Verification and Analysis of Sheep Tail Type-Associated PDGF-D Gene Polymorphisms
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. PCR Amplification and Mass Array Genotyping
2.3. Population Genetic Analysis of Polymorphisms in the PDGF-D Gene
2.4. Bioinformatics Analysis
2.5. Cell Culture and Transfection
2.6. Quantitative Real-Time PCR Analysis
2.7. Oil Red O Staining
2.8. Statistical Analysis
3. Results
3.1. SNP Detection and Genotyping
3.2. Genetic Parameters Calculation
3.3. Correlation Analysis between PDGF-D Gene Polymorphism and Tail Type in Sheep
3.4. Transcriptional Factor Binding Sites Prediction
3.5. Lentiviral Overexpression Efficiency Assay
3.6. Influence of Overexpressed PDGF-D on the Expression of Adipose Differentiation-Related Marker Genes
3.7. Oil Red O Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTB | β-actin |
| He | heterozygosity |
| LPL | lipoproteinlipase |
| MALDI-TOF-MS | matrix-assisted laser desorption/ionization time-of-flight mass spectrometer |
| Ne | effective allele number |
| PDGF-D | platelet-derived growth factor-D |
| PIC | polymorphic information content |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| qRT-PCR | quantitative real-time PCR |
| SNP | single nucleotide polymorphism |
| TF | transcriptional factor |
References
- Moradi, M.H.; Nejati-Javaremi, A.; Moradi-Shahrbabak, M.; Dodds, K.G.; McEwan, J.C. Genomic scan of selective sweeps in thin and fat tail sheep breeds for identifying of candidate regions associated with fat deposition. BMC Genet. 2012, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Rao, S.Q.; Lu, S.J.; Hou, G.Y.; Guan, W.J.; Li, H.B.; Li, X.; Zhao, Q.J.; Guo, J. Phylogeography and origin of sheep breeds in northern China. Conserv. Genet. 2006, 7, 117–127. [Google Scholar] [CrossRef]
- Nejati-Javaremi, A.R.; Izadi, F.A.; Rahmati, G.H.; Moradi, M.O.; Izadi, F. Selection in fat-tailed sheep based on two traits of fat-tail and body weight versus single-trait total body weight. Int. J. Agric. Biol. 2007, 9, 645–648. [Google Scholar]
