β-Sitosterol Attenuates High Grain Diet-Induced Inflammatory Stress and Modifies Rumen Fermentation and Microbiota in Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Diets and Experimental Design
2.2. Rumen Fluid Sampling and Analysis
2.3. Blood Sampling and Analysis
2.4. DNA Extraction, Illumina MiSeq Sequencing and Sequencing Data Processing
2.5. Statistical Analysis
3. Results
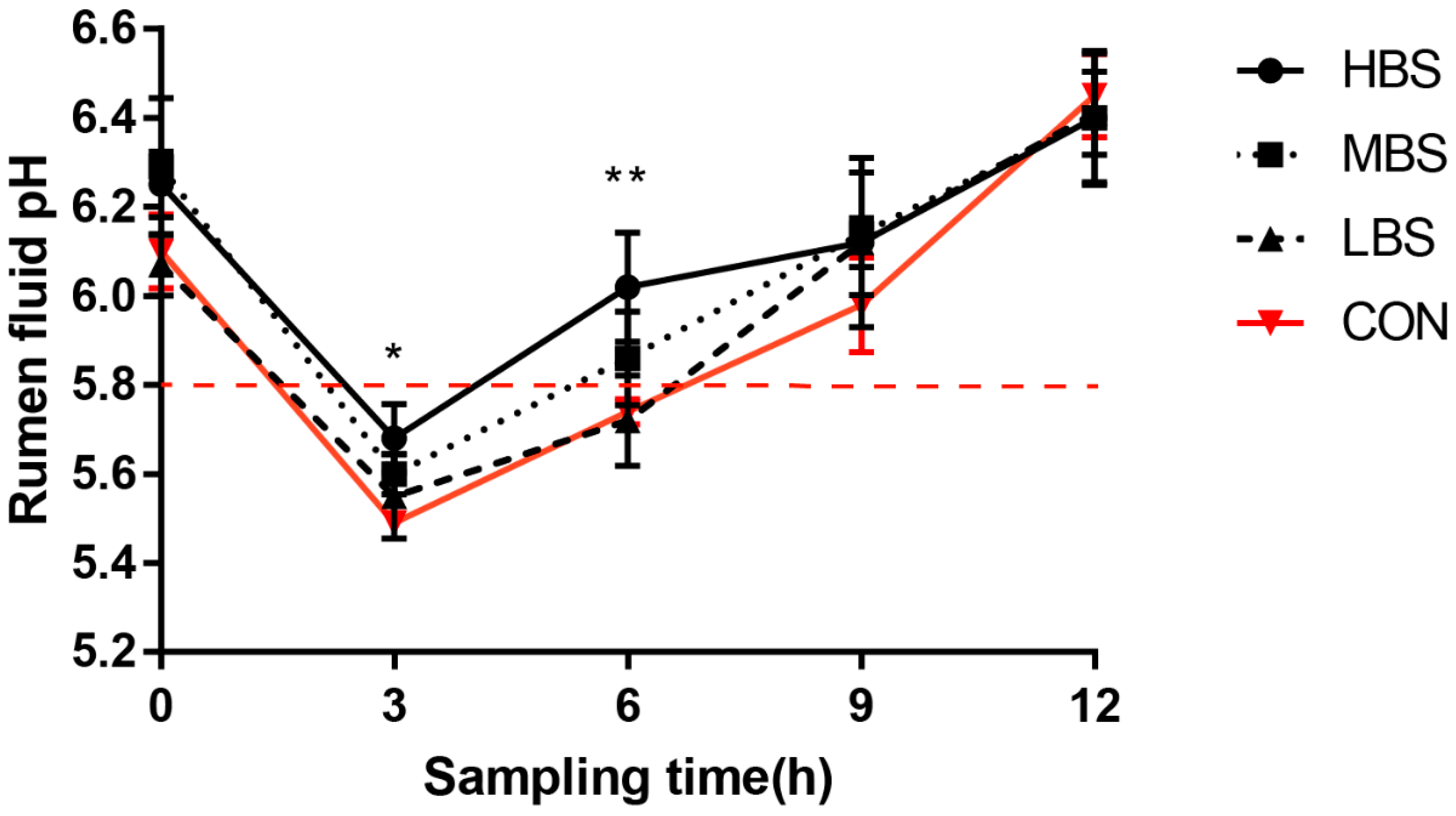
3.1. Induction of SARA Model
3.2. Blood Parameters
3.3. Ruminal Fermentation Characteristics
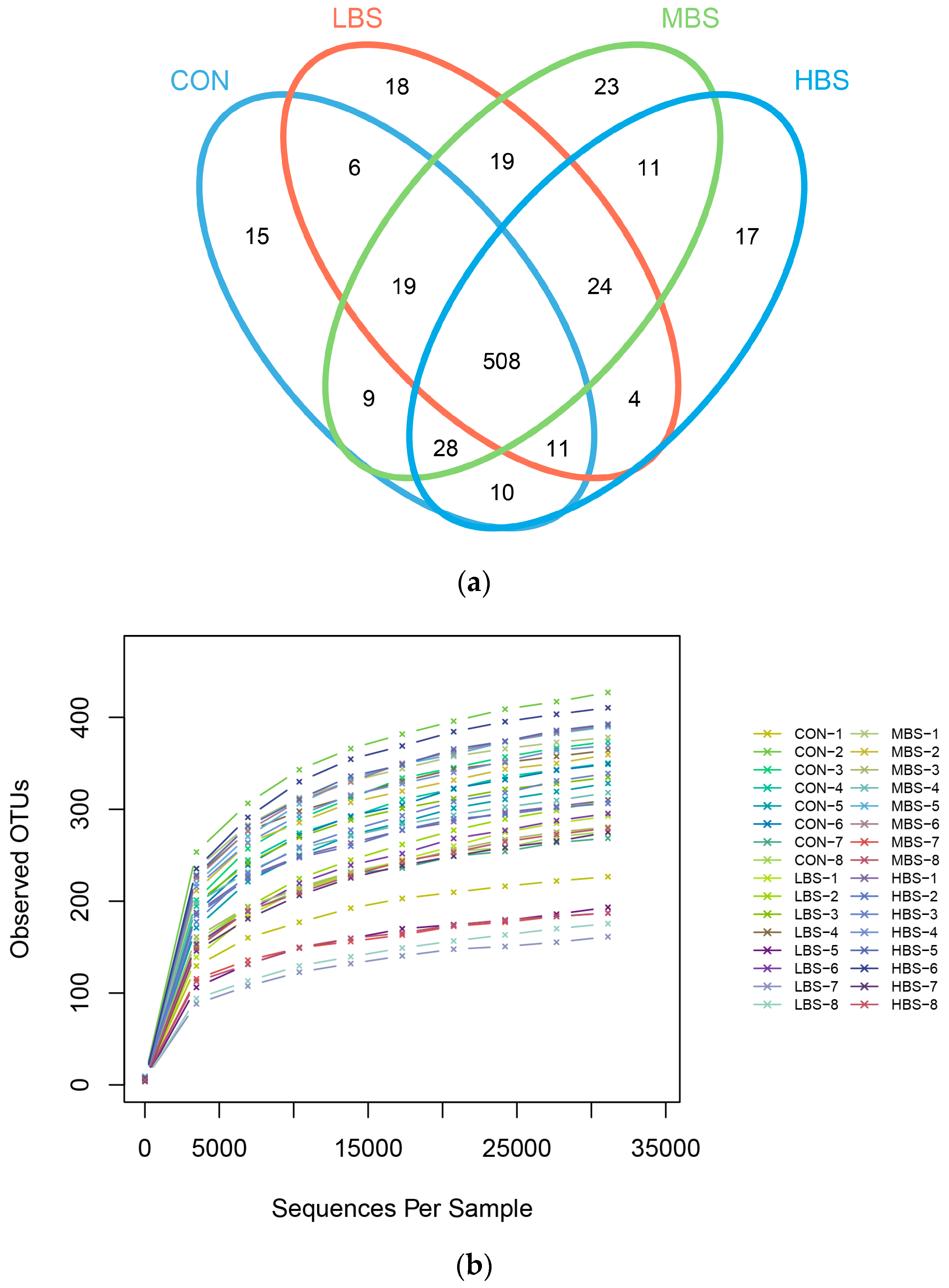
3.4. Diversity and Structure of the Ruminal Microbiota Composition
3.5. Effect of BSS Supplementation on Relative Abundance of Bacterial Communities
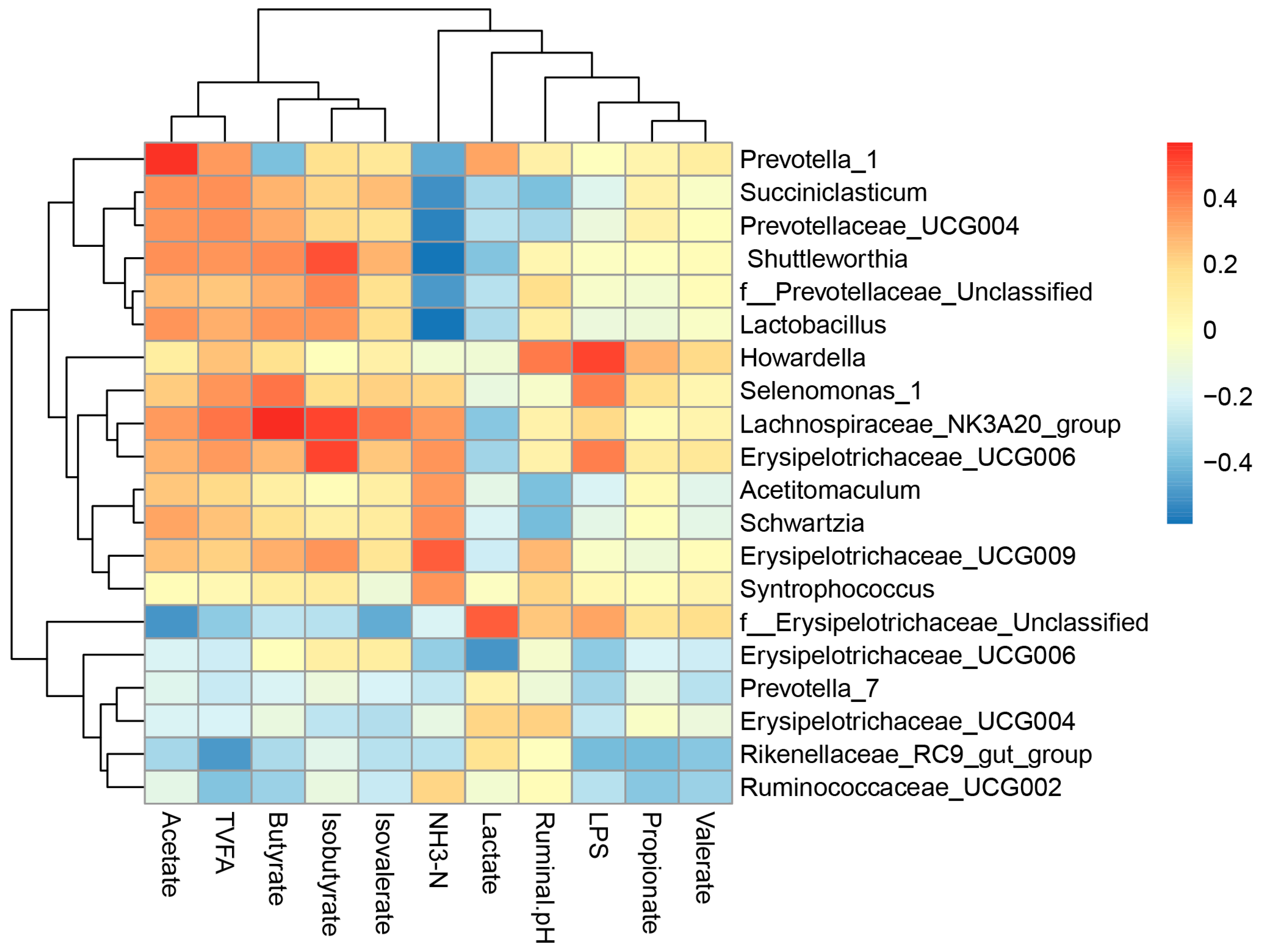
3.6. Correlations between Bacterial Communities and Ruminal Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90 (Suppl. 1), E17–E38. [Google Scholar] [CrossRef]
- Fernando, S.C.; Purvis, H.; Najar, F.; Sukharnikov, L.; Krehbiel, C.; Nagaraja, T.; Roe, B.; DeSilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.; Li, S.; Le Sciellour, M.; Schurmann, B.; Górka, P.; Penner, G. Effects of duration of moderate increases in grain feeding on endotoxins in the digestive tract and acute phase proteins in peripheral blood of yearling calves. J. Dairy Sci. 2014, 97, 7076–7084. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.; Khafipour, E.; Li, S.; Gozho, G.; Krause, D. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 2012, 172, 9–21. [Google Scholar] [CrossRef]
- Dong, H.; Wang, S.; Jia, Y.; Ni, Y.; Zhang, Y.; Zhuang, S.; Shen, X.; Zhao, R. Long-term effects of subacute ruminal acidosis (SARA) on milk quality and hepatic gene expression in lactating goats fed a high-concentrate diet. PLoS ONE 2013, 8, e82850. [Google Scholar] [CrossRef]
- Mao, S.; Huo, W.; Liu, J.; Zhang, R.; Zhu, W. In vitro effects of sodium bicarbonate buffer on rumen fermentation, levels of lipopolysaccharide and biogenic amine, and composition of rumen microbiota. J. Sci. Food Agric. 2017, 97, 1276–1285. [Google Scholar] [CrossRef]
