How Can Cattle Be Toilet Trained? Incorporating Reflexive Behaviours into a Behavioural Chain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
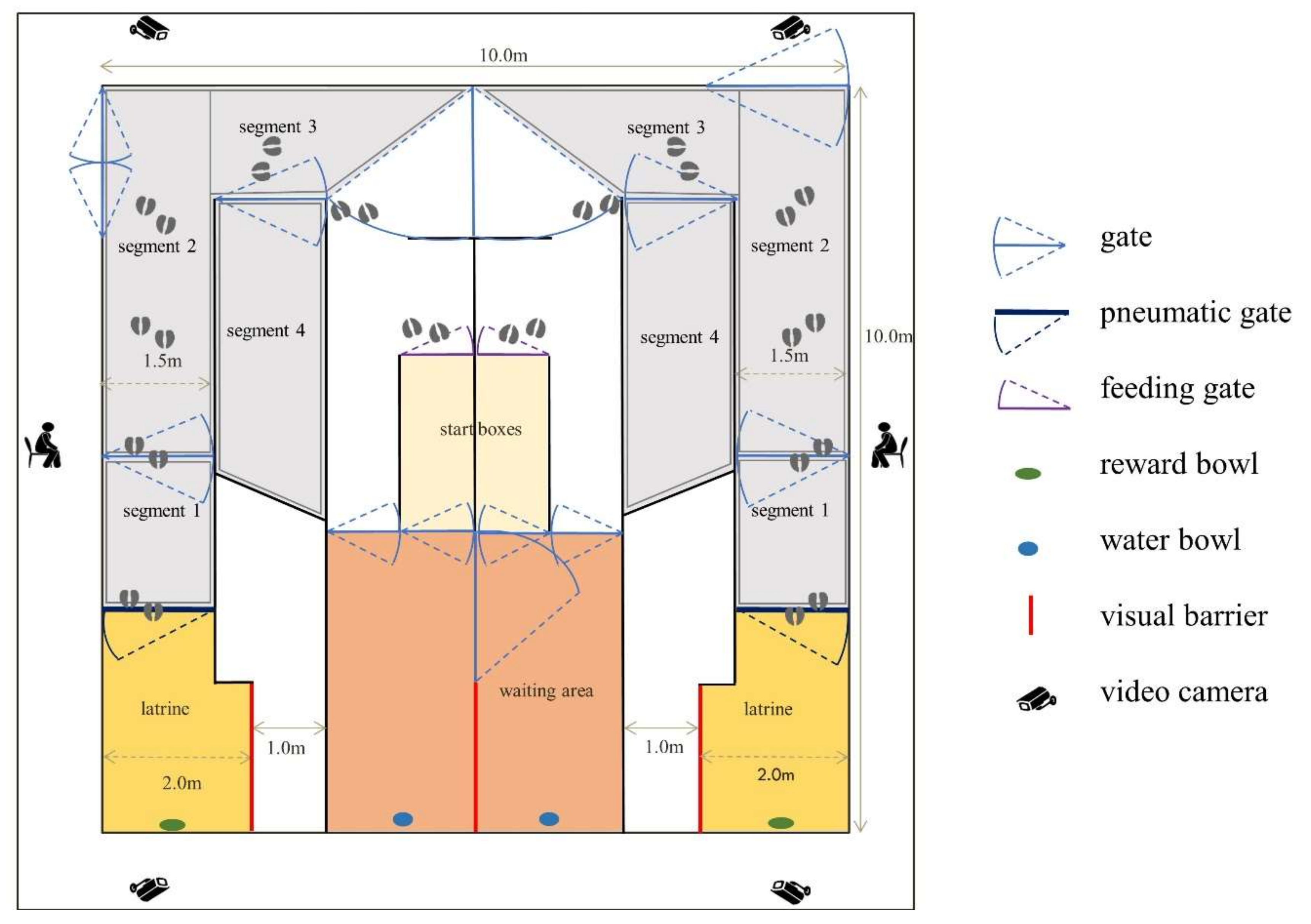
2.2. Experimental Facility
2.3. Experimental Procedure
2.3.1. Training Voluntary Behavioural Responses in the Latrine
2.3.2. Training Voluntary Behavioural Responses Outside of the Latrine
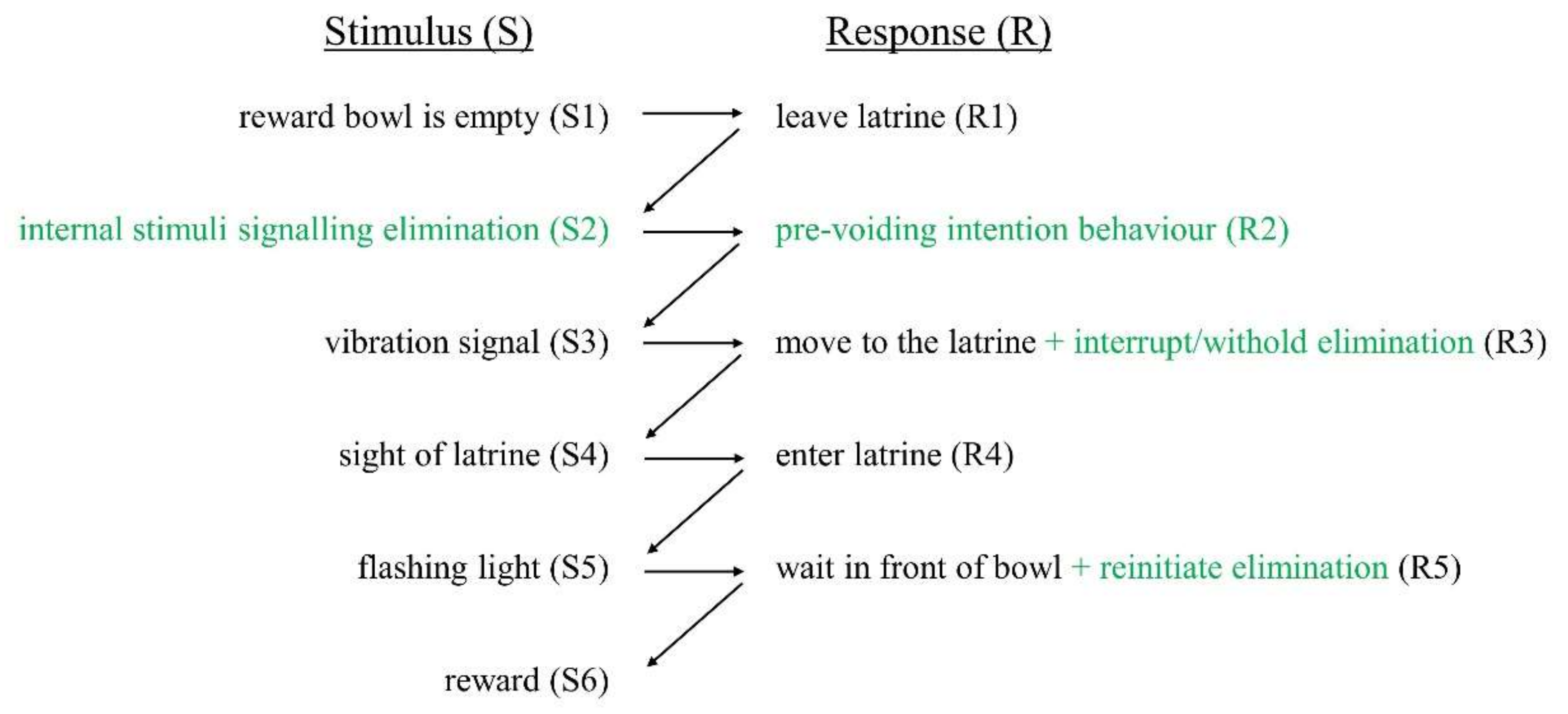
2.3.3. Incorporation of Reflexive Responses into the Chain
2.4. Data Analysis
3. Results
3.1. Training Voluntary Behavioural Responses in the Latrine
3.2. Training Voluntary Behavioural Responses Outside of the Latrine
3.3. Incorporation of Reflexive Responses into the Chain
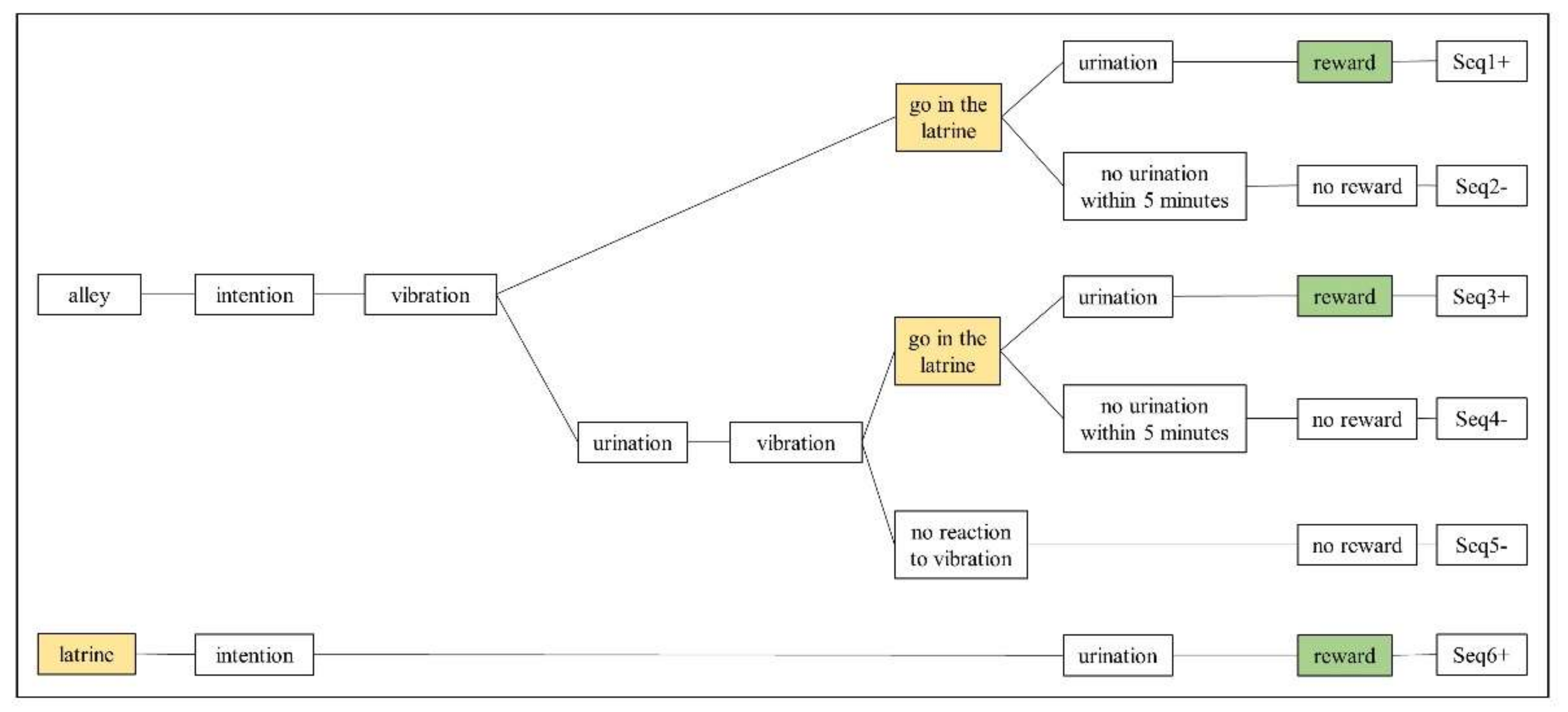
3.3.1. Behavioural Sequences
3.3.2. Duration of Urination and Time to Reinitiate Urination
3.3.3. Association of Urination (and Place) with Reward
3.3.4. Location of the Calves
4. Discussion
4.1. Initiation and Completion of Voluntary Behaviour Chains
4.2. Control and Learning of Reflexive Responses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Mendes, L.B.; Pieters, J.G.; Snoek, D.; Ogink, N.W.M.; Brusselman, E.; Demeyer, P. Reduction of ammonia emissions from dairy cattle cubicle houses via improved management- or design-based strategies: A modeling approach. Sci. Total Environ. 2017, 574, 520–531. [Google Scholar] [CrossRef]
- Braam, C.R.; Swierstra, D. Volatilization of ammonia from dairy housing floors with different surface characteristics. J. Agric. Eng. Res. 1999, 72, 59–69. [Google Scholar] [CrossRef]
- Vaddella, V.K.; Ndegwa, P.M.; Joo, H.S.; Ullman, J.L. Impact of Separating Dairy Cattle Excretions on Ammonia Emissions. J. Environ. Qual. 2010, 39, 1807–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, J.; Frankena, K.; Noordhuizen-Stassen, E.N.; Metz, J.H.M. Risk factors for interdigital dermatitis and heel erosion in dairy cows kept in cubicle houses in The Netherlands. Prev. Vet. Med. 2005, 71, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sarjokari, K.; Kaustell, K.O.; Hurme, T.; Kivinen, T.; Peltoniemi, O.A.T.; Saloniemi, H.; Rajala-Schultz, P.J. Prevalence and risk factors for lameness in insulated free stall barns in Finland. Livest. Sci. 2013, 156, 44–52. [Google Scholar] [CrossRef]
