Atmospheric Ammonia Affects Myofiber Development and Lipid Metabolism in Growing Pig Muscle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Management of Animals
2.2. Assessment of Meat Quality
2.3. Measurement of Serum Ammonia and Urea
2.4. Profile of Free Amino Acids in Serum
2.5. Free Amino Acids in Muscle
2.6. Quantitative Real-Time PCR for Genes Expression
2.7. Western Blot for Protein Expression
2.8. RNA-Seq
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Meat Quality
3.2. Serum Ammonia, Urea and Free Amino Acids
3.3. Free Amino Acids in Muscle
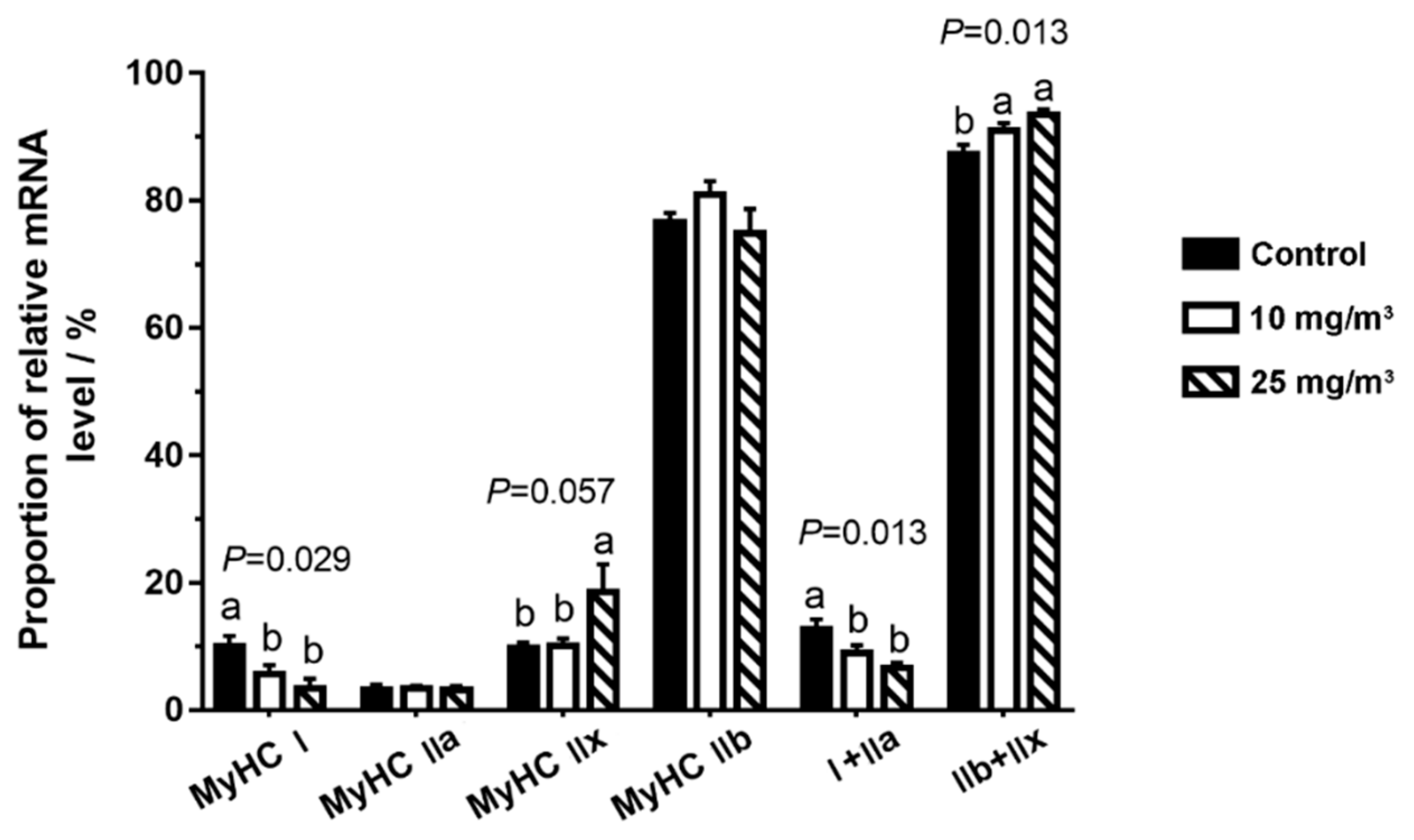
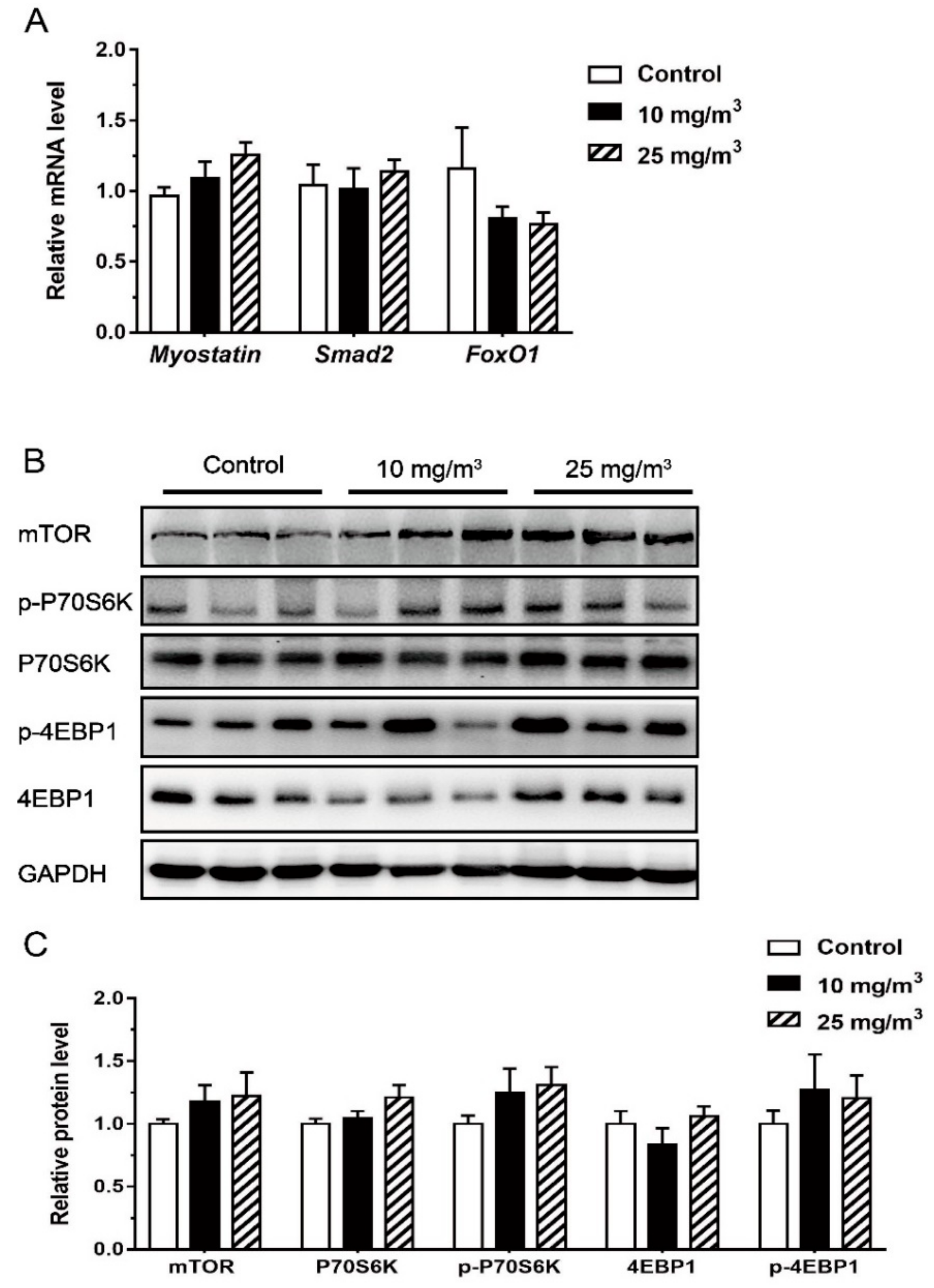
3.4. Expression of MSTN and Key Molecules Related to mTOR Pathway
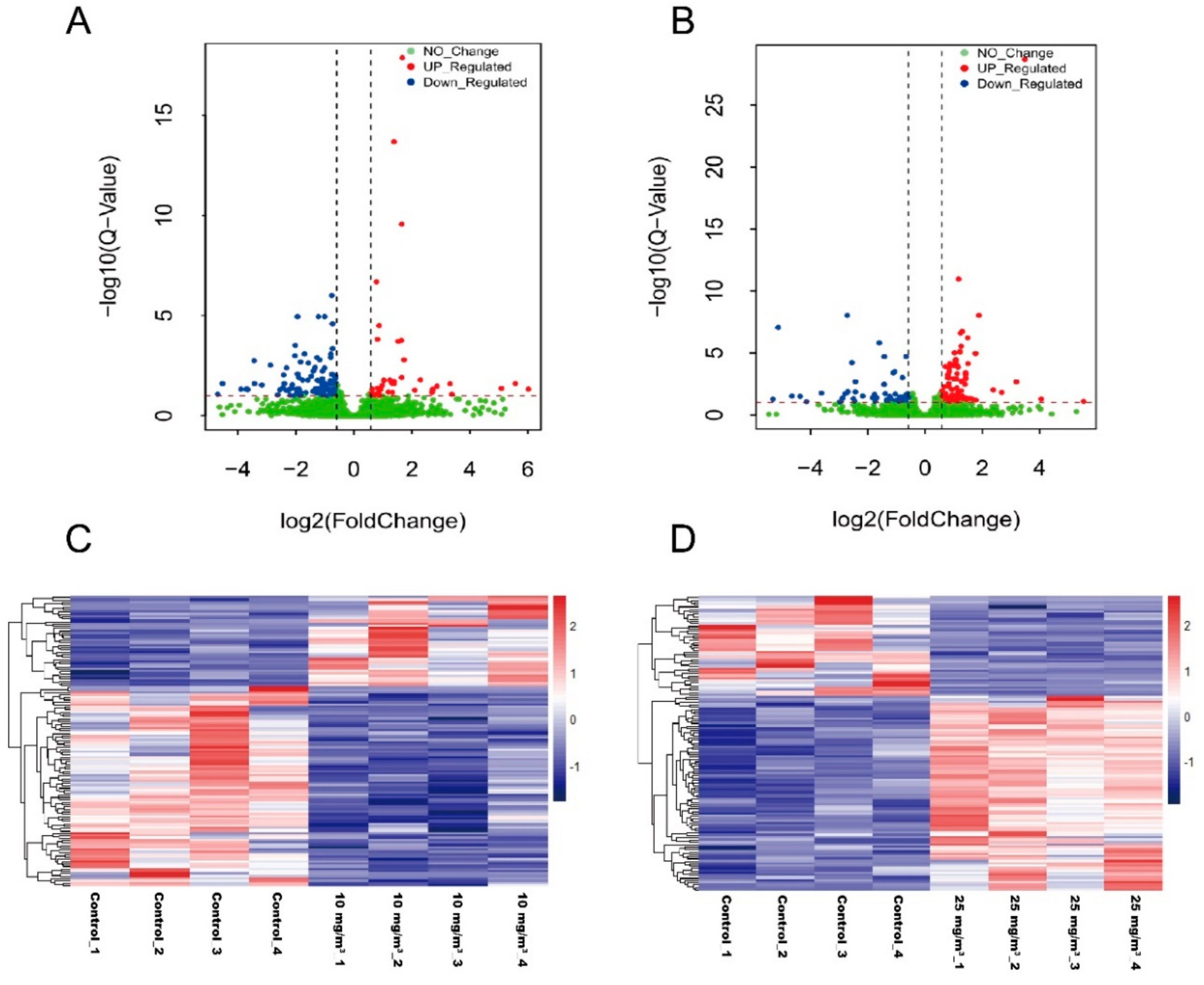
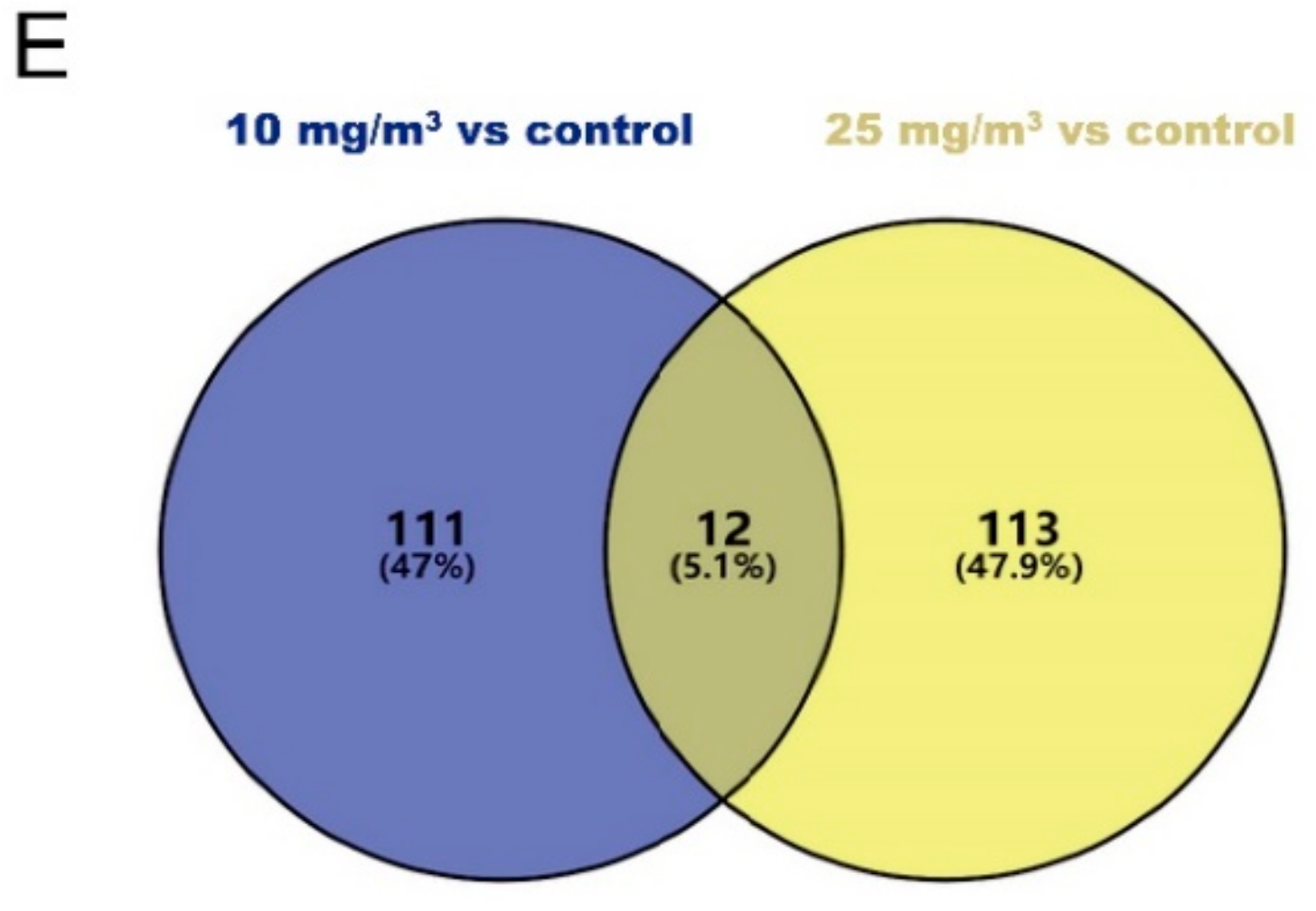
3.5. Differentially Expressed Genes (DEGs) Associated with Ammonia Exposure
3.6. GO Annotations and KEGG Pathway Analysis
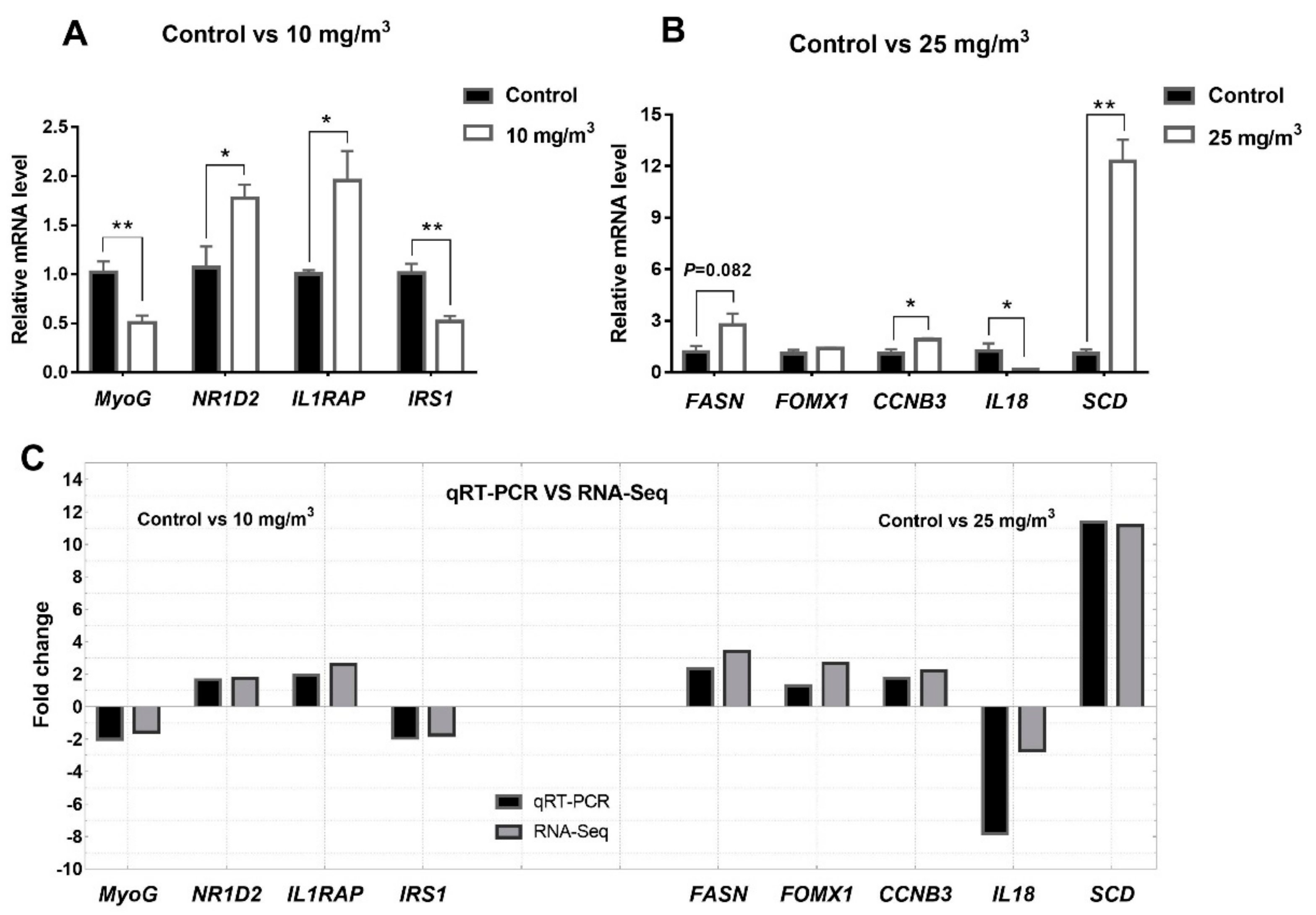
3.7. Verification of RNA-Seq by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Raw Data
References
- Yan, X.; Tang, X.; Meng, Q.; Zhang, H. Differential expression analysis of the broiler tracheal proteins responsible for the immune response and muscle contraction induced by high concentration of ammonia using iTRAQ-coupled 2D LC-MS/MS. Sci. China Life Sci. 2016, 59, 1166–1176. [Google Scholar]
- Heyden, C.V.D.; Demeyer, P.; Volcke, E.I.P. Mitigating emissions from pig and poultry housing facilities through air scrubbers and biofilters: State-of-the-art and perspectives. Biosyst. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Shepherd, T.A.; Li, H.; Xin, H. Environmental assessment of three egg production systems-Part I: Monitoring system and indoor air quality. Poult. Sci. 2015, 94, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; McLin, V.A.; Cudalbu, C. Ammonia toxicity to the brain. J. Inherit. Metab. Dis. 2013, 36, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, S.; Mccullough, A.J.; Muc, S.; Schneyer, A.; Bennett, C.D.; Dodig, M.; Kalhan, S.C. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J. Hepatol. 2011, 54, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, S. Consilience in sarcopenia of cirrhosis. J. Cachexia Sarcopenia Muscle 2012, 3, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Thrane, V.R.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef]
- Wei, F.X.; Hu, X.F.; Sa, R.N.; Liu, F.Z.; Li, S.Y.; Sun, Q.Y. Antioxidant capacity and meat quality of broilers exposed to different ambient humidity and ammonia concentrations. Genet. Mol. Res. 2014, 13, 3117–3127. [Google Scholar] [CrossRef]
- Sackett, B.A.M.; Fronting, G.W.; Deshazer, J.A.; Struwe, F.J. Effect of Gaseous Preslaughter Environment on Chicken Broiler Meat Quality. Poult. Sci. 1986, 65, 511–519. [Google Scholar] [CrossRef]
- Yi, B.; Chen, L.; Sa, R.; Zhong, R.; Xing, H.; Zhang, H. High concentrations of atmospheric ammonia induce alterations of gene expression in the breast muscle of broilers (Gallus gallus) based on RNA-Seq. BMC Genom. 2016, 17, 598. [Google Scholar] [CrossRef] [Green Version]
- Sa, R.N.; Xing, H.; Luan, S.J.; Sun, Y.B.; Sun, C.Y.; Zhang, H.F. Atmospheric ammonia alters lipid metabolism-related genes in the livers of broilers (Gallus gallus). J. Anim. Physiol. Anim. Nutr. 2018, 102, e941–e947. [Google Scholar] [CrossRef] [PubMed]
- Valerie, W. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar]
- Dimski, D.S. Ammonia metabolism and the urea cycle: Function and clinical implications. J. Vet. Intern. Med. 1994, 8, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D.; Sies, H.; Gerok, W. Functional hepatocyte heterogeneity in ammonia metabolism. The intercellular glutamine cycle. J. Hepatol. 