- Vyas, D.; Beauchemin, K.; Koenig, K. Using organic acids to control subacute ruminal acidosis and fermentation in feedlot cattle fed a high-grain diet. J. Anim. Sci. 2015, 93, 3950–3958. [Google Scholar] [CrossRef]
- Mutsvangwa, T.; Walton, J.; Plaizier, J.; Duffield, T.; Bagg, R.; Dick, P.; Vessie, G.; McBride, B. Effects of a monensin controlled-release capsule or premix on attenuation of subacute ruminal acidosis in dairy cows. J. Dairy Sci. 2002, 85, 3454–3461. [Google Scholar] [CrossRef]
- Henning, P.; Horn, C.; Leeuw, K.J.; Meissner, H.; Hagg, F. Effect of ruminal administration of the lactate-utilizing strain Megasphaera elsdenii (Me) NCIMB 41125 on abrupt or gradual transition from forage to concentrate diets. Anim. Feed Sci. Technol. 2010, 157, 20–29. [Google Scholar] [CrossRef]
- Lammie, S.L.; Hughes, J.M. Antimicrobial resistance, food safety, and one health: The need for convergence. Annu. Rev. Food Sci. Technol. 2016, 7, 287–312. [Google Scholar] [CrossRef]
- Mickdam, E.; Khiaosa-ard, R.; Metzler-Zebeli, B.U.; Klevenhusen, F.; Chizzola, R.; Zebeli, Q. Rumen microbial abundance and fermentation profile during severe subacute ruminal acidosis and its modulation by plant derived alkaloids in vitro. Anaerobe 2016, 39, 4–13. [Google Scholar] [CrossRef]
- Hutton, P.; Durmic, Z.; Ghisalberti, E.; Flematti, G.; Duncan, R.; Carson, C.; Riley, T.; Vercoe, P. Inhibition of ruminal bacteria involved in lactic acid metabolism by extracts from Australian plants. Anim. Feed Sci. Technol. 2012, 176, 170–177. [Google Scholar] [CrossRef]
- Agarwal, N.; Shekhar, C.; Kumar, R.; Chaudhary, L.; Kamra, D. Effect of peppermint (Mentha piperita) oil on in vitro methanogenesis and fermentation of feed with buffalo rumen liquor. Anim. Feed Sci. Technol. 2009, 148, 321–327. [Google Scholar] [CrossRef]