- Lv, F.H.; Peng, W.F.; Yang, J.; Zhao, Y.X.; Li, W.R.; Liu, M.J.; Ma, Y.H.; Zhao, Q.J.; Yang, G.L.; Wang, F.; et al. Mitogenomic meta-analysis identifies two phases of migration in the history of eastern Eurasian sheep. Mol. Biol. Evol. 2015, 32, 2515–2533. [Google Scholar] [CrossRef] [PubMed]
- Moioli, B.; Pilla, F.; Ciani, E. Signatures of selection identify loci associated with fat tail in sheep. J. Anim. Sci. 2015, 93, 4660–4669. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Bahbahani, H.; Moioli, B.; Ahbara, A.; Al Abri, M.; Almathen, F.; da Silva, A.; Belabdi, I.; Portolano, B.; Mwacharo, J.M.; et al. Novel and known signals of selection for fat deposition in domestic sheep breeds from Africa and Eurasia. PLoS ONE 2019, 14, e0209632. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, H.F.; Hu, W.X.; Li, S.R.; Yu, J.J. Over-expression of platelet-derived growth factor-D promotes tumor growth and invasion in endometrial cancer. Int. J. Mol. Sci. 2014, 15, 4780–4794. [Google Scholar] [CrossRef]
- Hye Kim, J.; Gyu Park, S.; Kim, W.K.; Song, S.U.; Sung, J.H. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells 2015, 33, 542–556. [Google Scholar] [CrossRef]
- Aguilar, A. Renal fibrosis: PDGF-D in renal fibrosis. Nat. Rev. Nephrol. 2016, 12, 257. [Google Scholar] [CrossRef]
- Gladh, H.; Folestad, E.B.; Muhl, L.; Ehnman, M.; Tannenberg, P.; Lawrence, A.L.; Betsholtz, C.; Eriksson, U. Mice lacking platelet-derived growth factor D display a mild vascular phenotype. PLoS ONE 2016, 11, e0152276. [Google Scholar] [CrossRef]
- Wei, C.H.; Wang, H.H.; Liu, G.; Wu, M.M.; Cao, J.X.; Liu, Z.; Liu, R.Z.; Zhao, F.P.; Zhang, L.; Lu, J.; et al. Genome-wide analysis reveals population structure and selection in Chinese indigenous sheep breeds. BMC Genet. 