1985, 1, 3–14. [Google Scholar] [CrossRef]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earnest, C.P.; Morss, G.M.; Wyatt, F.; Jordan, A.N.; Colson, S.; Church, T.S.; Fitzgerald, Y.; Autrey, L.; Jurca, R.; Lucia, A. Effects of a commercial herbal-based formula on exercise performance in cyclists. Med. Sci. Sports Exerc. 2004, 36, 504. [Google Scholar] [CrossRef]
- Zhaolai, D.; Zhenlong, W.; Junjun, W.; Xiaoqiu, W.; Sichao, J.; Bazer, F.W.; Guoyao, W. Analysis of polyamines in biological samples by HPLC involving pre-column derivatization with o-phthalaldehyde and N-acetyl-l-cysteine. Amino Acids 2014, 46, 1557–1564. [Google Scholar]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Ebrary, I. Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2008; pp. 58–115. [Google Scholar]
- Yoo, H.; Antoniewicz, M.; Stephanopoulos, G.; Kelleher, J. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J. Biol. Chem. 2008, 283, 20621–20627. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, S.; Zwingmann, C. Altered fatty acid metabolism and composition in cultured astrocytes under hyperammonemic conditions. J. Neurochem. 2010, 109, 258–264. [Google Scholar] [CrossRef]
- Stombaugh, D.P.; Teague, H.S.; Roller, W.L. Effects of atmospheric ammonia on the pig. J. Anim. Sci. 1969, 28, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.G.; Curtis, S.E.; Simon, J.; Norton, H.W. Effects of aerial ammonia on growth and health of young pigs. J. Anim. Sci. 1980, 50, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Colina, J.; Lewis, A.; Miller, P.S. A Review of the Ammonia Issue and Pork Production. In Nebraska Swine Reports; Animal Science Department: Lincoin, NE, USA, 2000; p. 108. [Google Scholar]
- Chang, K.C.; Da, C.N.; Blackley, R.; Southwood, O.; Evans, G.; Plastow, G.; Wood, J.D.; Richardson, R.I. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci. 2003, 64, 93–103. [Google Scholar] [CrossRef]
