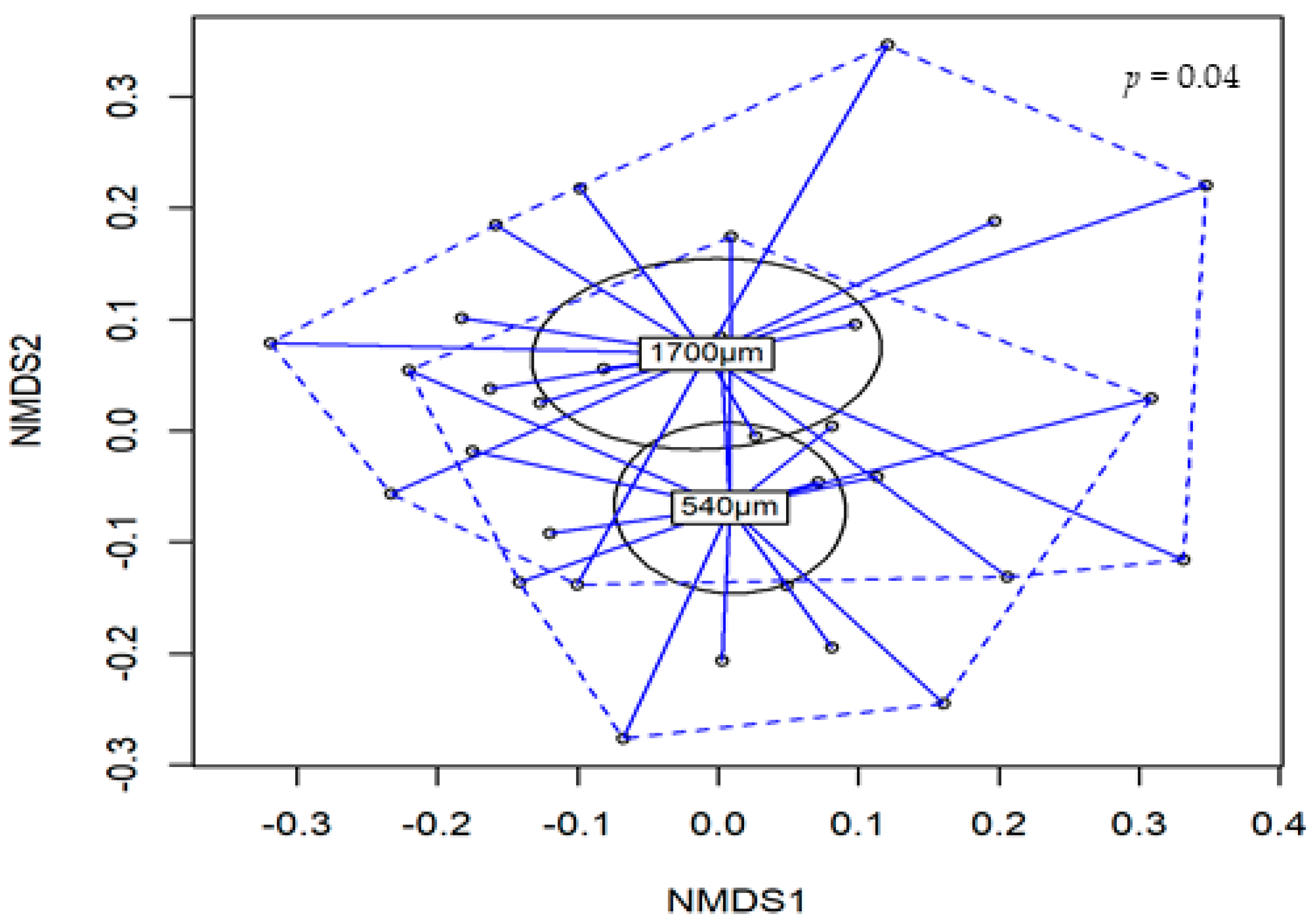
3.6. Microbial Diversity Analysis
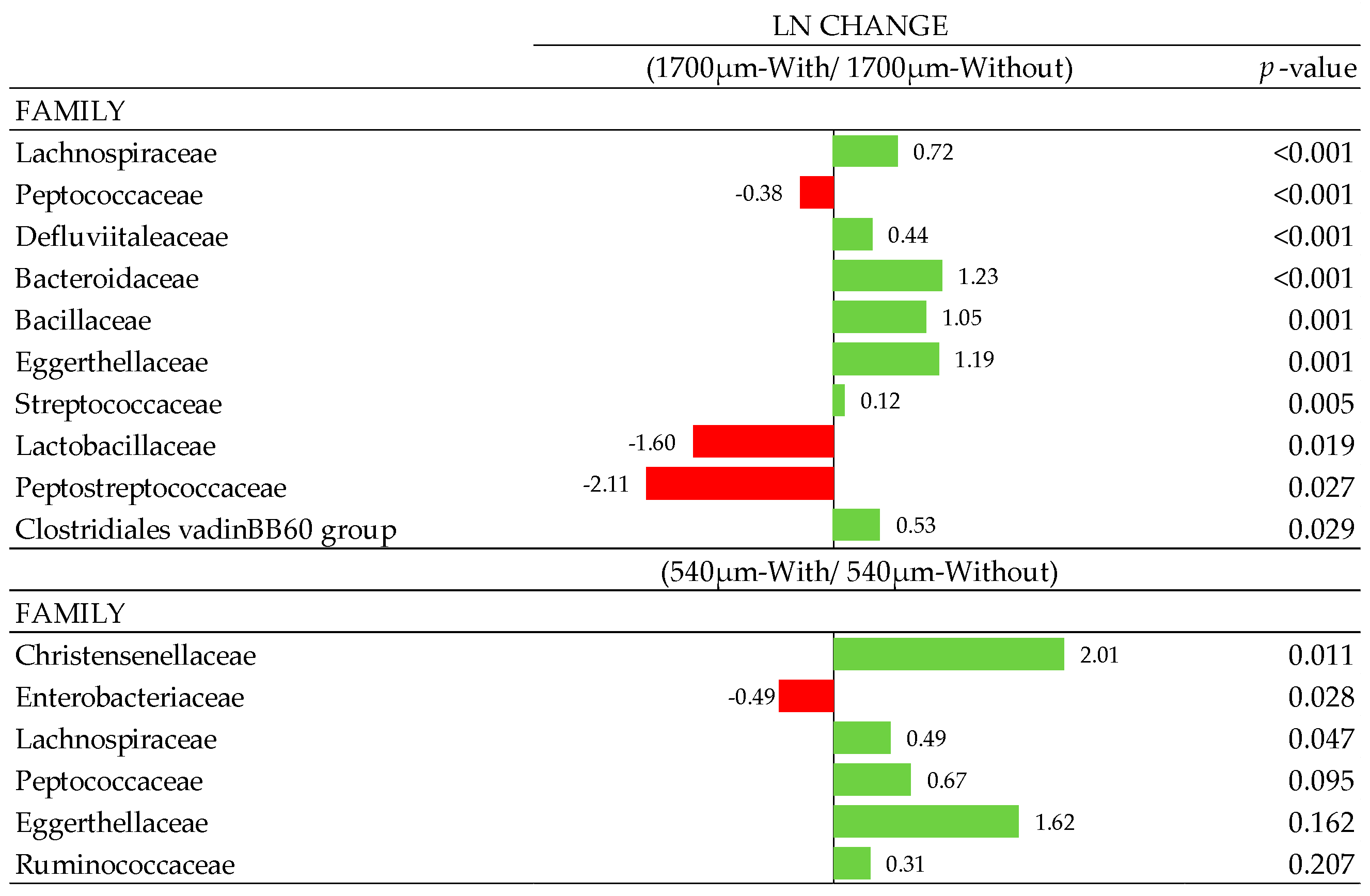
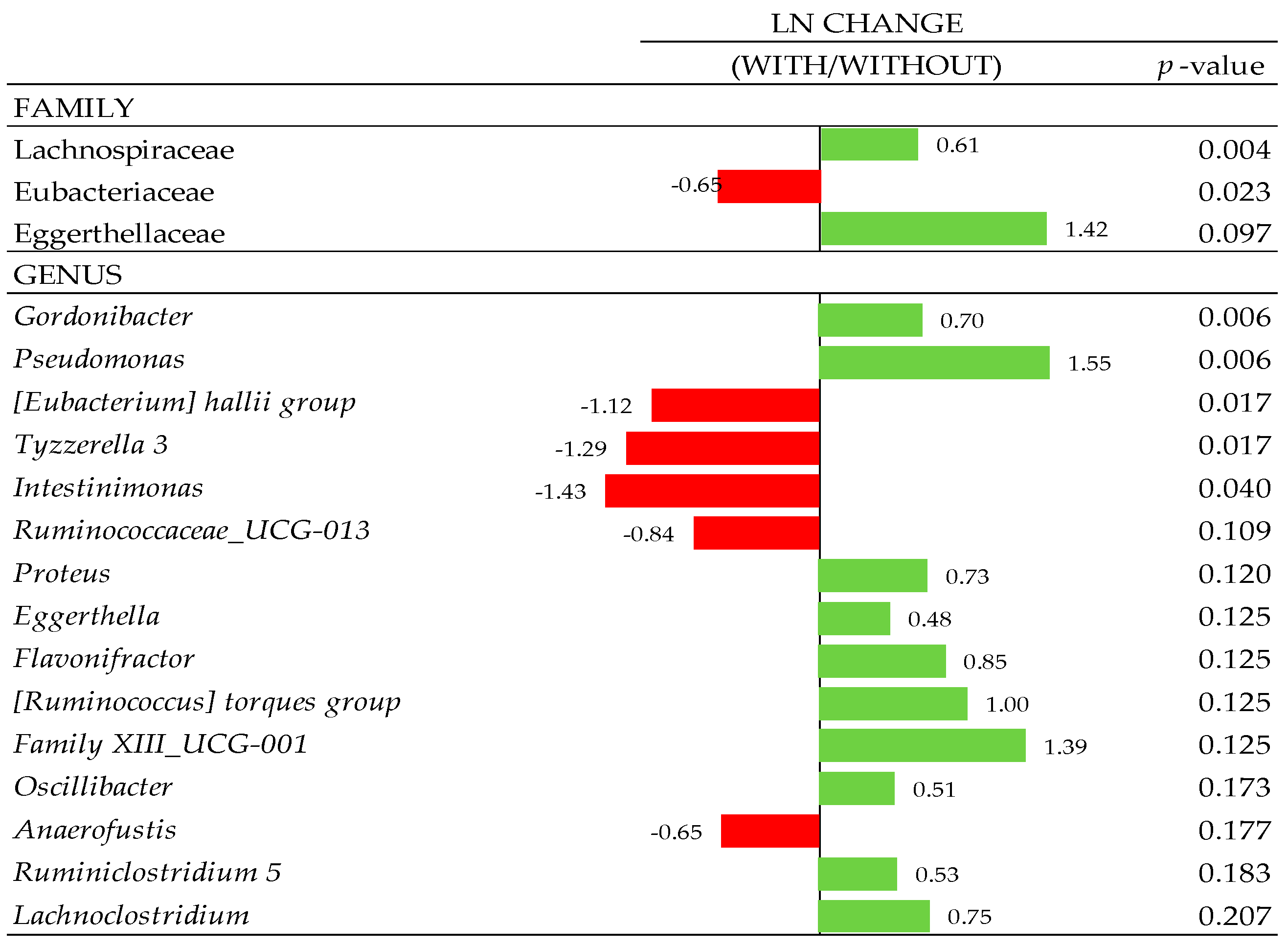
3.7. Composition of Gut Microbiota
3.8. Determination of Short-Chain Fatty Acids
4. Discussion
4.1. Particle Size
4.2. Xylanase
4.3. Particle Size and Xylanase Interaction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vukmirović, Đ.; Čolović, R.; Rakita, S.; Brlek, T.; Đuragić, O.; Solà-Oriol, D. Importance of feed structure (particle size) and feed form (mash vs. pellets) in pig nutrition–A review. Anim. Feed Sci. Technol. 2017, 233, 133–144. [Google Scholar] [CrossRef]
- Kersten, J.; Rohde, H.R.; Nef, E. Principles of Mixed Feed Production; Agrimedia: Bergen/Dumme, Germany, 2005. [Google Scholar]
- Roche, M. Comportement alimentaire et motricité digestive des oiseaux. Reprod. Nutr. Dev. 1981, 21, 781. [Google Scholar] [CrossRef]
- Córdova-Noboa, H.A.; Oviedo-Rondón, E.O.; Ortiz, A.; Matta, Y.; Hoyos, S.; Buitrago, G.D.; Martinez, J.D.; Yanquen, J.; Peñuela, L.; Sorbara, J.O.B.; et al. Corn drying temperature, particle size, and amylase supplementation influence growth performance, digestive tract development, and nutrient utilization of broilers. Poult. Sci. 2020. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.; Kriewitz, J.-P.; Hankel, J.; Chuppava, B.; Ratert, C.; Taube, V.; Visscher, C.; Kamphues, J. The Effects of Feed Particle Size and Floor Type on the Growth Performance, GIT Development, and Pododermatitis in Broiler Chickens. Animals 2020, 10, 1256. [Google Scholar] [CrossRef]
- Perera, W.N.U.; Abdollahi, M.R.; Zaefarian, F.; Wester, T.J.; Ravindran, V. The interactive influence of barley particle size and enzyme supplementation on growth performance, nutrient utilization, and intestinal morphometry of broiler starters. Poult. Sci. 2020, 99, 4466–4478. [Google Scholar] [CrossRef]
- Mtei, A.W.; Abdollahi, M.R.; Schreurs, N.M.; Ravindran, V. Impact of corn particle size on nutrient digestibility varies depending on bird type. Poult. Sci. 2019, 98, 5504–5513. [Google Scholar] [CrossRef]
- Svihus, B.; Kløvstad, K.H.; Perez, V.; Zimonja, O.; Sahlström, S.; Schüller, R.B.; Jeksrud, W.K.; Prestløkken, E. Physical and nutritional effects of pelleting of broiler chicken diets made from wheat ground to different coarsenesses by the use of roller mill and hammer mill. Anim. Feed Sci. Technol. 2004, 117, 281–293. [Google Scholar] [CrossRef]
- Vukmirović, D.; Fišteš, A.; Lević, J.; Čolović, R.; Rakić, D.; Brlek, T.; Banjac, V. Possibilities for preservation of coarse particles in pelleting process to improve feed quality characteristics. J. Anim. Physiol. Anim. Nutr. 2017, 101, 857–867. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Ravindran, V.; Svihus, B. Pelleting of broiler diets: An overview with emphasis on pellet quality and nutritional value. Anim. Feed Sci. Technol. 2013, 179, 1–23. [Google Scholar] [CrossRef]