- Klingberg, S.; Andersson, H.; Mulligan, A.; Bhaniani, A.; Welch, A.; Bingham, S.; Khaw, K.; Andersson, S.; Ellegard, L. Food sources of plant sterols in the EPIC Norfolk population. Eur. J. Clin. Nutr. 2008, 62, 695. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fiorito, A.; Panourgia, M.P.; Sangiorgi, Z.; Gaddi, A. Effects of a new soy/β-sitosterol supplement on plasma lipids in moderately hypercholesterolemic subjects. J. Am. Diet. Assoc. 2002, 102, 1807–1811. [Google Scholar] [CrossRef]
- Awad, A.; Downie, A.C.; Fink, C.S. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int. J. Mol. Med. 2000, 5, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Li, J.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary β-Sitosterol Improves Growth Performance, Meat Quality, Antioxidant Status, and Mitochondrial Biogenesis of Breast Muscle in Broilers. Animals 2019, 9, 71. [Google Scholar] [CrossRef]
- Ding, K.; Tan, Y.Y.; Ding, Y.; Fang, Y.; Yang, X.; Fang, J.; Xu, D.C.; Zhang, H.; Lu, W.; Li, M. β-Sitosterol improves experimental colitis in mice with a target against pathogenic bacteria. J. Cell Biochem. 2019, 120, 5687–5694. [Google Scholar] [CrossRef]
- Kim, K.A.; Lee, I.A.; Gu, W.; Hyam, S.R.; Kim, D.H. β-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway. Mol. Nutr. Food Res. 2014, 58, 963–972. [Google Scholar] [CrossRef]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K.P. Identification of β-sitosterol as in vitro anti-inflammatory constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Ministry of Agriculture (MOA), P.R.C. Feeding Standard of Meat-Producing Sheep and Goats (NY/T 816-2004); China Agricultural Press: Beijing, China, 2004. [Google Scholar]
- Pan, X.H.; Yang, L.; Xue, F.G.; Xin, H.R.; Jiang, L.S.; Xiong, B.H.; Beckers, Y. Relationship between thiamine and subacute ruminal acidosis induced by a high-grain diet in dairy cows. J. Dairy Sci. 2016, 99, 8790–8801. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.B.; Summerson, W.H. The colorimetric determination of lactic acid in biological material. J. Biol. Chem. 1941, 138, 535–554. [Google Scholar]