2015, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Y.; Fan, H.Y.; Yuan, Z.H.; Hu, S.J.; Ma, X.M.; Xuan, J.L.; Wang, H.W.; Zhang, L.; Wei, C.H.; Zhang, Q.; et al. Genome-wide detection of CNVs in Chinese indigenous sheep with different types of tails using ovine high-density 600K SNP arrays. Sci. Rep. 2016, 6, 27822. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.H.; Liu, E.M.; Liu, Z.; Kijas, J.W.; Zhu, C.Y.; Hu, S.J.; Ma, X.M.; Zhang, L.; Du, L.X.; Wang, H.H.; et al. Selection signature analysis reveals genes associated with tail type in Chinese indigenous sheep. Anim. Genet. 2017, 48, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, W.R.; Lv, F.H.; He, S.G.; Tian, S.L.; Peng, W.F.; Sun, Y.W.; Zhao, Y.X.; Tu, X.L.; Zhang, M.; et al. Whole-genome sequencing of native sheep provides insights into rapid adaptations to extreme environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yang, J.; Lv, F.H.; Hu, X.J.; Xie, X.L.; Zhang, M.; Li, W.R.; Liu, M.J.; Wang, Y.T.; Li, J.Q.; et al. Genomic reconstruction of the history of native sheep reveals the peopling patterns of nomads and the expansion of early pastoralism in East Asia. Mol. Biol. Evol. 2017, 34, 2380–2395. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Moioli, B.; Ahbara, A.; Latairish, S.; Portolano, B.; Pilla, F.; Ciani, E. Genome-wide scan of fat-tail sheep identifies signals of selection for fat deposition and adaptation. Anim. Prod. Sci. 2019, 59, 835–848. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, J.T.; Ma, Z.R.; Lu, J.X.; Zang, R.X.; Wu, J.P. Primary culture and differentiation of ovine preadipocytes. Chin. J. Anim. Nutr. 2010, 22, 1768–1774. [Google Scholar]
- Bhattacharyya, N.; Banerjee, D. Transcriptional regulatory sequences within the first intron of the chicken apolipoproteinAI (apoAI) gene. Gene 1999, 234, 371–380. [Google Scholar] [CrossRef]
- Horie, T.; Ono, K.; Horiguchi, M.; Nishi, H.; Nakamura, T.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Marusawa, H.; Iwanaga, Y.; et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17321–17326. [Google Scholar] [CrossRef]