- Choct, M.; Kocher, A.; Waters, D.L.E.; Pettersson, D.; Ross, G. A comparison of three xylanases on the nutritive value of two wheats for broiler chickens. Br. J. Nutr. 2004, 92, 53–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latham, R.E.; Williams, M.P.; Flores, C.; O’Neill, H.V.M.; York, T.W.; Lee, J.T. Impact of variable corn nutrient content, AME prediction, and xylanase inclusion on growth performance. J. Appl. Poult. Res. 2016, 25, 338–351. [Google Scholar] [CrossRef]
- Tang, D.F.; Liu, X.X.; Shi, X.G.; Aftab, U. Effect of cereal type and Xylanase supplementation on nutrient retention and growth performance of broilers. J. Appl. Poult. Res. 2017, 26, 529–535. [Google Scholar] [CrossRef]
- Bedford, M.R.; Apajalahti, J. Microbial interactions in the response to exogenous enzyme utilization. In Enzymes in Farm Animal Nutrition; Bedford, M.R., Gary, G., Eds.; CABI: Wallingford, UK, 2000; pp. 299–314. [Google Scholar]
- Bedford, M.R.; Classen, H.L. Reduction of Intestinal Viscosity through Manipulation of Dietary Rye and Pentosanase Concentration is Effected through Changes in the Carbohydrate Composition of the Intestinal Aqueous Phase and Results in Improved Growth Rate and Food Conversion Efficie. J. Nutr. 1992, 122, 560–569. [Google Scholar] [CrossRef]
- Khadem, A.; Lourenco, M.; Delezie, E.; Maertens, L.; Goderis, A.; Mombaerts, R.; Hofte, M.; Eeckhaut, V.; Van Immerseel, F. Does release of encapsulated nutrients have an important role in the efficacy of xylanase in broilers? Poult. Sci. 2016, 95, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Amerah, A.M.; Ravindran, V.; Lentle, R.G.; Thomas, D.G. Influence of particle size and xylanase supplementation on the performance, energy utilisation, digestive tract parameters and digesta viscosity of broiler starters. Br. Poult. Sci. 2008, 49, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Ravindran, V. Influence of whole wheat inclusion and xylanase supplementation on the performance, digestive tract measurements and carcass characteristics of broiler chickens. Anim. Feed Sci. Technol. 2004, 116, 129–139. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 of September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union. 2010, 33–79. [Google Scholar]
- Benz, C.; Goodband, B. Procedures for Three-Sieve Particle Analysis; Kansas State University: Manhattan, KS, USA, 2005; pp. 7–9. [Google Scholar]
- FEDNA. Necesidades Nutricionales Para Avicultura: Pollos de Carne y Aves de Puesta; Lázaro, R., Mateos, G.G., Eds.; FEDNA: Madrid, Spain, 2018. [Google Scholar]
- Baker, S.; Herrman, T. Evaluating Particle Size; Kansas State University: Manhattan, KS, USA, 2002; pp. 1–6. [Google Scholar]