- Bhandari, S.; Ominski, K.; Wittenberg, K.; Plaizier, J. Effects of chop length of alfalfa and corn silage on milk production and rumen fermentation of dairy cows. J. Dairy Sci. 2007, 90, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Plaizier, J.; Krause, D.; Gozho, G.; McBride, B. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Pan, X.H.; Yang, L.; Beckers, Y.; Xue, F.G.; Tang, Z.W.; Jiang, L.S.; Xiong, B.H. Thiamine supplementation facilitates thiamine transporter expression in the rumen epithelium and attenuates high-grain-induced inflammation in low-yielding dairy cows. J. Dairy Sci. 2017, 100, 5329–5342. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef]
- Fan, W.J.; Li, H.P.; Zhu, H.S.; Sui, S.P.; Chen, P.G.; Deng, Y.; Sui, T.M.; Wang, Y.Y. NF-κB is involved in the LPS-mediated proliferation and apoptosis of MAC-T epithelial cells as part of the subacute ruminal acidosis response in cows. Biotechnol. Lett. 2016, 38, 1839–1849. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, W.; Mao, S. Impact of subacute ruminal acidosis on the diversity of liquid and solid-associated bacteria in the rumen of goats. J. Microbiol. Biotechnol. 2014, 30, 669–680. [Google Scholar] [CrossRef]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Manubolu, M.; Madawala, S.R.; Dutta, P.C.; Malmlöf, K. In vitro biodegradation of cyanotoxins in the rumen fluid of cattle. BMC Vet. Res. 2014, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Tchinda, C.F.; Sonfack, G.; Simo, I.K.; Çelik, İ.; Voukeng, I.K.; Nganou, B.K.; Bitchagno, G.T.; Ekti, S.F.; Tene, M.; Tane, P. Antibacterial and antibiotic-modifying activities of fractions and compounds from Albizia adianthifolia against MDR Gram-negative enteric bacteria. BMC Complem. Altern. Med. 2019, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Wang, J.; Dong, Y.; Meng, S.; Li, R.; Niu, X.; Deng, X. β-sitosterol interacts with pneumolysin to prevent Streptococcus pneumoniae infection. Sci. Rep. 2015, 5, 17668. [Google Scholar] [CrossRef]