- Min, W.; Hui, Y.; Yong, S.K.; Bidwell, C.A.; Kuang, S. Myostatin facilitates slow and inhibits fast myosin heavy chain expression during myogenic differentiation. Biochem. Biophys. Res. Commun. 2012, 426, 83–88. [Google Scholar]
- Hennebry, A.; Berry, C.; Siriett, V.; O’Callaghan, P.; Chau, L.; Watson, T.; Sharma, M.; Kambadur, R. Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am. J. Physiol. Cell Physiol. 2009, 296, C525–C534. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Miyake, M.; Watanabe, K.; Aso, H.; Hayashi, S.; Ohwada, S.; Yamaguchi, T. Myostatin preferentially down-regulates the expression of fast 2x myosin heavy chain in cattle. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 354–362. [Google Scholar] [CrossRef]
- Wyzykowski, J.C.; Winata, T.I.; Mitin, N.; Taparowsky, E.J.; Konieczny, S.F. Identification of Novel MyoD Gene Targets in Proliferating Myogenic Stem Cells. Mol. Cell. Biol. 2002, 22, 6199–6208. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.L.; Sartorius, C.A.; Sycuro, L.K.; Leinwand, L.A. Different pathways regulate expression of the skeletal myosin heavy chain genes. J. Biol. Chem. 2001, 276, 43524–43533. [Google Scholar] [CrossRef] [Green Version]
- Talmadge, R.J. Myosin heavy chain isoform expression following reduced neuromuscular activity: Potential regulatory mechanisms. Muscle Nerve 2000, 23, 661–679. [Google Scholar] [CrossRef]
- Yan-Hong, S.U.; Wang, R.Y.; Lin, H. The effect of endurance training on heavy chain myosin as well as the regulating function of MyoD and Myogenin. J. Phys. Educ. 2007, 14, 48–52. (In Chinese) [Google Scholar]