- Miladinovic, D. Standard Wet Sieving Analysis Fôrtek; Norwegian University of Life Science (UMB): As, Norway, 2009; pp. 1–5. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2005; Volume 18, ISBN 1080-0344/0935584757 9780935584752. [Google Scholar]
- Anguita, M.; Gasa, J.; Martín-Orúe, S.M.; Pérez, J.F. Study of the effect of technological processes on starch hydrolysis, non-starch polysaccharides solubilization and physicochemical properties of different ingredients using a two-step in vitro system. Anim. Feed Sci. Technol. 2006, 129, 99–115. [Google Scholar] [CrossRef]
- Holben, W.E.; Williams, P.; Gilbert, M.A.; Saarinen, M.; Sarkilahti, L.K.; Apajalahti, J.H.A. Phylogenetic analysis of intestinal microflora indicates a novel Mycoplasma phylotype in farmed and wild salmon. Microb. Ecol. 2002, 44, 175–185. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, R.K.F.G.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, H.M.; Stevens, H.W. Package ‘vegan.’ In R Packag. Version 3.4.0. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 30 January 2020).
- Paulson, N.J.; Olson, N.D.; Braccia, D.J.; Wagner, J.; Talukder, H.; Pop, M.; Bravo, H.C. Package ‘metagenomeSeq’. 2019. Available online: https://www.bioconductor.org/packages/release/bioc/html/metagenomeSeq.html (accessed on 30 January 2020).
- Cumming, R.B. Opportunities for whole grain feeding. In Proceedings of the 9th European Poultry Conference; Worlds Poultry Science Association: Glasgow, UK, 1994; pp. 219–222. [Google Scholar]
- Engberg, R.M.; Hedemann, M.S.; Jensen, B.B. The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. Br. Poult. Sci. 2002, 43, 569–579. [Google Scholar] [CrossRef]
- Bjerrum, L.; Pedersen, K.; Engberg, R.M. The Influence of Whole Wheat Feeding on Salmonella Infection and Gut Flora Composition in Broilers. Avian Dis. 2005, 49, 9–15. [Google Scholar] [CrossRef]
- Gabriel, I.; Mallet, S.; Leconte, M. Differences in the digestive tract characteristics of broiler chickens fed on complete pelleted diet or on whole wheat added to pelleted protein concentrate. Br. Poult. Sci. 2003, 44, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Amerah, A.M.; Lentle, R.G.; Ravindran, V. Influence of Feed Form on Gizzard Morphology and Particle Size Spectra of Duodenal Digesta in Broiler Chickens. J. Poult. Sci. 2007, 44, 175–181. [Google Scholar] [CrossRef]
- Nir, I.; Hillel, R.; Ptichi, I.; Shefet, G. Effect of Particle Size on Performance. Grinding Pelleting Interactions. Poult. Sci. 1995, 74, 771–783. [Google Scholar] [CrossRef]
- Carré, B. Effets de la taille des particules alimentaires sur les processus digestifs chez les oiseaux d’élevage. Prod. Anim. 2000, 13, 131–136. [Google Scholar] [CrossRef]
- Singh, Y.; Ravindran, V. Influence of feeding whole maize, differing in endosperm hardness, on the performance, nutrient utilisation and digestive tract development of broiler starters. J. Appl. Anim. Nutr. 2019, 7, e1. [Google Scholar] [CrossRef]