- Pan, X.; Xue, F.; Nan, X.; Tang, Z.; Wang, K.; Beckers, Y.; Jiang, L.; Xiong, B. Illumina Sequencing Approach to Characterize Thiamine Metabolism Related Bacteria and the Impacts of Thiamine Supplementation on Ruminal Microbiota in Dairy Cows Fed High-Grain Diets. Front. Microbiol. 2017, 8, 1818. [Google Scholar] [CrossRef]
- Therion, J.J.; Kistner, A.; Kornelius, J. Effect of pH on growth rates of rumen amylolytic and lactilytic bacteria. Appl. Environ. Microbiol. 1982, 44, 428–434. [Google Scholar] [CrossRef]
- Mao, S.Y.; Huo, W.J.; Zhu, W.Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 2016, 18, 525–541. [Google Scholar] [CrossRef]
- Strobel, H.J. Vitamin B12-dependent propionate production by the ruminal bacterium Prevotella ruminicola 23. Appl. Environ. Microbiol. 1992, 58, 2331–2333. [Google Scholar] [CrossRef]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, gen. nov., sp. nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio 2014, 5, e00889-14. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Zhu, L.; Zhai, S.; Qin, S.; Du, Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. MicrobiologyOpen 2019, e825. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zuo, Q.; Hai, Y.; Sun, X.J. Lactulose: An indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med. Hypotheses 2011, 76, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Bian, G.R.; Zhu, W.Y.; Mao, S.Y. High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of Toll-like receptor genes in goats. Front. Microbiol. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
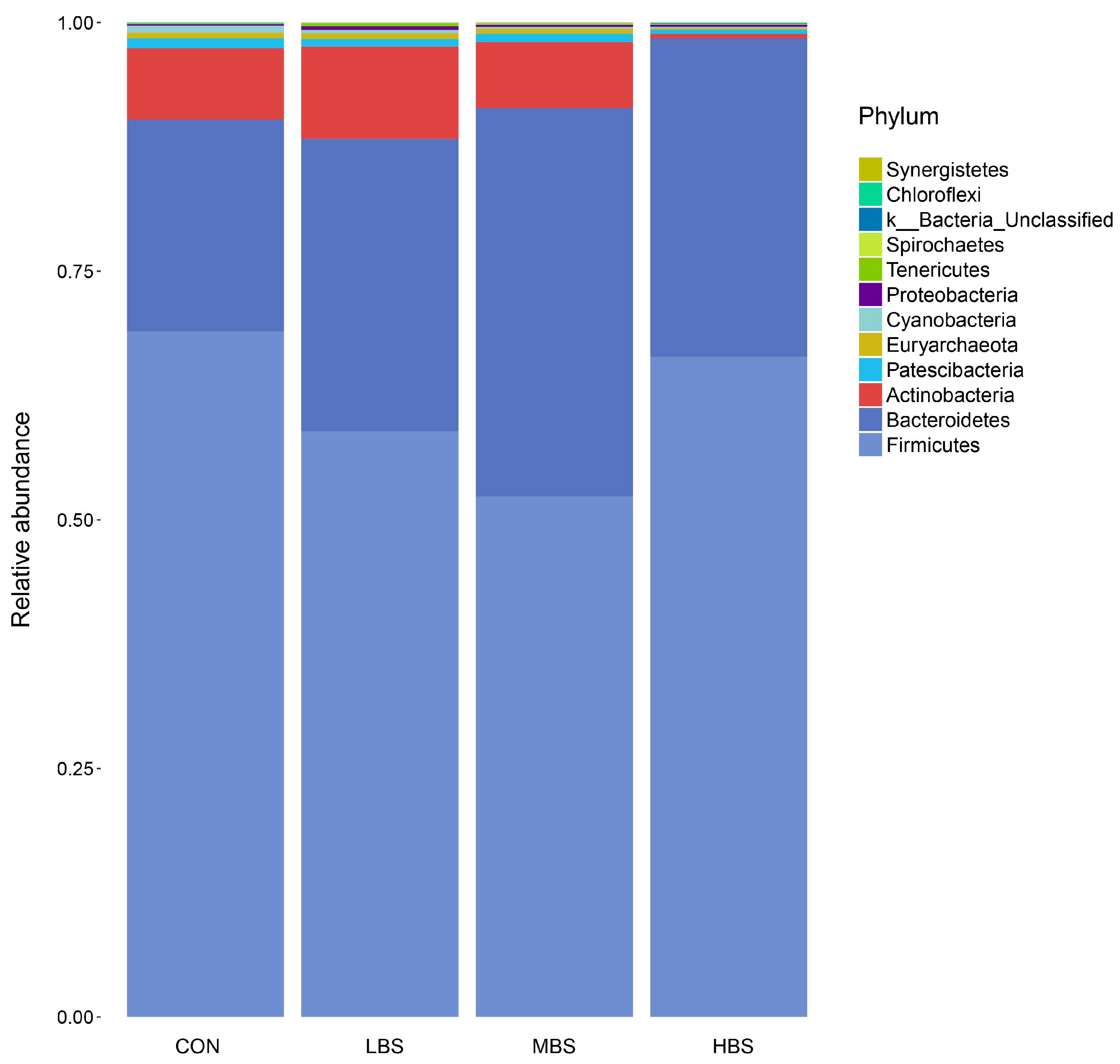
| Item (% of DM 1, Unless Otherwise Stated) | Compositions of Experimental Diet |
|---|---|
| Ingredient | |
| Oat grass hay | 11.7 |
| Alfalfa hay | 6.9 |
| Grounded corn | 62.7 |
| Soybean meal | 11.9 |
| CaHPO 4 | 1.1 |
| NaCl | 0.4 |
| Premix 2 | 5.3 |
| Total | 100.0 |
| Nutrient levels | |
