1. Introduction
The rescue and survival of the black-footed ferret (
Mustela nigripes) (BFF) have heavily relied on extensive studies of the reproductive biology and the development of assisted reproductive technologies for more than 35 years [
1]. This includes the establishment of optimized protocols for artificial insemination, semen cryopreservation, and systematic cryo-banking. BFFs are seasonal breeders, reaching puberty at the age of 1 year, followed by a relatively short reproductive life (3 to 4 years) [
2]. Therefore, it is critical to collect or rescue and store genetic materials from all individuals for later use. As seen in other rare and endangered species, long-term preservation of ovarian and testicular tissues from individuals sterilized for medical reasons or dying unexpectedly offers additional options to study and propagate genetically valuable animals over multiple generations [
3]. Cryopreservation of testicular tissues is now conducted in humans, laboratory models, domestic species, and some wildlife [
3,
4,
5]. However, there is no report in the BFF yet.
Among the multiple preservation protocols that are available for testicular tissues, vitrification or ultra-rapid freezing is the most convenient approach that has already provided encouraging results for carnivore tissues regarding the maintenance of histomorphometry [
3,
6]. While cryoprotectants, exposure conditions, tissue biopsy size, or freezing rates play critical roles in tissue survival [
3], warming is an essential step that is often overlooked. Proper warming can prevent devitrification/ice recrystallization and ensure optimal reanimation in preserved tissues. Recently, beneficial effects of short time exposure to high warming temperatures have been reported in testicular tissues from prepubertal cats [
7]. An additional short culture period of warmed tissues also promotes cell survival and mitigates DNA injuries [
7,
8].
Interestingly, spermatozoa from frozen-thawed testicular tissues are viable in the cat model (based on the birth of healthy offspring after transfer of embryos produced by sperm injection) [
9]. However, testicular tissues contain a large number of germ cells at early stages (in seminiferous tubules) that have the potential to develop into mature sperm cells after in vitro culture or xenografting as seen in other species, including the domestic ferret [
3,
10]. Spermatogenesis requires adequate germ cell support that may be compromised during freezing/warming as seen in humans [
5] or in the cat model [
6]. Sertoli cells are critical to fully support germ cell development and differentiation throughout spermatogenesis [
11]. Thus, the structure and functions of seminiferous tubules, including connections between germ cells (histomorphometry) and the integrity of Sertoli cells (assessed by the presence of vimentin), have to be properly preserved [
6,
7]. Recent studies in the cat model confirmed that markers such as Oct4 and Boule are present in pre-meiotic (spermatogonia) and meiotic (spermatocytes) germ cells, respectively [
7]. Those are very good indicators of germ cell composition in the tissue. However, the use of these critical markers to evaluate germ cell populations and progression has never been reported in BFFs.
Therefore, the objective of the study was to evaluate structural properties, cell survival, DNA fragmentation, and germ cell composition in vitrified testicular tissues from the endangered BFF directly after warming or after warming plus a short in vitro culture period.
2. Materials and Methods
2.1. Collection and Dissection of Testes
Tissues were sent to our laboratory for cryo-banking (collaboration with the US Fish and Wildlife Service’s National Black-Footed Ferret Conservation Center in Colorado). Testis and epididymis from BFFs (n = 5; 3–5 year old) were collected from routine orchiectomy of gonadal rescue (shortly after the unexpected death of the animal) from January to September 2018. The use of a subset of tissues for the present study was approved by the National Black-Footed Ferret Conservation Center and did not require approval from our Institutional Animal Care and Use Committee.
Testes were shipped overnight in phosphate buffered saline (PBS) at 4 °C to the laboratory. They were measured for length (L; cranial–caudal) and width (W; medial–lateral) and depth (D; dorso–ventral) using a caliper. Testes were extensively rinsed in PBS and dissected from surrounding tissues. Then, they were cut in pieces of approximately 1–3 mm3 using scalpel blades and forceps in Hepes–Ham’s F10 medium (Irvine Scientific, Santa Ana, CA, USA) supplemented with 1mM pyruvate, 2 mM L-glutamine, 100 IU/mL penicillin, 100 µg/mL streptomycin, 2.5% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA). A piece of epididymis was sliced with a scalpel blade in the same handling medium to detect the presence of spermatozoa.