- Slater, E.P.; Rabenau, O.; Karin, M.; Baxter, J.D.; Beato, M. Glucocorticoid receptor binding and activation of a heterologous promoter by dexamethasone by the first intron of the human growth hormone gene. Mol. Cell. Biol. 1985, 5, 2984–2992. [Google Scholar] [CrossRef]
- Mack, C.P.; Owens, G.K. Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5’ and first intron promoter regions. Circ. Res. 1999, 84, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.C.; Shukla, G.C.; Fuller, J.D.; Padgett, R.A. Alternative splicing of U12-dependent introns in vivo responds to purine-rich enhancers. RNA 2001, 7, 1378–1388. [Google Scholar] [PubMed]
- Schjerven, H.; Brandtzaeg, P.; Johansen, F.E. A novel NF-kappa B/Rel site in intron 1 cooperates with proximal promoter elements to mediate TNF-alpha-induced transcription of the human polymeric Ig receptor. J. Immunol. 2001, 167, 6412–6420. [Google Scholar] [CrossRef] [PubMed]
- Hube, F.; Guo, J.; Chooniedass-Kothari, S.; Cooper, C.; Hamedani, M.K.; Dibrov, A.A.; Blanchard, A.A.; Wang, X.M.; Deng, G.; Myal, Y.; et al. Alternative splicing of the first intron of the steroid receptor RNA activator (SRA) participates in the generation of coding and noncoding RNA isoforms in breast cancer cell lines. DNA Cell Biol. 2006, 25, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Pirskanen, R.; Giscombe, R.; Lefvert, A.K. Two SNPs in the promoter region of the CTLA-4 gene affect binding of transcription factors and are associated with human myasthenia gravis. J. Intern. Med. 2008, 263, 61–69. [Google Scholar] [CrossRef]
- Knappskog, S.; Lonning, P.E. Effects of the MDM2 promoter SNP285 and SNP309 on Sp1 transcription factor binding and cancer risk. Transcription 2011, 2, 207–210. [Google Scholar] [CrossRef]