- Chapinal, N.; Barrientos, A.K.; von Keyserlingk, M.A.G.; Galo, E.; Weary, D.M. Herd-level risk factors for lameness in freestall farms in the northeastern United States and California. J. Dairy Sci. 2013, 96, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, M.; Herlin, A.H.; Ventorp, M. Short Communication: Effect of Alley Floor Cleanliness on Free-Stall and Udder Hygiene. J. Dairy Sci. 2008, 91, 3927–3930. [Google Scholar] [CrossRef]
- Arya, N.G.; Weissbart, S.J. Central control of micturition in women: Brain-bladder pathways in continence and urgency urinary incontinence. Clin. Anat. 2017, 30, 373–384. [Google Scholar] [CrossRef]
- Salomon, F.V.; Geyer, H.; Gille, U. Anatomie für die Tiermedizin, 2nd ed.; Enke: Stuttgart, Germany, 2008; p. 884. [Google Scholar]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Wredle, E.; Munksgaard, L.; Spörndly, E. Training cows to approach the milking unit in response to acoustic signals in an automatic milking system during the grazing season. Appl. Anim. Behav. Sci. 2006, 101, 27–39. [Google Scholar] [CrossRef]
- Kiley-Worthington, M.; Savage, P. Learning in dairy cattle using a device for economical management of behaviour. Appl. Anim. Ethol. 1978, 4, 119–124. [Google Scholar] [CrossRef]
- Dirksen, N.; Langbein, J.; Matthews, L.; Puppe, B.; Elliffe, D.; Schrader, L. Conditionability of ‘voluntary’ and ‘reflexive-like’ behaviors, with special reference to elimination behavior in cattle. Neurosci. Biobehav. Rev. 2020, 115, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.K.; Reider, L.; Herron, M.E.; Graszak, K. Health and behavior problems in dogs and cats one week and one month after adoption from animal shelters. J. Am. Vet. Med. Assoc. 2008, 233, 1715–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, A. 5.05—Applied behavior analysis. In Comprehensive Clinical Psychology; Bellack, A.S., Hersen, M., Eds.; Pergamon: Oxford, UK, 1998; pp. 107–129. [Google Scholar] [CrossRef]