2.2. Vitrification of Testicular Tissue
Tissue biopsies were immediately evaluated (fresh control; see Experimental Design) or exposed to a mix of cryoprotectants (dimethylsulphoxide (DMSO) and glycerol (Sigma- Aldrich) following our standard protocol [
7]. Briefly, tissue biopsies were threaded onto a 30-G needle (BD Precision Glide needle, Fischer Scientific, Waltham, MA, USA) and immersed in an equilibrium (1.4 M of each cryoprotectant plus 0.25 M sucrose in Ham’s F10) for 10 min at room temperature (~22–24 °C). Tissues then were rapidly transferred to a vitrification solution (2.8 M of each cryoprotectant, 0.50 M sucrose, Ham’s F10, and 10% of FBS) for 5 min at room temperature. Threaded tissues then were plunged directly into liquid nitrogen and placed in sealed cryotubes before storage in a cryo-tank for at least one week.
2.3. Warming of Vitrified Tissues
Frozen tissue biopsies threaded onto needles were first transferred for 5 s into PBS solution pre-warmed to 50 °C. Progressive removal of the cryoprotectants was obtained by exposing tissues at room temperature to solutions containing decreasing concentrations of sucrose (0.50 M; 0.25 M; 0.00 M) in Hepes–Ham’s F10 and 20% of FBS for 5 min each.
2.4. Tissue Culture
Tissues were deposited on small cubes (1 cm3) of 1.5% agarose gel at the bottom of 4-well culture dishes filled with 400 µL of culture medium comprised of Hepes–Ham’s F10 (supplemented with 2 mM L-glutamine, 1 mM pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5% FBS). Two tissue biopsies were placed on each cube and were cultured for 24 h at 38.5 °C in a humidified atmosphere with 5% CO2.
2.5. Histomorphology
Tissues were fixed overnight in Bouin’s solution before embedding in paraffin and serial sections (5-µm thick). After mounting sections on frosted glass slides, staining with hematoxylin-eosin was performed according to a standard protocol [
7]. Slides then were observed using an upright microscope fitted with digital photomicrography (SPOT advanced software 5.0; Diagnostic Instruments, Sterling Heights, MI, USA). Seminiferous tubule and cell integrity were evaluated based on the attachment of cells to the basal membrane, absence of breaks in the stroma, no swelling of the lamina propria, and tight junctions between cells. Structures received a score of 1 in that case [