- Carré, B.; Muley, N.; Gomez, J.; Oury, F.-X.; Laffitte, E.; Guillou, D.; Signoret, C. Soft wheat instead of hard wheat in pelleted diets results in high starch digestibility in broiler chickens. Br. Poult. Sci. 2005, 46, 66–74. [Google Scholar] [CrossRef]
- Lin, H.; An, Y.; Hao, F.; Wang, Y.; Tang, H. Correlations of Fecal Metabonomic and Microbiomic Changes Induced by High-fat Diet in the Pre-Obesity State. Sci. Rep. 2016, 6, 21618. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. MBio 2014, 2, e00889-14. [Google Scholar] [CrossRef]
- Chai, L.-J.; Lu, Z.-M.; Zhang, X.-J.; Ma, J.; Xu, P.-X.; Qian, W.; Xiao, C.; Wang, S.-T.; Shen, C.-H.; Shi, J.-S.; et al. Zooming in on Butyrate-Producing Clostridial Consortia in the Fermented Grains of Baijiu via Gene Sequence-Guided Microbial Isolation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Hmani, H.; Daoud, L.; Jlidi, M.; Jalleli, K.; Ali, M.B.; Brahim, A.H.; Bargui, M.; Dammak, A.; Ali, M. A Bacillus subtilis strain as probiotic in poultry: Selection based on in vitro functional properties and enzymatic potentialities. J. Ind. Microbiol. Biotechnol. 2017, 44, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Nandha, N.K.; Kiarie, E.; Nyachoti, C.M.; Khafipour, E. Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult. Sci. 2016, 95, 528–540. [Google Scholar] [CrossRef]
- Bedford, M.R.; Cowieson, A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012, 173, 76–85. [Google Scholar] [CrossRef]
- Meng, X.; Slominski, B.A.; Nyachoti, C.M.; Campbell, L.D.; Guenter, W. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult. Sci. 2005, 84, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D.; Khattak, F.; Hastie, P.; Bedford, M.R.; Olukosi, O.A. Xylanase and xylo- oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens. Br. Poult. Sci. 2019, 61, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sou, H.; Lu, L.; Xu, G.; Xiao, L.; Chen, X.; Xia, R.; Zhang, L.; Luo, X. Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. J. Integr. Agric. 2015, 14, 2050–2057. [Google Scholar] [CrossRef]
- De Maesschalck, C.; Eeckhaut, V.; Maertens, L.; De Lange, L.; Marchal, L.; Nezer, C.; De Baere, S.; Croubels, S.; Daube, G.; Dewulf, J.; et al. Effects of Xylo-Oligosaccharides on Broiler Chicken Performance and Microbiota. Appl. Environ. Microbiol. 2015, 81, 5880–5888. [Google Scholar] [CrossRef]