- Slocum, S.K.; Tiger, J.H. An assessment of the efficiency of and child preference for forward and backward chaining. J. Appl. Behav. Anal. 2011, 44, 793–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.W.; Quintero, L.M. Comparing forward and backward chaining in teaching Olympic weightlifting. J. Appl. Behav. Anal. 2019, 52, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Steinfeld, M.; Alcalá, J.A.; Thrailkill, E.A.; Bouton, M.E. Renewal in a heterogeneous behavior chain: Extinction of the first response prevents renewal of a second response when it is separately extinguished and returned to the chain. Learn. Motiv. 2019, 68, 101587. [Google Scholar] [CrossRef]
- Mazur, J.E. Lernen und Gedächtnis, 5th ed.; Pearson Studium: München, Germany, 2004. [Google Scholar]
- Johnson, J.M.; Bailey, J.M.; Johnson, J.E.; Newland, M.C. Performance of BALB/c and C57BL/6 mice under an incremental repeated acquisition of behavioral chains procedure. Behav. Process. 2010, 84, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Pisacreta, R. A comparison of forward and backward procedures for the acquisition of response chains in pigeons. Bull. Psychon. Soc. 1982, 20, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Hundziak, M.; Maurer, R.A.; Watson, L.S. Operant conditioning in toilet training severely mentally retarded boys. Am. J. Ment. Defic. 1965, 70, 120–124. [Google Scholar]
- Van Wagenen, R.K.; Meyerson, L.; Kerr, N.J.; Mahoney, K. Field trials of a new procedure for toilet training. J. Exp. Child Psychol. 1969, 8, 147–159. [Google Scholar] [CrossRef]
- Vaughan, A.; de Passillé, A.M.; Stookey, J.; Rushen, J. Operant conditioning of urination by calves. Appl. Anim. Behav. Sci. 2014, 158, 8–15. [Google Scholar] [CrossRef]