- Knappskog, S.; Bjornslett, M.; Myklebust, L.M.; Huijts, P.E.; Vreeswijk, M.P.; Edvardsen, H.; Guo, Y.; Zhang, X.; Yang, M.; Ylisaukko-Oja, S.K.; et al. The MDM2 promoter SNP285C/309G haplotype diminishes Sp1 transcription factor binding and reduces risk for breast and ovarian cancer in Caucasians. Cancer Cell 2011, 19, 273–282. [Google Scholar] [CrossRef]
- Yeh, W.C.; Cao, Z.D.; Classon, M.; McKnight, S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995, 9, 168–181. [Google Scholar] [CrossRef]
- Ohlsson, E.; Hasemann, M.S.; Willer, A.; Lauridsen, F.K.; Rapin, N.; Jendholm, J.; Porse, B.T. Initiation of MLL-rearranged AML is dependent on C/EBPalpha. J. Exp. Med. 2014, 211, 5–13. [Google Scholar] [CrossRef]
- Hausman, G.J. The influence of dexamethasone and insulin on expression of CCAAT/enhancer binding protein isoforms during preadipocyte differentiation in porcine stromal-vascular cell cultures: Evidence for very early expression of C/EBPalpha. J. Anim. Sci. 2000, 78, 1227–1235. [Google Scholar] [CrossRef]
- Sakakura, Y.; Shimano, H.; Sone, H.; Takahashi, A.; Inoue, N.; Toyoshima, H.; Suzuki, S.; Yamada, N. Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem. Biophys. Res. Commun. 2001, 286, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Safdarian, M.; Zamiri, M.J.; Hashemi, M.; Noorolahi, H. Relationships of fat-tail dimensions with fat-tail weight and carcass characteristics at different slaughter weights of Torki-Ghashghaii sheep. Meat Sci. 2008, 80, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, Y.; Gagnon, A.; Aubin, D.; Sorisky, A. Anti-adipogenic effect of PDGF is reversed by PKC inhibition. J. Cell. Physiol. 2005, 204, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, T.E.; Mattsson, C.L.; Falting, J.M.; Nedergaard, J. Differential signalling pathways for EGF versus PDGF activation of Erk1/2 MAP kinase and cell proliferation in brown pre-adipocytes. Exp. Cell Res. 2008, 314, 3581–3592. [Google Scholar] [CrossRef]
- Virakul, S.; Dalm, V.A.; Paridaens, D.; van den Bosch, W.A.; Mulder, M.T.; Hirankarn, N.; van Hagen, P.M.; Dik, W.A. Platelet-derived growth factor-BB enhances adipogenesis in orbital fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5457–5464. [Google Scholar] [CrossRef] [PubMed]