- Zhu, L.N.; Ren, Y.; Chen, J.Q.; Wang, Y.Z. Effects of myogenin on muscle fiber types and key metabolic enzymes in gene transfer mice and C2C12 myoblasts. Gene 2013, 532, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Alapat, D.; Chaudhry, T.; Ardakany-Taghavi, R.; Kohtz, D. Fiber-types of sarcomeric proteins expressed in cultured myogenic cells are modulated by the dose of myogenin activity. Cell. Signal. 2009, 21, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kraus, B.; Pette, D. Quantification of MyoD, myogenin, MRF4 and Id-1 by reverse-transcriptase polymerase chain reaction in rat muscles-effects of hypothyroidism and chronic low-frequency stimulation. Eur. J. Biochem. 1997, 247, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Hughes, K.K.; Michael, R.; Alison, M.M. MyoD protein is differentially accumulated in fast and slow skeletal muscle fibres and required for normal fibre type balance in rodents. Mech. Dev. 1997, 61, 151–163. [Google Scholar]
- Hughes, S.M.; Chi, M.M.Y.; Lowry, O.H.; Gundersen, K. Myogenin Induces a Shift of Enzyme Activity from Glycolytic to Oxidative Metabolism in Muscles of Transgenic Mice. J. Cell Biol. 1999, 145, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.M.; Taylor, J.M.; Tapscott, S.J.; Gurley, C.M.; Carter, W.J.; Peterson, C.A. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development 1993, 118, 1137–1147. [Google Scholar] [PubMed]
- Thupari, J.N.; Landree, L.E.; Ronnett, G.V.; Kuhajda, F.P. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc. Natl. Acad. Sci. USA 2002, 99, 9498–9502. [Google Scholar] [CrossRef] [Green Version]
- Dridi, S.; Ververken, C.; Hillgartner, F.B.; Arckens, L.; Lutgarde, A.; Van, G.E.; Cnops, L.; Decuypere, E.; Buyse, J. FAS inhibitor cerulenin reduces food intake and melanocortin receptor gene expression without modulating the other (an)orexigenic neuropeptides in chickens. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 138–147. [Google Scholar] [CrossRef]