7]. If any change was observed in the previous criteria, tubules received a score of 0. For each individual and for each treatment group, a total of 50 seminiferous tubules (randomly selected on the slides) were assessed. Percentages of normal seminiferous tubules were calculated relative to the total number of observed tubules.
2.6. Vimentin, Oct4, and Boule Immunostaining
Following a standard protocol [
7], cells were isolated by slicing tissue pieces with a scalpel blade in modified Ham’s F-10 Basal Medium–HEPES (Irvine Scientific). Resulting cell suspensions were centrifuged (300×
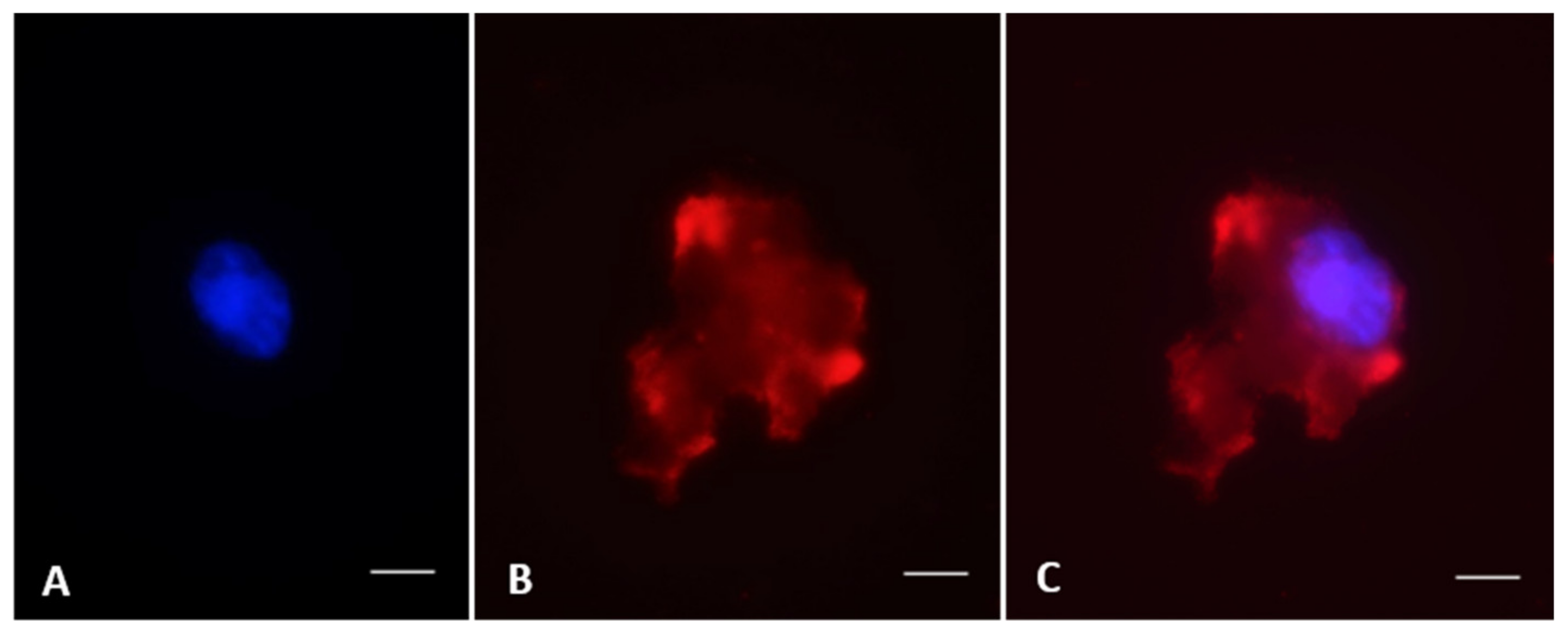
g) for 8 min and resuspended in fresh Ham’s F10 medium. After smearing 20 µL of suspension on a glass slide, cells were fixed in 4% paraformaldehyde for 1 h at room temperature followed by permeabilization with 0.1% Triton X-100 in PBS (PBS-T) for 3 min. After saturation of non-specific sites in PBS with 5% Bovine Serum Albumin (BSA, Sigma-Aldrich) for 1 h at room temperature, cells were incubated overnight at 4 °C in a humidified chamber with a primary antibody: anti-vimentin (1:500, Abcam #ab8069), pre-meiotic marker anti-OCT4 (1:200, Abcam #ab137427), or meiotic marker anti-Boule (1:100, Abcam #ab28745, Cambridge, MA, USA). After two washing in PBS and PBS-T for 5 min each, cells were incubated for 1 h at 37 °C with secondary antibodies: anti-mouse IgG (1:500, Invitrogen #62-6520, Carlsbad, CA, USA), donkey anti-goat IgG-FITC (1:500, Santa Cruz Biotechnology #2024, Dallas, TX, USA), or goat anti-rabbit IgG-TR (1:100; Santa Cruz Biotechnology #2780). Nuclear chromatin then was counterstained with Hoechst 33342 (1:100, Sigma-Aldrich) for 10 min at room temperature before mounting slides with Vectashield (Vector laboratories, Malvern, PA, USA). Slides were observed and images were captured using a microscope fitted with epifluorescence (Olympus BX41, Olympus Corporation, Cemter Valley, PA, USA) and with a SPOT camera and advanced software 5.0 (Diagnostic Instruments). For each individual and for each treatment group, 200 cells were observed for each staining. Percentages of stained cells were calculated relative to the total number of observed cells.