- Kurasawa, K.; Arai, S.; Owada, T.; Maezawa, R.; Kumano, K.; Fukuda, T. Autoantibodies against platelet-derived growth factor receptor alpha in patients with systemic lupus erythematosus. Mod. Rheumatol. 2010, 20, 458–465. [Google Scholar] [CrossRef]
- Xu, S.S.; Li, M.H. Recent advances in understanding genetic variants associated with economically important traits in sheep (Ovis aries) revealed by high-throughput screening technologies. Front. Agric. Sci. Eng. 2017, 4, 279–288. [Google Scholar] [CrossRef]
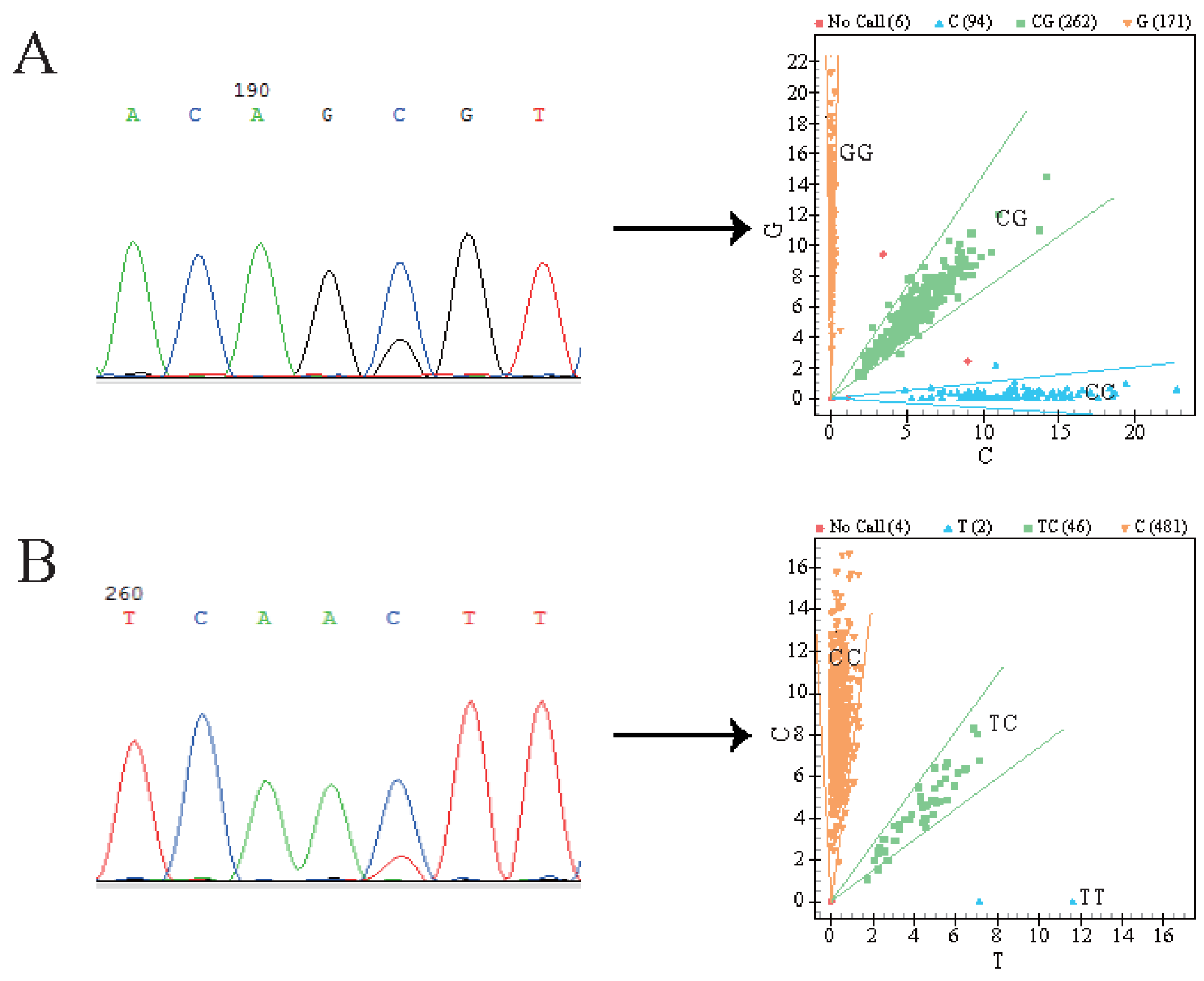
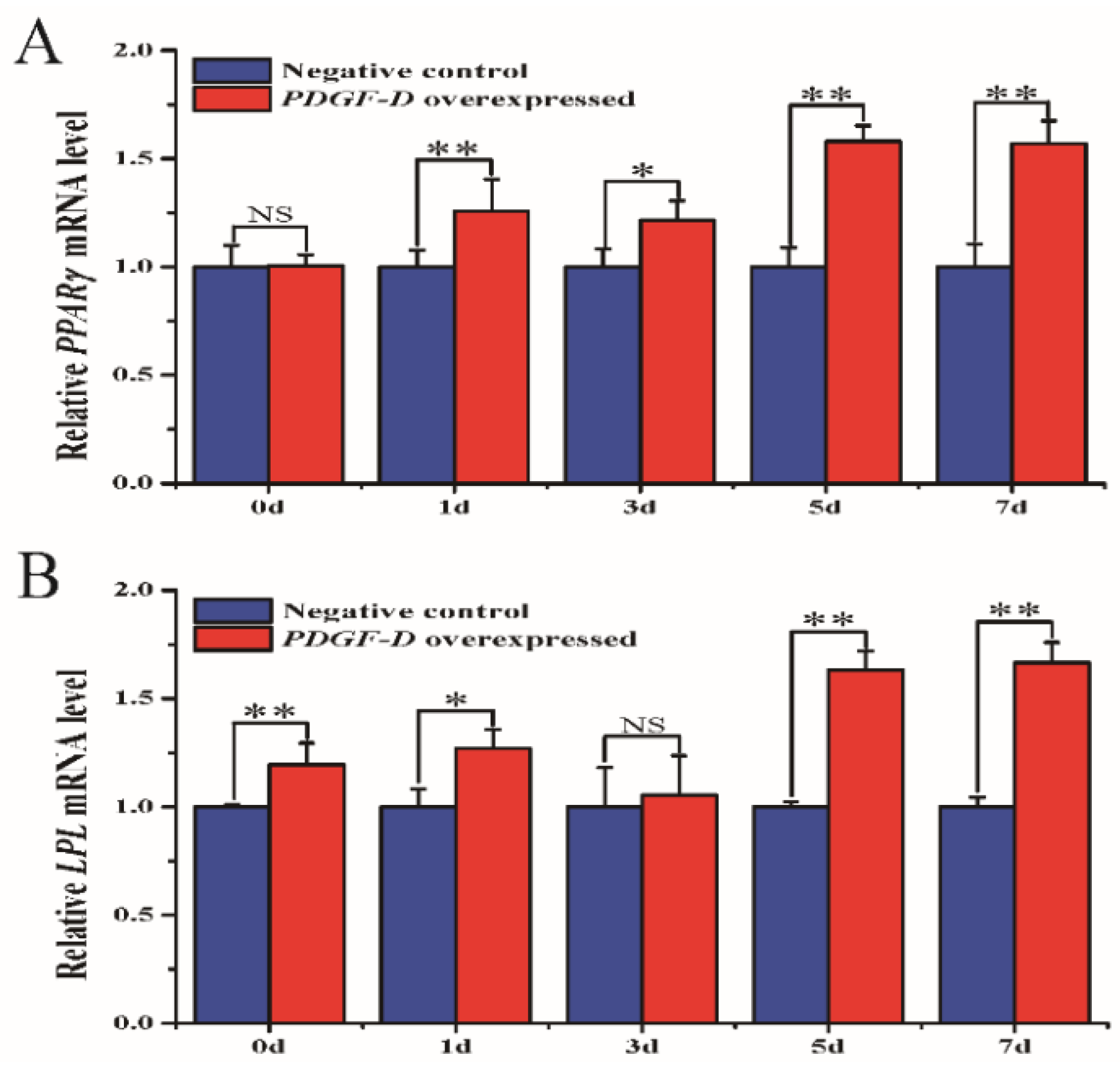
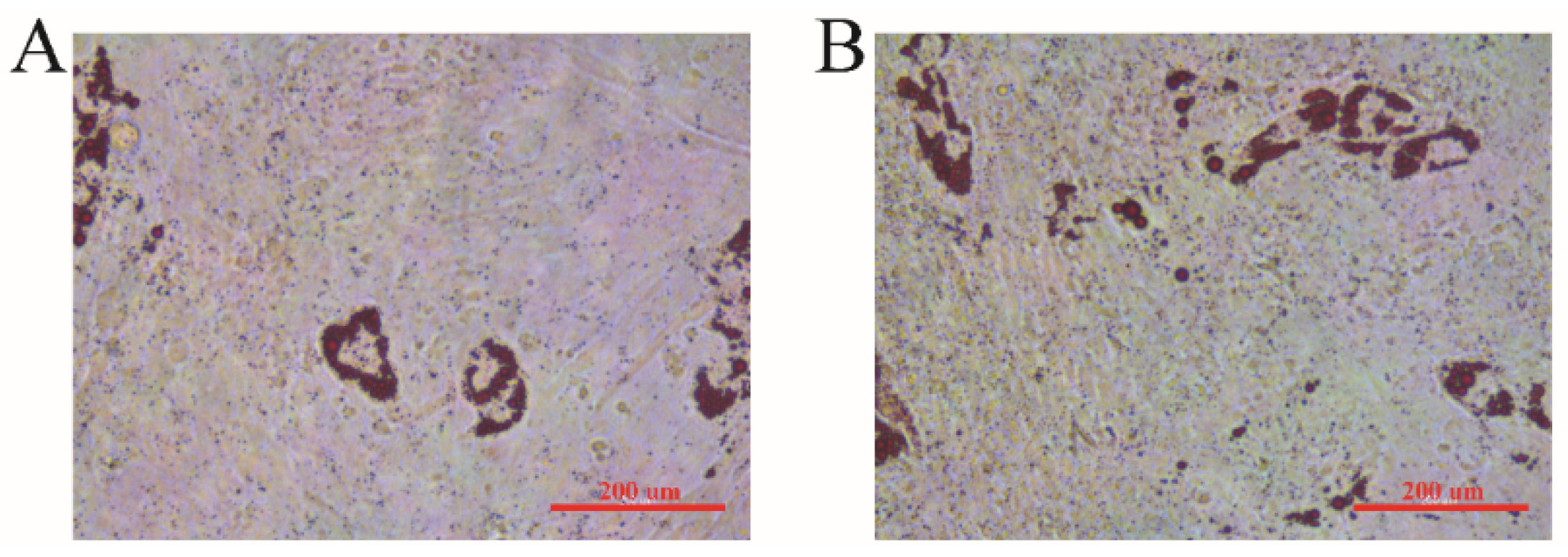
| Locus | Breed | Genotype | Genotype Frequency | Allele Frequency | He | Ne | PIC | Hardy–Weinberg Test (p-Value) |
|---|---|---|---|---|---|---|---|---|
| g.4122606 C > G | Hu sheep | CC (23) | 0.110577 | C (0.38) G (0.63) | 0.47 | 1.88 | 0.36 | 0.064457718 |
| CG (110) | 0.528846 | |||||||
| GG (75) | 0.360577 | |||||||
| Tibetan sheep | CC (49) | 0.286550 | C (0.52) G (0.48) | 0.5 | 2.0 | 0.37 | 0.50856548 | |
| CG (81) | 0.473684 | |||||||
| GG (41) | 0.239766 | |||||||
| Hybrid sheep | CC (24) | 0.155844 | C (0.39) G (0.61) | 0.48 | 1.91 | 0.36 | 0.832708982 | |
| CG (72) | 0.467532 | |||||||
| GG (58) | 0.376623 | |||||||
| g.3852134 C > T | Hu sheep | CC (187) | 0.899038 | C (0.94) T (0.06) | 0.10 | 1.12 | 0.10 | 0.070148151 |
| CT (19) | 0.091346 | |||||||
| TT (2) | 0.009615 | |||||||
| Tibetan sheep | CC (157) | 0.918129 | C (0.96) T (0.04) | 0.08 | 1.09 | 0.08 | 0.576740804 | |
| CT (14) | 0.081871 | |||||||
| TT (0) | 0.000000 | |||||||
| Hybrid sheep | CC (141) | 0.915584 | C (0.96) T (0.04) | 0.08 | 1.09 | 0.08 | 0.584470126 | |
| CT (13) | 0.084416 | |||||||
| TT (0) | 0.000000 |
| Locus | Genotype | Tail Length (cm) | Tail Width (cm) | Tail Circumference (cm) |