- Whistance, L.K.; Sinclair, L.A.; Arney, D.R.; Phillips, C.J.C. Trainability of eliminative behaviour in dairy heifers using a secondary reinforcer. Appl. Anim. Behav. Sci. 2009, 117, 128–136. [Google Scholar] [CrossRef]
- Elias, P.K.; Elias, M.F. Effects of age on learning ability: Contributions from the animal literature. Exp. Aging Res. 1976, 2, 165–186. [Google Scholar] [CrossRef]
- Kovalčik, K.; Kovalčik, M. Learning ability and memory testing in cattle of different ages. Appl. Anim. Behav. Sci. 1986, 15, 27–29. [Google Scholar] [CrossRef]
- Aland, A.; Lidfors, L.; Ekesbo, I. Diurnal distribution of dairy cow defecation and urination. Appl. Anim. Behav. Sci. 2002, 78, 43–54. [Google Scholar] [CrossRef]
- Villettaz Robichaud, M.; de Passillé, A.M.; Pellerin, D.; Rushen, J. When and where do dairy cows defecate and urinate? J. Dairy Sci. 2011, 94, 4889–4896. [Google Scholar] [CrossRef]
- Hirata, M.; Higashiyama, M.; Hasegawa, N. Diurnal pattern of excretion in grazing cattle. Livest. Sci. 2011, 142, 23–32. [Google Scholar] [CrossRef]
- Shahan, T.A.; Chase, P.N. Novelty, stimulus control, and operant variability. Behav. Anal. 2002, 25, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Kayser, J.E.; Billingsley, F.F.; Neel, R.S. A comparison of in-context and traditional instructional approaches: Total task, single trial versus backward chaining, multiple trials. J. Assoc. Pers. Sev. Handicaps 1986, 11, 28–38. [Google Scholar] [CrossRef]
- Touchette, P.E. Transfer of stimulus control: Measuring the moment of transfer. J. Exp. Anal. Behav. 1971, 15, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.H.; Rilling, M.E. Stimulus delay and the reduction of errors in the transfer of stimulus control. Anim. Learn. Behav. 1975, 3, 21–27. [Google Scholar] [CrossRef]
- MacDonall, J.S.; Marcucella, H. Cross-modal transfer of stimulus control in the albino rat: A stimulus delay procedure. Anim. Learn. Behav. 1976, 4, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Thrailkill, E.A.; Shahan, T.A. Temporal integration and instrumental conditioned reinforcement. Learn. Behav. 