- Mobbs, C.V.; Makimura, H. Block the FAS, lose the fat. Nat. Med. 2002, 8, 335–336. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Dobrzyn, P.; Lee, SH.; Miyazaki, M. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 11110–11115. [Google Scholar] [CrossRef] [Green Version]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metab. Clin. Exp. 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Holz, B.; Jenke, B.; Binczek, E.; Günter, R.H.; Kiss, C.; Karakesisoglou, I.; Thevis, M.; Weber, A.-A.; Arnhold, S. Δ6-Desaturase (FADS2) deficiency unveils the role of ω3- and ω6-polyunsaturated fatty acids. EMBO J. 2008, 27, 2281–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjögren, P.; Sierra-Johnson, J.; Gertow, K.; Rosell, M.; Vessby, B.; De, F.U.; Hamsten, A.; Hellenius, M.L.; Fisher, R.M. Fatty acid desaturases in human adipose tissue: Relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008, 51, 328–335. [Google Scholar] [CrossRef] [Green Version]
| Items | Ammonia Concentration, mg/m3 | SEM | p Value | ||
|---|---|---|---|---|---|
| 0 | 10 | 25 | |||
| Initial body weight, kg | 20.33 | 21.20 | 20.72 | 0.565 | 0.566 |
| End body weight, kg | 30.55 | 32.70 | 31.87 | 1.003 | 0.338 |
| Body weight gain, kg | 10.22 | 11.25 | 11.10 | 0.441 | 0.478 |
| Average daily feed intake, g/d | 1028.02 | 1104.04 | 1048.24 | 34.980 | 0.310 |
| pH45 min | 7.01 | 7.13 | 7.00 | 0.061 | 0.283 |
| Drip loss, % | 3.84 | 3.87 | 3.37 | 0.276 | 0.384 |
| Meat color | 85.36 a | 87.54 a | 77.82 b | 1.444 | 0.001 |
| Fat content, g/100 g freeze-dried muscle | 5.81 b | 9.05 a | 8.84 a | 0.683 | 0.007 |
| Items | Ammonia Concentration, mg/m3 | SEM | p Value | ||
|---|---|---|---|---|---|
| 0 | 10 | 25 | |||
| Serum ammonia and urea | |||||
| Serum ammonia, umol/L | 135.70 | 135.50 | 112.97 | 7.085 | 0.067 |
| Serum urea, mmol/L | 2.94 | 3.09 | 3.15 | 0.167 | 0.660 |
| Serum-free amino acid, nmol/mL | |||||
| Glutamic acid | 240.93 | 234.74 | 177.90 | 23.611 | 0.157 |