|---|---|---|---|---|
| g.4122606 C > G | CC | 19.940 ± 0.276 a | 9.897 ± 0.202 | 20.424 ± 0.414 |
| CG | 19.733 ± 0.166 a,b | 9.751 ± 0.121 | 20.230 ± 0.249 | |
| GG | 19.168 ± 0.204 b | 9.480 ± 0.149 | 19.611 ± 0.306 | |
| g.3852134 C > T | CC | 19.543 ± 0.123 | 9.657 ± 0.089 a | 20.026 ± 0.183 |
| CT | 20.060 ± 0.396 | 9.912 ± 0.288 a,b | 20.257 ± 0.590 | |
| TT | 19.581 ± 1.903 | 12.996 ± 1.382 b | 25.756 ± 2.837 |
| Group | Model ID | Melel Name | Score | Relative Score | Start | End | Strand | Predicted Site Sequence |
|---|---|---|---|---|---|---|---|---|
| Transcriptional factor binding sites before mutation | MA0484.1 | HNF4G | 5.173 | 0.81593069560891 | 884 | 898 | −1 | GAAGTTGAGGGGGCA |
| MA0155.1 | INSM1 | 9.035 | 0.833563663883174 | 884 | 895 | −1 | GTTGAGGGGGCA | |
| MA0504.1 | NR2C2 | 10.447 | 0.852923410523265 | 884 | 898 | −1 | GAAGTTGAGGGGGCA | |
| MA0528.1 | ZNF263 | 7.665 | 0.818705635466418 | 884 | 904 | −1 | GGTGAAGAAGTTGAGGGGGCA | |
| MA0503.1 | Nkx2-5 | 4.672 | 0.826755938995108 | 885 | 895 | −1 | GCCCCCTCAAC | |
| MA0528.1 | ZNF263 | 8.684 | 0.827686489190624 | 885 | 905 | −1 | AGGTGAAGAAGTTGAGGGGGC | |
| MA0027.1 | En1 | 4.660 | 0.810707218857438 | 887 | 897 | −1 | AAGTTGAGGGG | |
| MA0158.1 | HOXA5 | 4.749 | 0.82019043611391 | 890 | 897 | −1 | CTCAACTT | |
| MA0130.1 | ZNF354C | 4.636 | 0.812679270758179 | 890 | 895 | −1 | CTCAAC | |
| MA0468.1 | DUX4 | 0.491 | 0.811042995629078 | 892 | 902 | −1 | CAACTTCTTCA | |
| MA0109.1 | Hltf | 5.162 | 0.86314820468518 | 892 | 901 | −1 | CAACTTCTTC | |
| MA0080.3 | Spi1 | 8.605 | 0.859723524619004 | 892 | 906 | −1 | AAGGTGAAGAAGTTG | |
| MA0466.1 | CEBPB | −1.589 | 0.803283232723208 | 893 | 903 | 1 | AACTTCTTCAC | |
| MA0158.1 | HOXA5 | 4.377 | 0.807088931935232 | 893 | 900 | −1 | AAGAAGTT | |
| MA0598.1 | EHF | 6.510 | 0.869437914477032 | 894 | 901 | 1 | ACTTCTTC | |
| Transcriptional factor binding sites after mutation | MA0155.1 | INSM1 | 7.803 | 0.807735310463782 | 884 | 895 | −1 | ATTGAGGGGGCA |