- Cordero, G.; Kim, J.C.; Whenham, N.; Masey-O’Neill, H.; Srinongkote, S.; González-Ortiz, G. 163 Xylanase and fermentable xylo-oligosaccharides improve performance in grower-finisher pigs fed a corn-soybean meal based diet. J. Anim. Sci. 2019, 97, 91–92. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Rochat, F.; Jann, A.; Meirim, I.; Sanchez-Garcia, J.-L.; Ornstein, K.; German, B.; Ballèvre, O. Chicory increases acetate turnover, but not propionate and butyrate peripheral turnovers in rats. Br. J. Nutr. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Melo-Duran, D.; Gonzalez-Ortiz, G.; Sola-Oriol, D.; Martinez-Mora, M.; Perez, J.F.; Bedford, M.R. Relationship between peptide YY, cholecystokinin and fermentation products in fasted, re-fed and ad libitum fed broiler chickens. Anim. Feed Sci. Technol. 2019, 247, 141–148. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar] [CrossRef]
- Kheravii, S.K.; Swick, R.A.; Choct, M.; Wu, S.B. Upregulation of genes encoding digestive enzymes and nutrient transporters in the digestive system of broiler chickens by dietary supplementation of fiber and inclusion of coarse particle size corn. BMC Genom. 2018, 19, 1–14. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering Endogenous Butyrate Production in the Intestinal Tract of Broilers as a Tool to Improve Gut Health. Front. Vet. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of Early-Life Fecal Microbiota in Susceptible and Healthy Pigs to Post-Weaning Diarrhoea. PLoS ONE 2017, 12, e0169851. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Velge, P.; Bottreau, E.; Fievez, V.; Haesebrouck, F.; Ducatelle, R. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 2003, 85, 237–248. [Google Scholar] [CrossRef]
- Vermeulen, K.; Verspreet, J.; Courtin, C.M.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Reduced particle size wheat bran is butyrogenic and lowers Salmonella colonization, when added to poultry feed. Vet. Microbiol. 2017, 198, 64–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Items | Geometric Mean of Corn (µm) | |||
|---|---|---|---|---|
| 540 | 660 | 1390 | 1700 | |
| Ingredients, % | ||||
| Corn | 53.4 | |||
| Soybean meal 47 | 38.4 | |||
| Palm oil | 2.50 | |||
| Soy oil | 2.69 | |||
| Salt | 0.31 | |||
| DL-Methionine | 0.29 | |||
| Lysine HCl | 0.14 | |||
| Threonine | 0.03 | |||
| Limestone | 0.97 | |||
| Mono dicalcium phosphate | 0.94 | |||
| Vitamin premix 2 | 0.40 | |||
| Calculated composition (%, as feed) | ||||
| Apparent Metabolizable Energy, kcal/kg | 2900 | |||
| Crude protein | 22.3 | |||
| Calcium | 0.90 | |||
| Available P | 0.45 | |||
| Crude fat | 7.68 | |||
| D Me + Cys | 0.98 | |||
| D Lys | 1.18 | |||
| Analyzed composition 3, % | ||||
| Gross energy, kcal/kg | 4264 | |||
| Dry matter | 90.3 | |||
| Crude protein | 22.3 | |||
| Starch | 27.7 | |||
| Ash | 5.6 | |||
| Diets 1 | Xylanase 2 (BXU/kg) |
|---|---|
| 540 | <2000 |
| 660 | <2000 |
| 1390 | <2000 |
| 1700 | <2000 |
| 540 + Xylanase | 12,400 |
| 660 + Xylanase | 13,900 |
| 1390 + Xylanase | 12,700 |
| 1700 + Xylanase | 14,200 |
| Dgw (µm) | Sgw (µm) | ||||
|---|---|---|---|---|---|
| Corn | Mash Diet | Pellet Diet | Corn | Mash Diet | Pellet Diet |
| 540 | 617 | 488 | 2.33 | 2.79 | 2.03 |
| 660 | 701 | 549 | 2.37 | 2.85 | 2.13 |