| Glutamine | 512.90 | 470.88 | 517.98 | 29.658 | 0.527 |
| Alanine | 490.18 | 484.06 | 479.92 | 38.717 | 0.982 |
| b-Alanine | 111.35 | 107.82 | 104.08 | 5.833 | 0.685 |
| Citrulline | 65.17 | 62.22 | 64.43 | 4.840 | 0.915 |
| Arginine | 153.23 | 195.36 | 152.37 | 32.177 | 0.609 |
| Ornithine | 105.22 | 101.02 | 101.43 | 10.606 | 0.956 |
| Isoleucine | 82.48 | 75.88 | 83.70 | 3.586 | 0.325 |
| Leucine | 146.57 | 147.76 | 159.48 | 6.494 | 0.335 |
| Aspartic acid | 42.63 | 43.68 | 32.45 | 3.513 | 0.082 |
| Asparagine | 39.57 | 36.80 | 44.62 | 3.134 | 0.260 |
| Serine | 135.53 | 122.86 | 114.68 | 7.156 | 0.153 |
| Histidine | 48.92 | 51.22 | 47.13 | 3.699 | 0.762 |
| Glycine | 1383.90 | 838.80 | 822.43 | 259.720 | 0.263 |
| Threonine | 95.05 | 107.44 | 101.87 | 10.615 | 0.736 |
| Taurine | 80.00 b | 112.40 a | 76.00 b | 9.508 | 0.045 |
| Tyrosine | 59.15 b | 57.64 b | 77.17 a | 4.358 | 0.013 |
| Tryptophan | 42.78 | 36.08 | 43.10 | 2.913 | 0.230 |
| Methionine | 26.42 | 22.06 | 28.12 | 2.756 | 0.220 |
| Valine | 187.08 ab | 155.96 b | 198.23 a | 10.073 | 0.037 |
| Phenylalanine | 67.88 | 67.34 | 73.45 | 2.851 | 0.290 |
| Lysine | 100.20 | 105.64 | 105.18 | 5.919 | 0.782 |
| Items | Ammonia Concentration, mg/m3 | SEM | p Value | ||
|---|---|---|---|---|---|
| 0 | 10 | 25 | |||
| Glutamic acid | 267.54 | 258.35 | 282.51 | 35.637 | 0.891 |
| Glutamine | 5182.77 a | 2828.16 b | 2336.01 b | 577.525 | 0.008 |
| Alanine | 1281.96 | 1336.08 | 1111.67 | 149.186 | 0.557 |
| Citrulline | 121.24 | 118.13 | 105.61 | 9.348 | 0.493 |
| Arginine | 73.17 | 72.39 | 69.76 | 8.509 | 0.959 |
| Ornithine | 73.52 | 84.44 | 67.51 | 12.788 | 0.647 |
| Isoleucine | 51.56 a | 44.03 a,b | 34.45 b | 3.567 | 0.019 |
| Leucine | ND | ND | ND | ||
| Category | Term | Enriched Genes | p Value |
|---|---|---|---|
| 0 ppm VS 10 mg/m3 | |||
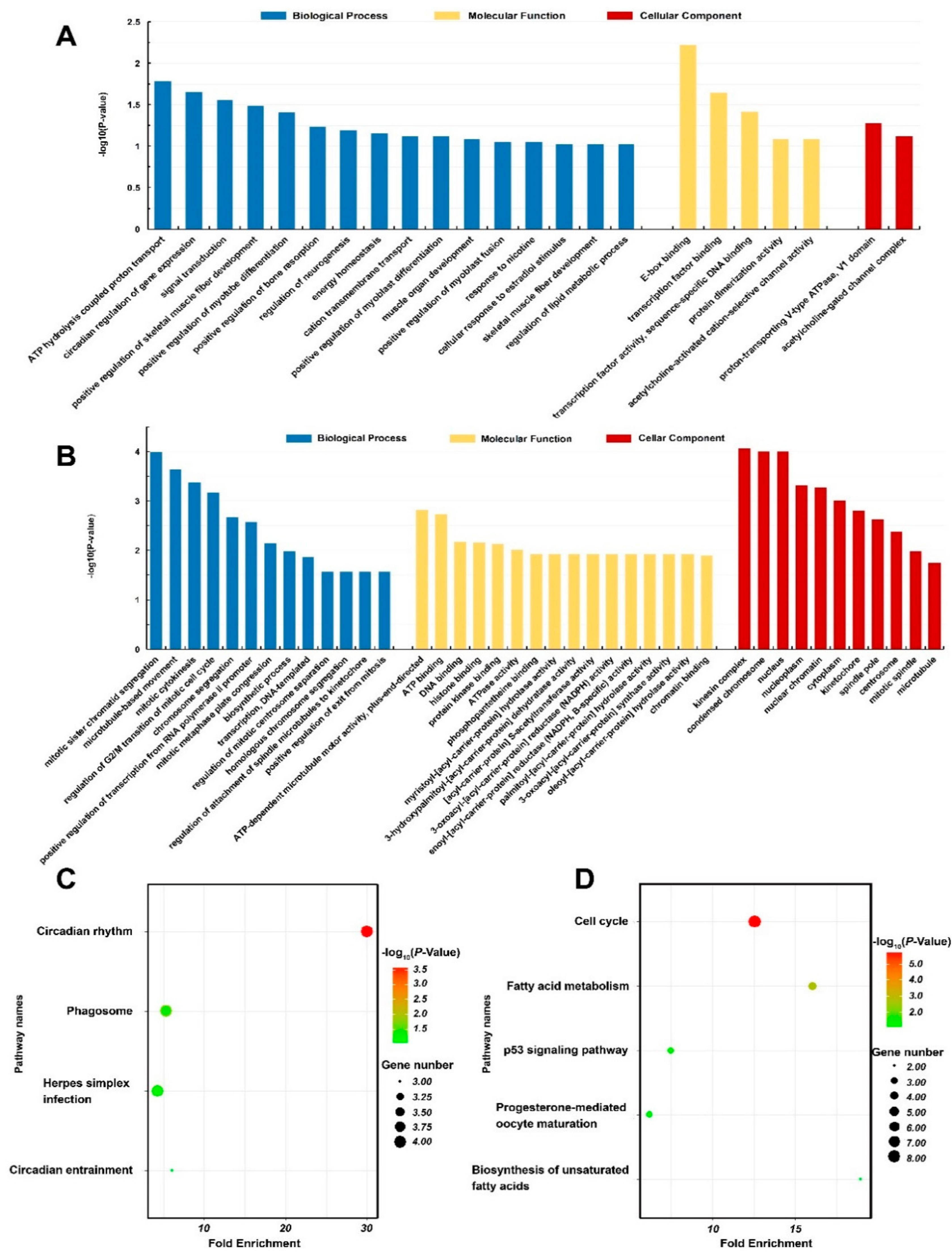