2014, 42, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.J.; Timberlake, W.; Gont, R.S. Spatiotemporal characteristics of serial CSs and their relation to search modes and response form. Anim. Learn. Behav. 1998, 26, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Dabrowska, B.; Harmata, W.; Lenkiewicz, Z.; Schiffer, Z.; Wojtusiak, R.J. Colour perception in cows. Behav. Process. 1981, 6, 1–10. [Google Scholar] [CrossRef]
- Heffner, H.E. Auditory awareness. Appl. Anim. Behav. Sci. 1998, 57, 259–268. [Google Scholar] [CrossRef]
- Wredle, E.; Rushen, J.; de Passillé, A.M.; Munksgaard, L. Training cattle to approach a feed source in response to auditory signals. Can. J. Anim. Sci. 2004, 84, 567–572. [Google Scholar] [CrossRef]
- Ouweltjes, W.; van der Werf, J. Detectie van eliminaties (lozing van mest en urine) van melkvee. In Scheiding van Urine en Feces Bij Melkvee: Fysiologie, Gedragsherkenning en Techniek; Verdoes, N., Bokma, S., Eds.; Wageningen Livestock Research: Wageningen, The Netherlands, 2017; Volume 1041, pp. 24–31. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.J.; Pham, J.C.; Choo, J.; Hu, D.L. Law of urination: All mammals empty their bladders over the same duration. arXiv 2013, arXiv:1310.3737. [Google Scholar]
- Al-Marashdeh, O.; Gregorini, P.; Maxwell, T.M.R.; Cheng, L.; Beltran, I.E.; Hussein, A.N.; Chen, A.; Guinot, L.; Hodge, S.; Cameron, K.C.; et al. Short-term grazing and urination behaviour of dairy cows differing in their genetic merit. New Zealand J. Agric. Res. 2020, 63, 260–267. [Google Scholar] [CrossRef]
- Amici, F.; Cacchione, T.; Bueno-Guerra, N. Understanding of object properties by sloth bears, Melursus ursinus ursinus. Anim. Behav. 2017, 134, 217–222. [Google Scholar] [CrossRef]
- Mastellone, V.; Scandurra, A.; D’Aniello, B.; Nawroth, C.; Saggese, F.; Silvestre, P.; Lombardi, P. Long-term socialization with humans affects human-directed behavior in goats. Animals 2020, 10, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noldus Information Technology. Reliability analysis. In Reference Manual—The Observer XT 13; Noldus Information Technology: Wageningen, The Netherlands, 2016; pp. 327–369. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Hirata, M.; Tomita, C.; Yamada, K. Use of a maze test to assess spatial learning and memory in cattle: Can cattle traverse a complex maze? Appl. Anim. Behav. Sci. 2016, 180, 18–25. [Google Scholar] [CrossRef]
- Seo, T.; Takahashi, S.; Ohtani, M.; Kashiwamura, F. Can Individual vibratory pagers be used to leading cows to concentrate feeding stations efficiently? In Proceedings of the 36th International Congress of the ISAE, Wageningen, The Netherlands, 9 August 2002; p. 193. [Google Scholar]
- Lattal, K.A. Delayed reinforcement of operant behavior. J. Exp. Anal. Behav. 2010, 93, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Recording Type 1 | Behavioural Parameter | Definition |
|---|---|---|
| D, F, T | intention (to urinate) | arching the back, raising the tail and/or spreading the hind legs |
| D, F, T | urination | urinating (urine is voided) |
| D, F, T | location of the calf | >50% of the calf’s body is in a given segment or in the latrine |
| T | orientation towards the bowl | moving the head in the direction of the bowl, and/or starting locomotion towards the bowl (only during a urination event) |
| Phase | Blue | Black | Turquoise | Red | Brown |
|---|---|---|---|---|---|
| Phase 1.1 | 6 | 18 | 14 | 11 | 6 |
| Phase 1.2 | 5 | 19 | 13 | 5 | 31 |
| Phase 1.3 | 8 | 7 | 10 | 14 | 5 |
| Phase | Blue | Black | Turquoise | Red | Brown |
|---|---|---|---|---|---|
| Test/Control | Test/Control | Test/Control | Test/Control | Test/Control | |
| Phase 1.1 | 5/2 | 12/0 | 10/3 | 5/2 | 5/1 |
| Phase 1.2 | 5/0 | 12/8 | 8/0 | 5/1 | 20/0 |
| Phase 1.3 | 6/4 | 7/2 | 7/0 | 13/5 | 5/0 |
| Test Calf | Session 1 | Session 2 | Session 3 | |||
|---|---|---|---|---|---|---|
| Events with Orientation | Total Number of Events | Events with Orientation | Total Number of Events | Events with Orientation | Total Number of Events | |
| Blue | 0 | 3 | 2 | 3 | 8 | 8 |