| ME 3 (MJ/kg of DM 1) | 11.6 |
| CP 4 | 10.0 |
| NDF 5 | 32.5 |
| NFC 3 | 66.0 |
| NFC/NDF | 2.0 |
| Ca | 0.6 |
| P | 0.3 |
| Items | Treatments 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LBS | MBS | HBS | |||
| TNF-α (ng/L) 1 | 347.48 a | 342.11 ab | 323.35 b | 324.60 b | 6.86 | 0.044 |
| IL-6 (ng/L) 4 | 29.38 a | 29.43 a | 26.86 b | 26.82 b | 0.62 | 0.005 |
| IL-8 (ng/L) 5 | 254.17 | 252.96 | 251.49 | 245.19 | 7.19 | 0.611 |
| IL-1β (ng/L) 6 | 119.21 a | 106.69 ab | 104.70 b | 105.87 b | 2.14 | <0.001 |
| TLR4 (ng/L) 7 | 34.94 b | 36.38 a | 33.32 b | 35.78 b | 0.50 | 0.002 |
| LPS (EU/mL) | 0.20 a | 0.15 ab | 0.06c | 0.06 c | 0.02 | <0.001 |
| Items | Treatment 3 | SEM 4 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LBS | MBS | HBS | |||
| Ruminal pH | 5.75 b | 5.72 b | 5.86 ab | 6.02 a | 0.08 | 0.006 |
| NH3-N (mg/dL) | 5.73 b | 7.39 ab | 9.63 a | 10.19 a | 1.03 | 0.021 |
| Lactate (mmol/L) | 1.12 a | 0.96 ab | 0.65 b | 0.67 b | 0.12 | 0.023 |
| LPS (×103 EU/mL) | 138.99 a | 92.45 ab | 66.41 b | 56.07 b | 4.80 | <0.001 |
| TVFA (mmol/L) 1 | 109.32 | 114.89 | 109.79 | 114.34 | 0.81 | 0.074 |
| Acetate (%) 2 | 48.29 b | 49.67 b | 58.27 a | 56.60 a | 1.84 | <0.001 |
| Propionate (%) 2 | 28.34 a | 29.72 a | 24.66 ab | 21.09 b | 1.89 | 0.017 |
| Isobutyrate (%) 2 | 0.84 | 0.66 | 0.77 | 1.25 | 0.16 | 0.060 |
| Butyrate (%) 2 | 19.30 a | 17.28 ab | 14.13 b | 15.92 ab | 1.20 | 0.037 |
| Isovalerate (%) 2 | 1.37 ab | 1.07 b | 1.03 b | 1.61 a | 0.15 | 0.037 |
| Valerate (%) 2 | 1.85 a | 1.59 a | 1.14 b | 1.53 a | 0.12 | 0.004 |
| Items | Treatments 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LBS | MBS | HBS | |||
| OUT 3 | 331.13 a | 272.38 b | 317.25 ab | 342.25 a | 17.15 | 0.043 |
| ACE index 4 | 377.38 ab | 329.63 b | 361.55 ab | 392.92 a | 13.74 | 0.023 |
| Chao1 index | 384.70 ab | 333.00 c | 359.23 b | 405.78 a | 14.07 | 0.008 |
| Shannon index | 4.65 | 4.17 | 5.13 | 4.35 | 0.30 | 0.149 |
| Simpson index | 0.85 | 0.82 | 0.90 | 0.78 | 0.06 | 0.292 |