| GO term | ATP hydrolysis coupled proton transport | ATP6V1B2↑, ATP1A1↓, ATP6V1C2↓ | 0.017 |
| GO term | Signal transduction | CHRNG↓, LVRN↓, ARHGAP20↓, RREB1↓, IL1RAP↑ | 0.027 |
| GO term | Positive regulation of skeletal muscle fiber development | MYOD1↓, MYOG↓ | 0.033 |
| GO term | Positive regulation of myotube differentiation | MAMSTR↓, MYOG↓ | 0.039 |
| GO term | Energy homeostasis | MRAP2↓, AMPD3↓ | 0.070 |
| GO term | Regulation of lipid metabolic process | NR1D2↑, IRS1↓ | 0.095 |
| 0 ppm VS 25 mg/m3 | |||
| GO term | ATPase activity | ABCA1↓, ATAD2↑, KIF20A↑, KIF22↑ | 0.010 |
| KEGG pathway | Cell cycle | CCNA2↑, PTTG1↑, MCM3↑, CCNB1↑, E2F1↑, CCNB3↑, ESPL1↑, MCM2↑ | 0.000 |
| KEGG pathway | Fatty acid metabolism | FADS1↑, ACADL↓, FASN↑, SCD↑ | 0.002 |
| KEGG pathway | p53 signaling pathway | GTSE1↑, CCNB1↑, CCNB3↑ | 0.057 |
| KEGG pathway | Biosynthesis of unsaturated fatty acids | FADS1↑, SCD↑ | 0.098 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Xie, J.; Zhang, S.; Wu, W.; Yi, B.; Zhang, H. Atmospheric Ammonia Affects Myofiber Development and Lipid Metabolism in Growing Pig Muscle. Animals 2020, 10, 2. https://doi.org/10.3390/ani10010002
Tang S, Xie J, Zhang S, Wu W, Yi B, Zhang H. Atmospheric Ammonia Affects Myofiber Development and Lipid Metabolism in Growing Pig Muscle. Animals. 2020; 10(1):2. https://doi.org/10.3390/ani10010002
Chicago/Turabian StyleTang, Shanlong, Jingjing Xie, Sheng Zhang, Weida Wu, Bao Yi, and Hongfu Zhang. 2020. "Atmospheric Ammonia Affects Myofiber Development and Lipid Metabolism in Growing Pig Muscle" Animals 10, no. 1: 2. https://doi.org/10.3390/ani10010002
APA StyleTang, S., Xie, J., Zhang, S., Wu, W., Yi, B., & Zhang, H. (2020). Atmospheric Ammonia Affects Myofiber Development and Lipid Metabolism in Growing Pig Muscle. Animals, 10(1), 2. https://doi.org/10.3390/ani10010002