2.7. Evaluations of DNA Fragmentation and Viability
DNA integrity and cell viability were simultaneously assessed using the In-Situ Cell Death Detection kit (Roche, Burlington, NC, USA), as previously reported [
7]. Briefly, cell suspensions were obtained as mentioned above. After smearing 20 µL of suspension on a glass slide, cells were fixed in 4% paraformaldehyde for 1 h at room temperature and then permeabilized on ice for 2 min in PBS with 0.1% Triton X-100 and 0.1% sodium citrate (PBS-T). After two washing in PBS, cells were incubated with Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reaction mixture (prepared according to the manufacturer) for 1 h at 37 °C in humid environment. A negative control slide (omission of terminal deoxynucleotidyl transferase, TdT, in the TUNEL reaction mixture) was included in each staining. A positive control slide was added in each staining as well by pre-incubating the cells in DNase I recombinant (Sigma-Aldrich) for 10 min before adding the TUNEL reaction mixture. Final counterstaining with Hoechst 33342 (1:100, Sigma-Aldrich; for nuclear chromatin) and propidium iodide (1:100, Invitrogen; for dead cell nuclei) lasted 10 min at room temperature before mounting slides in Vectashield (Vector laboratories). Slides were observed and images were captured using a microscope fitted with epifluorescence (Olympus BX41, Olympus Corporation) and with a SPOT camera and advanced software 5.0 (Diagnostic Instruments). Cells with DNA fragmentation had a bright green nucleus and dead cells had a bright red nucleus. For each individual and for each treatment group, we observed 200 cells. Proportions of cells with fragmented DNA were calculated relative to the total number of live cells. Percentages of viable cells were calculated based on the number of viable cells (normal DNA integrity or fragmented DNA) relative to the total number of cells.
2.8. Experimental Design and Statistical Analysis
On a given day, tissue biopsies from one individual were randomly allocated to different groups: control (fresh tissue), vitrification, or vitrification and culture. For histomorphological evaluation, 1-2 biopsies were fixed. For vimentin, DNA fragmentation, viability, Boule, or Oct4, 1-2 testicular pieces were dissected. Experiments were repeated five times (n = 5 individuals) on different days. Data were expressed as mean and standard error. All data analyses were conducted with statistical software GraphPad Prism® version 5.01 (GraphPad Software Inc., San Diego, CA, USA). Data distribution was systematically tested with the Shapiro–Wilk test. When data distributions were normal, average proportions were compared between groups using analysis of variance (ANOVA) followed by a Tukey test. When data distributions were not normal, values were analyzed using a Wilcoxon or Mann Whitney test (non-parametrical test). Differences were considered statistically significant when p < 0.05.
4. Discussion
This is the first report about testicular tissue cryopreservation in the BFF. Tissue pieces subjected to vitrification/warning followed by a short period of in vitro culture kept an overall normal structure and maintained the ability to resume germ cell progression.
Testicular morphometry and the presence of sperm cells in the tissue and the epididymis were comparable to previous reports in the same species [