| Coverage (%) | 99.8 | 99.8 | 99.8 | 99.8 | 0.00 | 0.895 |
| Phylum | Treatment 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LBS | MBS | HBS | |||
| Firmicutes | 68.94 | 58.88 | 52.32 | 66.38 | 5.81 | 0.200 |
| Bacteroidetes | 21.34 b | 29.43 ab | 39.11 a | 32.04 ab | 4.38 | 0.041 |
| Actinobacteria | 7.17 | 9.30 | 6.62 | 0.46 | 4.43 | 0.543 |
| Patescibacteria | 0.99 | 0.76 | 0.83 | 0.42 | 0.22 | 0.324 |
| Euryarchaeota | 0.58 | 0.58 | 0.51 | 0.13 | 0.26 | 0.292 |
| Cyanobacteria | 0.66 a | 0.33 ab | 0.16 b | 0.13 b | 0.22 | 0.046 |
| Proteobacteria | 0.18 | 0.33 | 0.23 | 0.24 | 0.09 | 0.675 |
| Tenericutes | 0.02 | 0.31 | 0.07 | 0.07 | 0.15 | 0.435 |
| Spirochaetes | 0.05 | 0.04 | 0.14 | 0.09 | 0.028 | 0.097 |
| k__Bacteria_Unclassified | 0.02 | 0.03 | 0.02 | 0.06 | 0.02 | 0.124 |
| Chloroflexi | 0.06 | 0.01 | 0.03 | 0.02 | 0.02 | 0.361 |
| Synergistetes | 0.005 | 0.011 | 0.006 | 0.008 | 0.03 | 0.465 |
| Phylum | Family | Genus | Treatments 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| CON | LBS | MBS | HBS | |||||
| Bacteroidetes | Prevotellaceae | Prevotella_1 | 7.06 b | 6.53 b | 9.18 a | 13.84 a | 2.09 | 0.041 |
| f__Prevotellaceae_Unclassified | 0.41 b | 8.33 a | 2.62 ab | 0.26 b | 1.99 | 0.030 | ||
| Prevotella_7 | 0.04 b | 0.37 b | 1.77 a | 0.23 b | 0.41 | 0.028 | ||
| Prevotellaceae_UCG-003 | 0.23 b | 0.19 b | 0.51 ab | 0.80 a | 0.17 | 0.045 | ||
| Prevotellaceae_UCG004 | 0.07 b | 0.26 ab | 0.52 a | 0.06 b | 0.12 | 0.018 | ||
| Rikenellaceae | Rikenellaceae_RC9_gut_group | 2.67 b | 3.45 b | 7.21 ab | 11.33 a | 1.75 | 0.008 | |
| Firmicutes | Erysipelotrichaceae | Erysipelotrichaceae_UCG006 | 4.85 a | 3.23 ab | 0.46 b | 0.004 b | 1.16 | 0.022 |
| Erysipelotrichaceae_UCG-004 | 0.25 b | 0.21 b | 0.77 a | 0.33 b | 0.17 | 0.049 | ||
| Erysipelotrichaceae_UCG-009 | 0.07 b | 0.99 a | 0.20 b | 0.05 b | 0.19 | 0.005 | ||
| f__Erysipelotrichaceae_Unclassified | 0.19 a | 0.19 a | 0.09 b | 0.08 b | 0.02 | 0.017 | ||