| 1390 | 1032 | 583 | 2.54 | 3.01 | 2.11 |
| 1700 | 1170 | 637 | 2.48 | 3.04 | 2.32 |
| pH 2 | pH 5 | ||||
|---|---|---|---|---|---|
| Particle Size | Xylanase | SolDM | WRC | SolDM | WRC |
| 540 | - | 0.136 | 2.16 | 0.149 a | 2.25 |
| + | 0.146 | 2.21 | 0.157 a | 2.27 | |
| 660 | - | 0.126 | 2.22 | 0.135 b | 2.28 |
| + | 0.143 | 2.11 | 0.153 a | 2.23 | |
| 1390 | - | 0.122 | 2.23 | 0.133 b | 2.37 |
| + | 0.141 | 2.27 | 0.156 a | 2.31 | |
| 1700 | - | 0.120 | 2.26 | 0.122 b | 2.35 |
| + | 0.147 | 2.28 | 0.154 a | 2.24 | |
| SEM 3 | 0.0033 | 0.044 | 0.0031 | 0.058 | |
| Main effect | |||||
| Particle size | 540 | 0.144 a | 2.18 b | 0.153 | 2.27 |
| 660 | 0.135 ab | 2.17 b | 0.144 | 2.26 | |
| 1390 | 0.132 b | 2.25 a | 0.144 | 2.34 | |
| 1700 | 0.133 b | 2.27 a | 0.138 | 2.30 | |
| SEM | 0.0021 | 0.031 | 0.0002 | 0.043 | |
| Xylanase | - | 0.126 b | 2.22 | 0.135 | 2.32 |
| + | 0.144 a | 2.22 | 0.155 | 2.28 | |
| SEM | 0.0013 | 0.018 | 0.0014 | 0.030 | |
| p-value 4 | |||||
| Particle Size | 0.013 | 0.015 | <0.0001 | 0.443 | |
| Xylanase | <0.0001 | 0.993 | <0.0001 | 0.164 | |
| Particle size x Xylanase | 0.051 | 0.105 | 0.002 | 0.880 | |
| Particle Size | Xylanase | BW (g) | ADG (g/d/bird) | ADFI (g/d/bird) | FCR (g/g) | CV (%) |
|---|---|---|---|---|---|---|
| 540 | - | 1032 | 47.5 | 55.4 | 1.17 | 12.1 |
| + | 1048 | 48.0 | 56.1 | 1.18 | 7.6 | |
| 660 | - | 1050 | 48.0 | 56.6 | 1.18 | 10.4 |
| + | 1059 | 48.8 | 56.9 | 1.17 | 8.1 | |
| 1390 | - | 1076 | 49.5 | 58.2 | 1.18 | 8.1 |
| + | 1053 | 48.2 | 57.5 | 1.19 | 8.5 | |
| 1700 | - | 1060 | 48.6 | 56.9 | 1.17 | 8.1 |
| + | 1066 | 48.6 | 57.8 | 1.18 | 6.6 | |
| SEM 3 | 14.0 | 0.69 | 0.84 | 0.014 | 0.89 | |
| Main effect | ||||||
| Particle size | 540 | 1040 | 47.4 | 55.7 | 1.18 | 9.9 b |
| 660 | 1055 | 48.4 | 56.8 | 1.17 | 9.2 b | |
| 1390 | 1064 | 48.8 | 57.9 | 1.18 | 8.3 a | |
| 1700 | 1063 | 48.6 | 57.3 | 1.17 | 7.4 a | |
| SEM | 9.9 | 0.48 | 0.59 | 0.010 | 0.63 | |
| Xylanase | - | 1055 | 48.4 | 56.8 | 1.17 | 9.7 b |
| SEM | + | 1057 | 48.3 | 57.1 | 1.18 | 7.7 a |
| 7.0 | 0.34 | 0.42 | 0.007 | 0.44 | ||
| p-value 4 | ||||||
| Particle Size | 0.280 | 0.189 | 0.083 | 0.886 | 0.044 | |
| Xylanase | 0.836 | 0.848 | 0.637 | 0.486 | 0.003 | |
| Particle size x Xylanase | 0.520 | 0.537 | 0.762 | 0.835 | 0.074 | |
| Main Effects | Ileal Digestibility (%) | Intake of Digestible Nutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Energy | OM | Starch | Protein g | Energy kcal | OM g | Starch G | ||
| Particle size | 540 | 83.8 b | 76.6 b | 74.1 | 96.8 | 220 b | 3848 b | 776 b | 319 |
| 660 | 84.4 b | 76.4 b | 73.5 | 96.1 | 221 b | 3839 b | 770 b | 320 | |
| 1390 | 86.2 a | 78.1 a | 75.1 | 96.5 | 235 a | 4045 a | 811 a | 328 | |
| 1700 | 86.2 a | 78.8 a | 76.2 | 96.5 | 233 a | 4033 a | 810 a | 326 | |
| SEM | 0.47 | 0.66 | 0.78 | 0.31 | 3.7 | 53.1 | 10.9 | 3.6 | |