| Black | 1 | 2 | 2 | 3 | 4 | 4 |
| Turquoise | 3 | 5 | 4 | 4 | 9 | 9 |
| Red | 1 | 3 | 0 | 3 | 2 | 4 |
| Brown | 3 | 3 | 9 | 9 | 6 | 6 |
| Session | Seq1+ | Seq2- | Seq3+ | Seq4- | Seq5- | Seq6+ |
|---|---|---|---|---|---|---|
| Session 1 | 2 | 0 | 6 | 2 | 2 | 4 |
| Session 2 | 3 | 0 | 7 | 0 | 0 | 4 |
| Session 3 | 3 | 0 | 5 | 5 | 1 | 6 |
| Session 4 | 1 | 0 | 5 | 5 | 0 | 1 |
| Session 5 | 0 | 0 | 4 | 3 | 0 | 6 |
| Session 6 | 1 | 0 | 1 | 4 | 0 | 3 |
| Session 7 | 2 | 0 | 1 | 2 | 0 | 5 |
| Test Calf | Seq1+ | Seq2- | Seq3+ | Seq4- | Seq5- | Seq6+ | Total |
|---|---|---|---|---|---|---|---|
| Blue | 0 | 0 | 6 | 6 | 0 | 6 | 18 |
| Black | 6 | 0 | 2 | 1 | 0 | 0 | 9 |
| Turquoise | 4 | 0 | 11 | 6 | 3 | 1 | 25 |
| Red | 0 | 0 | 3 | 4 | 0 | 14 | 21 |
| Brown | 2 | 0 | 7 | 4 | 0 | 8 | 21 |
| total | 12(13%) | 0(0%) | 29(31%) | 21(22%) | 3(3%) | 29(31%) | 94(100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirksen, N.; Langbein, J.; Schrader, L.; Puppe, B.; Elliffe, D.; Siebert, K.; Röttgen, V.; Matthews, L. How Can Cattle Be Toilet Trained? Incorporating Reflexive Behaviours into a Behavioural Chain. Animals 2020, 10, 1889. https://doi.org/10.3390/ani10101889
Dirksen N, Langbein J, Schrader L, Puppe B, Elliffe D, Siebert K, Röttgen V, Matthews L. How Can Cattle Be Toilet Trained? Incorporating Reflexive Behaviours into a Behavioural Chain. Animals. 2020; 10(10):1889. https://doi.org/10.3390/ani10101889
Chicago/Turabian StyleDirksen, Neele, Jan Langbein, Lars Schrader, Birger Puppe, Douglas Elliffe, Katrin Siebert, Volker Röttgen, and Lindsay Matthews. 2020. "How Can Cattle Be Toilet Trained? Incorporating Reflexive Behaviours into a Behavioural Chain" Animals 10, no. 10: 1889. https://doi.org/10.3390/ani10101889
APA StyleDirksen, N., Langbein, J., Schrader, L., Puppe, B., Elliffe, D., Siebert, K., Röttgen, V., & Matthews, L. (2020). How Can Cattle Be Toilet Trained? Incorporating Reflexive Behaviours into a Behavioural Chain. Animals, 10(10), 1889. https://doi.org/10.3390/ani10101889