| MA0528.1 | ZNF263 | 7.211 | 0.814704352256615 | 884 | 904 | −1 | GGTGAAGAAATTGAGGGGGCA | |
| MA0528.1 | ZNF263 | 7.080 | 0.813549796969205 | 885 | 905 | −1 | AGGTGAAGAAATTGAGGGGGC | |
| MA0158.1 | HOXA5 | 4.749 | 0.82019043611391 | 890 | 897 | 1 | CTCAATTT | |
| MA0063.1 | Nkx2-5 | 6.260 | 0.876914864771809 | 891 | 897 | 1 | TCAATTT | |
| MA0468.1 | DUX4 | 1.215 | 0.818924066022953 | 892 | 902 | 1 | CAATTTCTTCA | |
| MA0075.1 | Prrx2 | 4.766 | 0.819222411057098 | 892 | 896 | −1 | AATTG | |
| MA0080.3 | Spi1 | 4.762 | 0.817634453369339 | 892 | 906 | −1 | AAGGTGAAGAAATTG | |
| MA0466.1 | CEBPB | 8.714 | 0.911280728265531 | 893 | 903 | 1 | AATTTCTTCAC | |
| MA0158.1 | HOXA5 | 6.700 | 0.888902894857458 | 893 | 900 | −1 | AAGAAATT | |
| MA0102.3 | CEBPA | 8.325 | 0.897723669989696 | 894 | 904 | 1 | ATTTCTTCACC | |
| MA0488.1 | JUN | 3.746 | 0.828518034950643 | 894 | 906 | −1 | AAGGTGAAGAAAT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Lu, Z.; Jin, M.; Fei, X.; Quan, K.; Liu, Y.; Ma, L.; Chu, M.; Wang, H.; Wei, C. Verification and Analysis of Sheep Tail Type-Associated PDGF-D Gene Polymorphisms. Animals 2020, 10, 89. https://doi.org/10.3390/ani10010089
Li Q, Lu Z, Jin M, Fei X, Quan K, Liu Y, Ma L, Chu M, Wang H, Wei C. Verification and Analysis of Sheep Tail Type-Associated PDGF-D Gene Polymorphisms. Animals. 2020; 10(1):89. https://doi.org/10.3390/ani10010089
Chicago/Turabian StyleLi, Qing, Zengkui Lu, Meilin Jin, Xiaojuan Fei, Kai Quan, Yongbin Liu, Lin Ma, Mingxing Chu, Huihua Wang, and Caihong Wei. 2020. "Verification and Analysis of Sheep Tail Type-Associated PDGF-D Gene Polymorphisms" Animals 10, no. 1: 89. https://doi.org/10.3390/ani10010089
APA StyleLi, Q., Lu, Z., Jin, M., Fei, X., Quan, K., Liu, Y., Ma, L., Chu, M., Wang, H., & Wei, C. (2020). Verification and Analysis of Sheep Tail Type-Associated PDGF-D Gene Polymorphisms. Animals, 10(1), 89. https://doi.org/10.3390/ani10010089