| Xylanase | - | 85.4 | 78.2 a | 75.8 a | 96.7 | 225 | 3968 | 797 | 322 |
| + | 84.9 | 76.8 b | 73.7 b | 96.3 | 229 | 3914 | 786 | 324 | |
| SEM2 | 0.35 | 0.47 | 0.55 | 0.22 | 2.9 | 36.6 | 7.9 | 2.5 | |
| p-value 3 | |||||||||
| Particle Size | 0.001 | 0.029 | 0.086 | 0.525 | 0.011 | 0.003 | 0.013 | 0.135 | |
| Xylanase | 0.29 | 0.037 | 0.007 | 0.139 | 0.429 | 0.277 | 0.311 | 0.605 | |
| Particle size x Xylanase | 0.515 | 0.775 | 0.867 | 0.099 | 0.537 | 0.645 | 0.486 | 0.511 | |
| Main Effects | Total Tract Digestibility (%) | Intake of Digestible Nutrients | |||||
|---|---|---|---|---|---|---|---|
| Protein | Energy | OM | Protein g | Energy kcal | OM g | ||
| Particle size | 540 | 66.8 b | 77.6 | 74.6 | 171 c | 3862 b | 794 b |
| 660 | 69.5 ab | 78.5 | 74.9 | 182 b | 4016 ab | 822 a | |
| 1390 | 70.2 a | 78.1 | 74.9 | 189 a | 4066 a | 836 a | |
| 1700 | 70.1 a | 78.7 | 74.9 | 185 ab | 4128 a | 827 | |
| SEM | 0.61 | 0.44 | 0.55 | 2.2 | 64.1 | 9.4 | |
| Xylanase | - | 69.5 | 78.7 a | 75.3 | 181 | 3987 | 818 |
| + | 68.9 | 77.7 b | 74.3 | 183 | 4050 | 821 | |
| SEM 3 | 0.41 | 0.3 | 0.37 | 1.5 | 42.6 | 6.5 | |
| p-value 4 | |||||||
| Particle Size | 0.001 | 0.296 | 0.961 | <0.0001 | 0.031 | 0.016 | |
| Xylanase | 0.367 | 0.025 | 0.058 | 0.434 | 0.317 | 0.700 | |
| Particle size x Xylanase | 0.788 | 0.633 | 0.765 | 0.857 | 0.293 | 0.485 | |
| Main Effects | Gizzard | Duodenum | Jejunum | Ileum | Ceca | Small Intestine 2 | |
|---|---|---|---|---|---|---|---|
| g/100 g of Body Weight | |||||||
| Particle size | 540 | 1.54 c | 1.11 | 1.41 | 1.29 | 0.42 a | 3.82 |
| 660 | 1.66 bc | 1.14 | 1.38 | 1.28 | 0.40 ab | 3.83 | |
| 1390 | 1.78 ab | 1.15 | 1.36 | 1.22 | 0.38 b | 3.75 | |
| 1700 | 1.91 a | 1.13 | 1.27 | 1.29 | 0.40 ab | 3.71 | |
| SEM 3 | 0.051 | 0.033 | 0.042 | 0.032 | 0.011 | 0.089 | |
| Xylanase | - | 1.75 | 1.15 | 1.35 | 1.28 | 0.39 | 3.80 |
| SEM | + | 1.69 | 1.11 | 1.37 | 1.25 | 0.39 | 3.76 |
| 0.035 | 0.023 | 0.029 | 0.024 | 0.007 | 0.063 | ||
| p-value 4 | |||||||
| Particle Size | <0.0001 | 0.826 | 0.102 | 0.353 | 0.004 | 0.767 | |
| Xylanase | 0.288 | 0.227 | 0.599 | 0.247 | 0.831 | 0.407 | |
| Particle size x Xylanase | 0.721 | 0.646 | 0.805 | 0.353 | 0.314 | 0.917 | |
| Main Effects | Shannon | Simpson | Inverse-Simpson | |
|---|---|---|---|---|
| Particle size | 540 | 4.22 b | 0.96 | 31.0 b |
| 1700 | 4.44 a | 0.97 | 44.1 a | |
| SEM 3 | 0.068 | 0.004 | 4.49 | |
| Xylanase | - | 4.28 | 0.96 | 35.4 |
| + | 4.38 | 0.96 | 39.4 | |
| SEM | 0.068 | 0.004 | 4.49 | |
| p-value 4 | ||||
| Particle size | 0.034 | 0.146 | 0.048 | |
| Xylanase | 0.282 | 0.471 | 0.566 | |
| Particle size x Xylanase | 0.092 | 0.155 | 0.290 | |
| Acid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SCFA | VFA | BCFA | Acetic | Propionic | Butyric | Valeric | Lactic | ||
| Particle Size | Xylanase | mM | % | ||||||