2]. Histology in fresh tissues also was similar to earlier studies conducted during the BFF breeding season [
2]. Vimentin—a major component of the Sertoli cell cytoskeleton—was detected like in rodents, ungulates, or other carnivores [
7,
12,
13]. The high prevalence of vimentin confirmed that critical cells supporting spermatogenesis were resilient and recovered after vitrification/warming plus culture as observed in the domestic cat [
7].
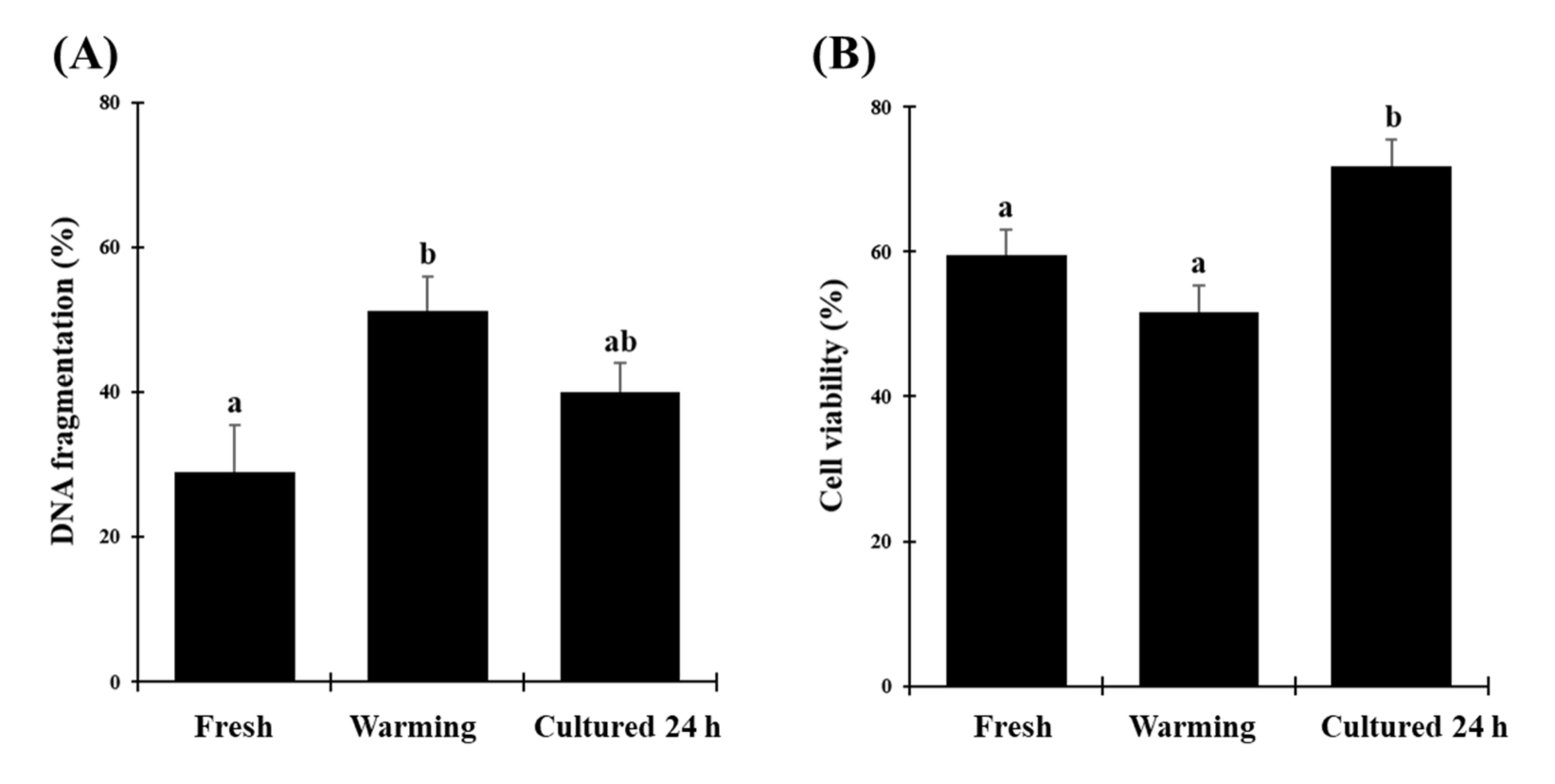
Incidence of DNA fragmentation in testicular cells increased right after warming but returned to the same level after culture as in the fresh tissue. This likely involved DNA repair mechanisms during the additional culture period as previously observed in cat testes [
7] but also in ovarian tissues exposed to comparable conditions [
14]. Previous studies reported that germ cells at the early stages of spermatogenesis are more sensitive to DNA damage than sperm cells. Non-physiological conditions created by the preservation methods break hydrogen bonds between the bases of the DNA strands [
15]. Our next step is to decipher and quantify DNA repair mechanisms in tissues through staining of proliferating cell nuclear antigen (PCNA) or RAD51 protein [
16] and detection of heat shock proteins [
17]. Similarly, testicular cell viability improved after culture like in other species, which is considered a good sign of functional reanimations [
3]. Interestingly, viability after vitrification and culture was even better than in fresh tissues. We hypothesize that more cells had time to revive over 24 h of culture and eventually were present in higher proportions than in the refrigerated/fresh tissue shipped overnight to our laboratory. This is a phenomenon that has been already reported in gonadal tissues in many species (from ungulates to carnivores) following vitrification and warming [
3,
18].
The increase in Boule staining after culture was previously observed in domestic cat tissues [
7]. This also was consistent with germ cell composition (progression from spermatogonia to spermatocytes) reported in the domestic ferret [
19]. The higher incidence of Boule-labeled cells after culture could also be due to easier isolation of spermatocytes compared to the refrigerated/fresh tissues.
Present results were obtained in adult tissues, but it is expected that structural or functional properties in testes from prepubertal or younger BFFs would be comparable or even better, as seen in the preservation of cat testicular tissues [
15]. Collective results helped to better characterize the resilience of BFF testicular tissues to freezing temperatures and the essential role of rapid warming followed by a 24-h reanimation period. These findings will have major implications for the development of cryo-banking in that species and future technologies involving the in vitro culture of testicular tissues (to produce mature sperm cells for in vitro fertilization, including sperm injection). It also is crucial to properly preserve testicular tissues because semen collection is not always possible (prepubertal individuals dying unexpectedly, castration for medical reasons during the non-breeding season, or obstructive azoospermia) [
3].