| Veillonellaceae | Selenomonas_1 | 0.32 b | 0.27 b | 1.04 b | 3.56 a | 0.74 | 0.015 | |
| Succiniclasticum | 0.23 b | 0.25 b | 1.75 a | 0.42 b | 0.32 | 0.009 | ||
| Unclassified | Howardella | 0.60 a | 0.28 ab | 0.18 b | 0.13 b | 0.13 | 0.038 | |
| Lachnospiraceae | Lachnospiraceae_NK3A20_group | 5.09 ab | 8.03 a | 4.19 ab | 1.77 b | 1.60 | 0.046 | |
| Acetitomaculum | 1.42 a | 0.63 ab | 1.09 ab | 0.44 b | 0.27 | 0.043 | ||
| Shuttleworthia | 0.16 b | 0.52 a | 0.32 ab | 0.09 b | 0.11 | 0.044 | ||
| Syntrophococcus | 0.13 b | 0.31 a | 0.14 b | 0.06 b | 0.04 | 0.005 | ||
| lactobacillaceae | Lactobacillus | 0.11 b | 0.71 a | 0.35 ab | 0.03 b | 0.15 | 0.010 | |
| Selenomonadaceae | Schwartzia | 0.02 b | 0.07 b | 0.49 a | 0.02 b | 0.10 | 0.010 | |
| Ruminococcaceae | Ruminococcaceae_UCG002 | 0.02 b | 0.03 b | 0.14 ab | 0.38 a | 0.09 | 0.032 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, G.; Sun, J.; Fan, Y.; Zhao, F.; Ahmed, G.; Jin, Y.; Zhang, Y.; Wang, H. β-Sitosterol Attenuates High Grain Diet-Induced Inflammatory Stress and Modifies Rumen Fermentation and Microbiota in Sheep. Animals 2020, 10, 171. https://doi.org/10.3390/ani10010171
Xia G, Sun J, Fan Y, Zhao F, Ahmed G, Jin Y, Zhang Y, Wang H. β-Sitosterol Attenuates High Grain Diet-Induced Inflammatory Stress and Modifies Rumen Fermentation and Microbiota in Sheep. Animals. 2020; 10(1):171. https://doi.org/10.3390/ani10010171
Chicago/Turabian StyleXia, Guangliang, Jie Sun, Yaotian Fan, Fangfang Zhao, Gulzar Ahmed, Yaqian Jin, Ying Zhang, and Hongrong Wang. 2020. "β-Sitosterol Attenuates High Grain Diet-Induced Inflammatory Stress and Modifies Rumen Fermentation and Microbiota in Sheep" Animals 10, no. 1: 171. https://doi.org/10.3390/ani10010171
APA StyleXia, G., Sun, J., Fan, Y., Zhao, F., Ahmed, G., Jin, Y., Zhang, Y., & Wang, H. (2020). β-Sitosterol Attenuates High Grain Diet-Induced Inflammatory Stress and Modifies Rumen Fermentation and Microbiota in Sheep. Animals, 10(1), 171. https://doi.org/10.3390/ani10010171