| 540 | - | 97.3 | 90.5 | 1.93 | 71.3 | 4.1 bc | 12.1 | 1.07 | 9.5 |
| + | 98.5 | 90.7 | 1.80 | 71.4 | 4.7 abc | 11.7 | 0.97 | 9.3 | |
| 660 | - | 96.9 | 91.5 | 1.73 | 72.3 | 4.4 abc | 12.0 | 0.95 | 8.5 |
| + | 93.0 | 92.1 | 1.95 | 71.5 | 5.3 a | 12.3 | 1.01 | 7.8 | |
| 1700 | - | 93.1 | 92.5 | 1.85 | 71.7 | 5.0 ab | 12.9 | 1.03 | 7.5 |
| + | 91.8 | 92.9 | 1.81 | 74.7 | 3.8 c | 11.7 | 0.83 | 7.0 | |
| SEM 5 | 4.33 | 0.802 | 0.159 | 1.43 | 0.40 | 0.88 | 0.090 | 0.80 | |
| Main effect | |||||||||
| Particle size | 540 | 97.9 | 90.6 b | 1.86 | 71.3 | 4.5 | 11.9 | 1.03 | 9.4 a |
| 660 | 95.0 | 91.8 ab | 1.84 | 71.9 | 4.8 | 12.2 | 0.98 | 8.1 ab | |
| 1700 | 92.5 | 92.8 a | 1.83 | 73.2 | 4.4 | 12.3 | 0.94 | 7.3 b | |
| SEM | 3.06 | 0.56 | 0.112 | 1.01 | 0.28 | 0.62 | 0.063 | 0.56 | |
| Xylanase | - | 95.8 | 87.7 | 1.83 | 71.8 | 4.5 | 12.4 | 1.02 | 8.48 |
| + | 94.4 | 86.9 | 1.85 | 72.5 | 4.6 | 11.9 | 0.94 | 8.03 | |
| SEM4 | 2.50 | 2.52 | 0.092 | 0.82 | 0.23 | 0.51 | 0.052 | 0.462 | |
| p-value 5 | |||||||||
| Particle Size | 0.459 | 0.039 | 0.981 | 0.416 | 0.519 | 0.911 | 0.628 | 0.039 | |
| Xylanase | 0.711 | 0.565 | 0.898 | 0.527 | 0.697 | 0.552 | 0.288 | 0.507 | |
| Particle size*Xylanase | 0.836 | 0.947 | 0.552 | 0.403 | 0.028 | 0.721 | 0.349 | 0.954 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo-Durán, D.; Pérez, J.F.; González-Ortiz, G.; Sala, R.; Villagómez-Estrada, S.; Bedford, M.R.; Graham, H.; Solà-Oriol, D. Influence of Particle Size and Xylanase in Corn-Soybean Pelleted Diets on Performance, Nutrient Utilization, Microbiota and Short-Chain Fatty Acid Production in Young Broilers. Animals 2020, 10, 1904. https://doi.org/10.3390/ani10101904
Melo-Durán D, Pérez JF, González-Ortiz G, Sala R, Villagómez-Estrada S, Bedford MR, Graham H, Solà-Oriol D. Influence of Particle Size and Xylanase in Corn-Soybean Pelleted Diets on Performance, Nutrient Utilization, Microbiota and Short-Chain Fatty Acid Production in Young Broilers. Animals. 2020; 10(10):1904. https://doi.org/10.3390/ani10101904
Chicago/Turabian StyleMelo-Durán, Diego, José Francisco Pérez, Gemma González-Ortiz, Roser Sala, Sandra Villagómez-Estrada, Michael R. Bedford, Hadden Graham, and David Solà-Oriol. 2020. "Influence of Particle Size and Xylanase in Corn-Soybean Pelleted Diets on Performance, Nutrient Utilization, Microbiota and Short-Chain Fatty Acid Production in Young Broilers" Animals 10, no. 10: 1904. https://doi.org/10.3390/ani10101904
APA StyleMelo-Durán, D., Pérez, J. F., González-Ortiz, G., Sala, R., Villagómez-Estrada, S., Bedford, M. R., Graham, H., & Solà-Oriol, D. (2020). Influence of Particle Size and Xylanase in Corn-Soybean Pelleted Diets on Performance, Nutrient Utilization, Microbiota and Short-Chain Fatty Acid Production in Young Broilers. Animals, 10(10), 1904. https://doi.org/10.3390/ani10101904





